


The article asserts that clinical research partnerships significantly enhance the efficiency and effectiveness of medical trials, resulting in faster approvals and improved patient outcomes. It elaborates on various benefits, including:
These elements collectively contribute to a streamlined process for bringing new therapies to market, underscoring the critical role of collaboration in advancing medical research.
In the rapidly evolving landscape of medical innovation, clinical research partnerships emerge as a pivotal force for accelerating trial timelines and enhancing the quality of outcomes. These collaborations streamline processes and foster patient diversity, ensuring that new therapies are effective across varied demographics. However, the pressing question remains: how can organizations leverage these partnerships to overcome the inherent challenges of clinical trials and achieve faster, more reliable results?
The company adeptly navigates the regulatory landscapes of Latin America, the Balkans, and Australia, securing ethical approvals in an impressive 4-6 weeks. This expedited process is crucial for Medtech, Biopharma, and Radiopharma innovators aiming to fast-track their trials.
With over 20 years of experience, the company employs a tailored strategy to enhance the approval process, not only accelerating the time to market for innovative therapies but also expanding patient access to essential medical advancements.
In a landscape where the average duration for ethical approvals can extend significantly longer, this organization distinguishes itself as a leader in establishing clinical research partnerships, empowering companies to respond swiftly to patient needs and market demands.
Industry leaders have underscored the significance of rapid ethical approvals, highlighting that timely access to new therapies can profoundly influence patient outcomes.
Through a clinical research partnership with various healthcare institutions and community organizations, such as the Caribbean Health Group, bioaccess® is able to engage effectively with diverse patient populations. This strategic approach not only enhances the data collected during tests but also ensures that the resulting treatments are effective across various demographics.
With the support of Colombia's Minister of Health, these collaborations aim to create a clinical research partnership that positions Barranquilla as a leading site for medical trials in Latin America. By prioritizing patient diversity, this method bolsters the reliability of medical study results, paving the way for innovative treatments that cater to a broader spectrum of patients.
The service delivers cost-effective clinical research partnership solutions that empower Medtech and Biopharma companies to manage their budgets efficiently. By identifying funding opportunities and enhancing resource distribution, bioaccess® supports clients in conducting high-quality studies through a clinical research partnership without exceeding financial limits. This financial agility is crucial for startups that aim to maximize their impact while minimizing costs.
Notably, funding opportunities in Latin America are particularly advantageous due to the region's diverse patient populations and regulatory efficiency, which can significantly reduce study costs. Industry leaders emphasize the importance of leveraging available funding through a clinical research partnership to enhance the sustainability of Medtech startups, ensuring they can effectively navigate the complexities of research processes.
With over 20 years of experience in Medtech, the team is well-prepared to guide clients through the funding landscape. Given that the typical expense of medical studies ranges from $1 million to over $100 million depending on the phase and therapeutic field, identifying and securing funding is vital for sustaining innovation and advancing medical breakthroughs.
To effectively pinpoint funding opportunities, startups should:
An advanced method is employed to simplify medical studies, significantly enhancing efficiency from participant recruitment to information management. By leveraging cutting-edge technologies and optimizing workflows, the organization minimizes the time needed to bring new therapies to market. This acceleration in the development process ensures that patients gain quicker access to potentially life-saving treatments.
The implementation of centralized electronic solutions and real-time milestone tracking facilitates superior project management and oversight, effectively reducing delays in site initiation and patient enrollment. Furthermore, innovative software solutions address challenges in research supply and production, promoting a smoother operational flow.
Consequently, this approach illustrates how technology and efficient processes can transform the medical study landscape, ultimately benefiting both sponsors and patients alike.
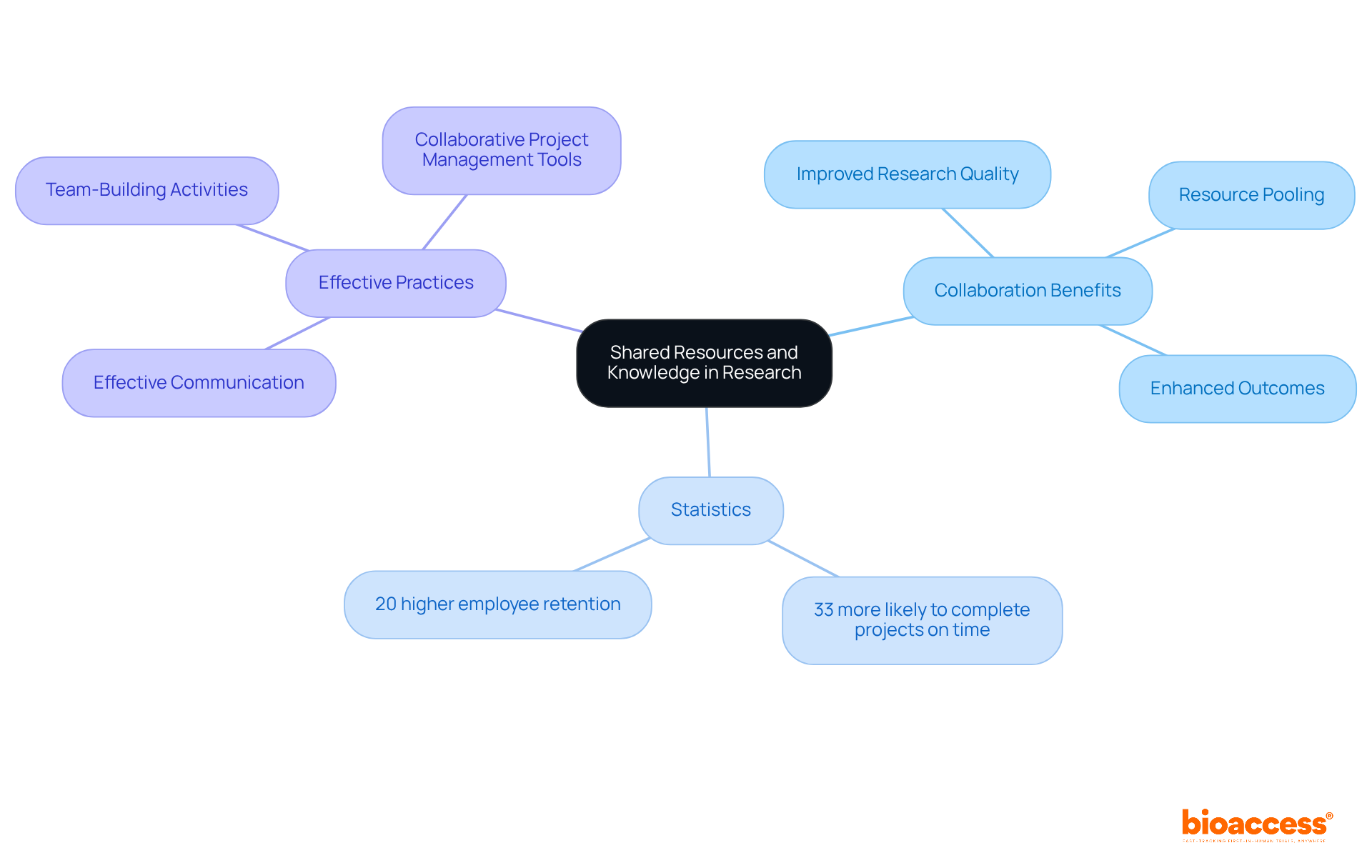
This organization forges collaborations that facilitate the exchange of resources and expertise among study groups, significantly elevating the quality of clinical assessments. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its inaugural human trial in Colombia, underscoring the effectiveness of collaborative environments in integrating diverse expertise and advanced technologies. Such clinical research partnerships are essential for addressing complex challenges within the field.
Research indicates that collaborative teams are 33% more likely to complete projects on time, highlighting the efficiency gained through teamwork. Furthermore, organizations that foster collaboration enjoy a 20% higher employee retention rate, as noted by Deloitte, which cultivates a stable environment conducive to high-quality studies.
In a clinical research partnership, pooling resources allows partners to leverage each other's strengths, resulting in more reliable outcomes and enhanced statistical power in medical trials. This synergy not only bolsters the credibility of study results but also accelerates the development of innovative therapies, ultimately benefiting patient care. Effective communication can further reduce the time spent on conflict resolution by 50%, enhancing the collaborative process.
To fully capitalize on these advantages, research directors must proactively implement collaborative practices within their teams.
By leveraging collaborative networks, bioaccess® significantly broadens the geographical and demographic scope of clinical research partnerships. This strategic approach not only amplifies the potential participant pool but also enhances the diversity of participants, which is vital for generating data that accurately mirrors real-world patient populations in a clinical research partnership.
For instance, the partnership with Caribbean Health Group aims to position Barranquilla as a leading hub for medical research in Latin America, a move supported by Colombia's Minister of Health. Such collaborations within a clinical research partnership streamline patient enrollment, addressing a challenge faced by approximately 80% of research studies due to recruitment difficulties. Notably, nearly 40% of research studies experience delays or terminations as a result of these issues, highlighting the necessity of a clinical research partnership.
By engaging diverse communities and utilizing proven recruitment strategies, the organization enhances the inclusivity of its studies, ultimately yielding more reliable and applicable outcomes. This approach aligns with findings that suggest effective clinical research partnerships can significantly enhance participant diversity, thereby elevating the overall quality and credibility of research results.
Moreover, collaborations such as the one with GlobalCare Clinical Trials highlight how a clinical research partnership can achieve over a 50% reduction in recruitment time and 95% retention rates, further emphasizing the critical role of these collective efforts in securing regulatory approvals.

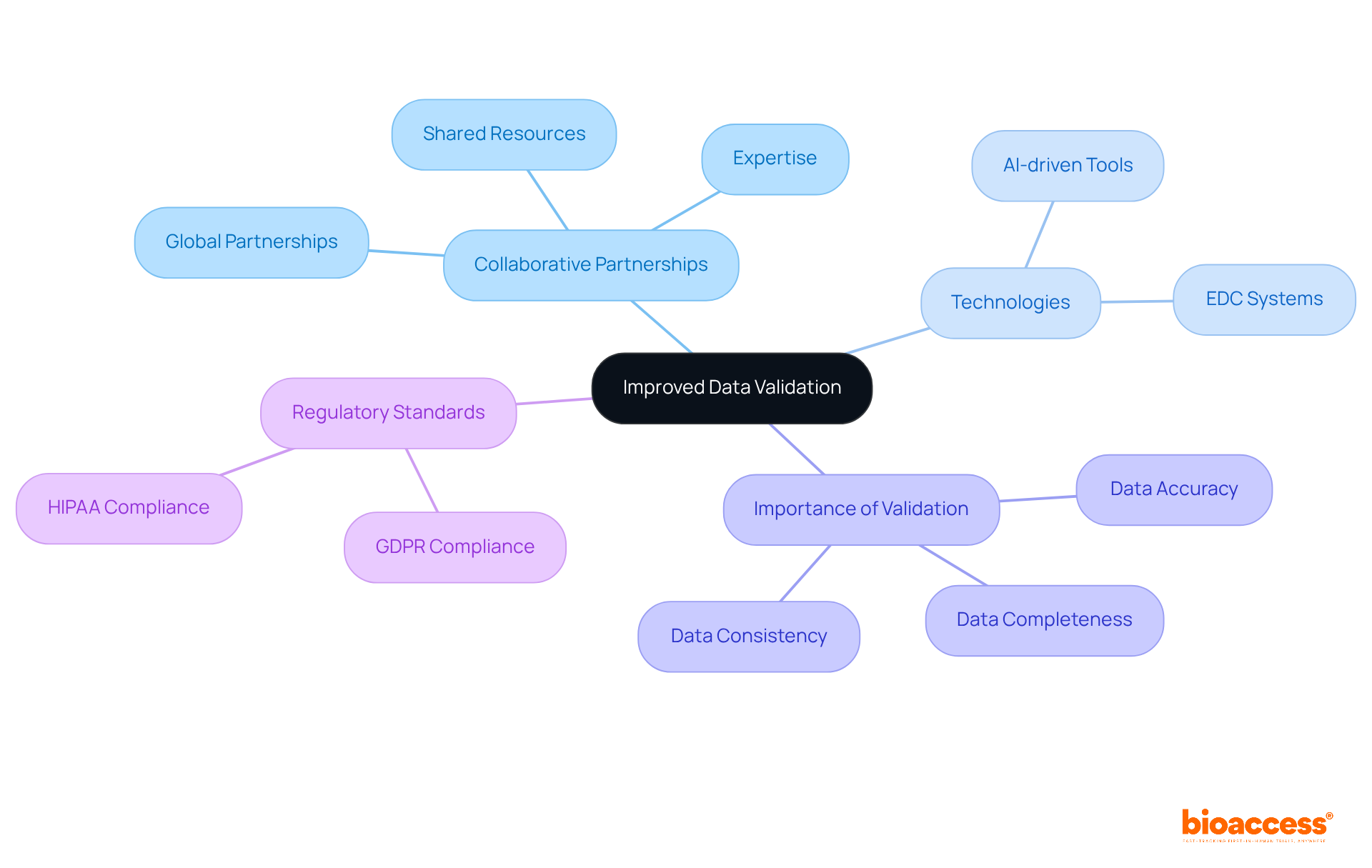
Collaborative partnerships empower bioaccess® to establish robust data validation processes that enhance the precision and reliability of data within the clinical research partnership. Through a clinical research partnership, partners can leverage shared resources and expertise to implement comprehensive validation checks, ensuring that the data collected is not only complete but also consistent across various sources. This rigorous methodology is crucial, as research indicates that data errors in medical studies can range from 2.3% to 26.9% due to human input mistakes. Such discrepancies underscore the necessity for meticulous validation to maintain the integrity of findings.
Moreover, innovative connections link cutting-edge Medtech, Biopharma, and Radiopharma startups with esteemed experimental sites in Latin America, Eastern Europe, and Australia, facilitating expedited studies through a clinical research partnership. Collaborative efforts also enable the integration of advanced technologies, such as AI-driven tools, which automate source data verification and enhance data quality. For example, these tools improve accuracy verification by aligning information from multiple sources and swiftly addressing discrepancies. Additionally, electronic data capture (EDC) systems play a vital role in facilitating real-time validation checks, further ensuring data integrity.
By fostering a culture of continuous improvement and adherence to regulatory standards, including compliance with global guidelines like GDPR and HIPAA, these clinical research partnerships significantly enhance the credibility of research. Ultimately, this ensures that stakeholders can place their trust in the results of studies.
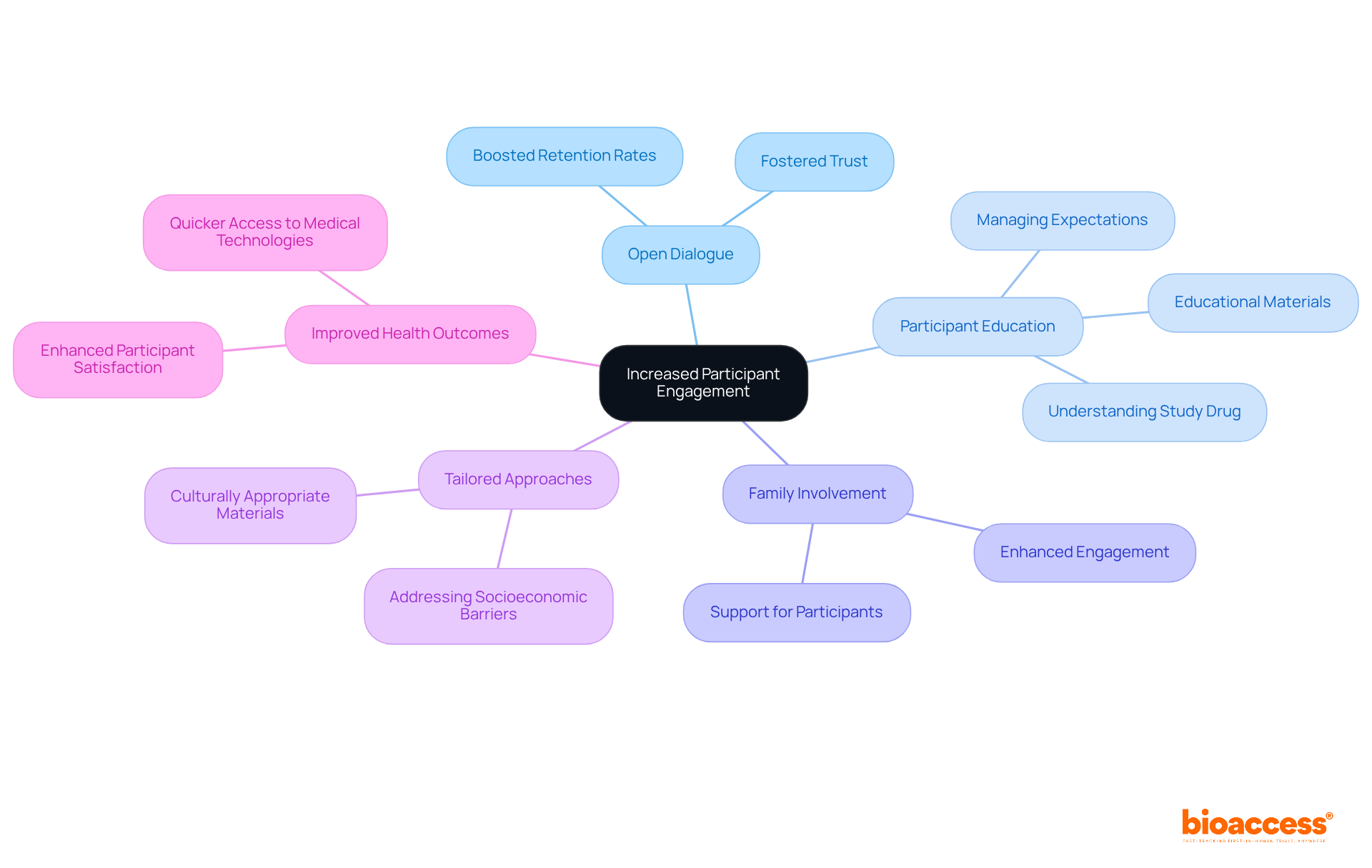
The company employs innovative strategies to enhance participant involvement throughout the clinical study process, particularly in the diverse and complex landscape of Latin America. By promoting open dialogue and engaging participants in decision-making, the program significantly boosts retention rates, ensuring that individuals feel valued. This involvement is crucial in a region where understanding local healthcare systems and cultural nuances can greatly influence study success.
For instance, akin to the approach taken by the Madras Diabetes Research Foundation, the program recognizes the importance of participant education and family involvement in sustaining engagement and minimizing dropout rates. By leveraging regional knowledge and tailoring approaches to meet specific national requirements, the organization not only enhances the study but also contributes to improved health outcomes for participants, ultimately facilitating quicker access to novel medical technologies.
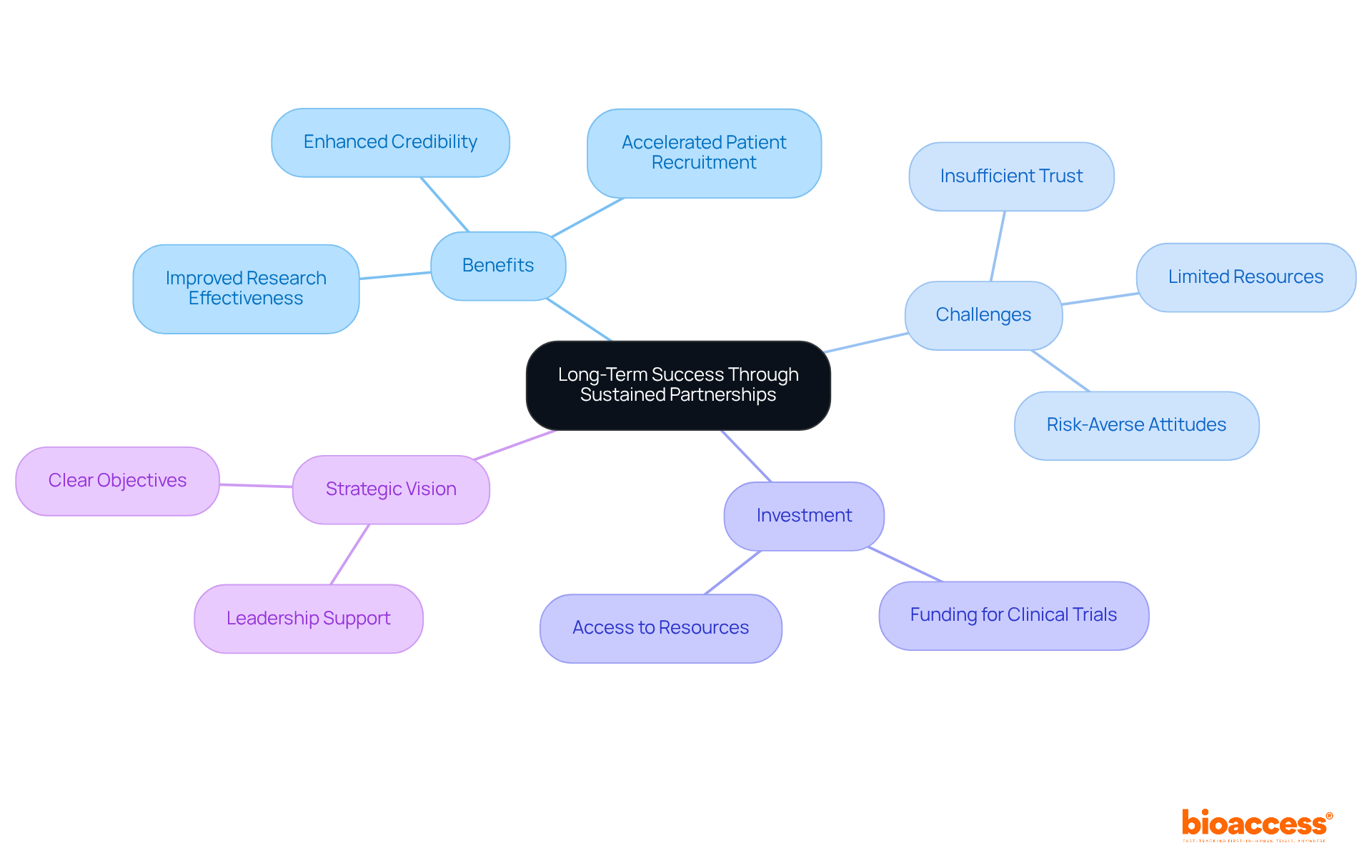
The company emphasizes the essential role of long-term clinical research partnerships with clients and collaborators in the Medtech and Biopharma sectors. These relationships cultivate a dynamic environment conducive to continuous improvement and innovation, allowing all parties to effectively leverage shared knowledge and resources. By nurturing these clinical research partnerships, the organization improves the effectiveness of research studies and significantly increases the likelihood of favorable outcomes.
With over 50 pre-qualified locations activated in less than eight weeks, it accelerates patient recruitment—a notable challenge in research studies—by utilizing broader networks and established connections. Research indicates that approximately 80% of medical trials do not meet initial enrollment targets, underscoring the critical need for effective collaboration.
Successful clinical research partnerships also enhance credibility, attracting more investors and facilitating faster market access. As David Shoultz aptly noted, "Success factors often relate to aspects of management, leadership, and/or people involved," emphasizing the importance of strategic vision and support from senior leaders in ensuring the success of these collaborations.
By investing in long-term clinical research partnerships and providing FDA/EMA/MDR-ready datasets with centralized monitoring, the company guarantees that its initiatives are not only successful but also positioned for sustained growth and innovation.
Advancing Medical Technologies Through Collaborative Research
Collaborative research efforts are essential in propelling medical technologies forward, with bioaccess® at the forefront of these initiatives. By bringing together diverse expertise and resources, the organization and its partners promote innovation and create new therapies that effectively tackle unmet medical needs. This synergy not only streamlines the investigation process but also significantly enhances healthcare results, aligning with our commitment to innovation and quality in healthcare.
At bioaccess®, we invite you to join us in this mission to advance medical technologies. Our distinctive approaches in collaborative studies utilize advanced technologies and regulatory excellence, ensuring that we stay at the forefront of Medtech clinical trials in Latin America.
For instance, partnerships like Apple's Digital Health Records Initiative, which has engaged over 40 hospitals globally, have improved patient data management and accessibility. Likewise, Google Cloud's partnership with Massachusetts General Hospital has enabled secure data sharing while complying with HIPAA regulations, thus improving investigative capabilities.
Statistics underscore the importance of these collaborations:
However, challenges remain, as many healthcare institutions struggle to navigate the complexities of integrating these advanced technologies into their existing systems.
The role of cooperative study in developing new therapies cannot be overstated. By leveraging the strengths of various stakeholders, this initiative exemplifies how a clinical research partnership can drive innovation, ultimately resulting in faster trials and better healthcare solutions for patients worldwide. This collaborative approach not only enhances healthcare outcomes but also contributes to local economies through job creation and economic growth, thereby reinforcing bioaccess®'s position as a leader in Medtech clinical research partnership in Latin America. As Tressa Springmann noted, 'The cat's out of the bag here on consumerism. Patients are going to drive us based on their comfort and utilization of technology,' highlighting the critical need for innovation in response to patient engagement.

The advantages of clinical research partnerships are multifaceted, significantly influencing the speed and effectiveness of medical trials. By fostering collaborations that prioritize ethical approvals, enhance patient diversity, and streamline processes, these partnerships not only accelerate the time to market for new therapies but also ensure that they are accessible to a wider range of patients. The strategic alliances formed through these partnerships create a robust framework for innovation, ultimately benefiting both healthcare providers and those in need of new medical solutions.
Key insights from the article highlight the importance of:
The ability to navigate regulatory landscapes efficiently, engage with varied demographics, and leverage shared resources enhances the quality of research outcomes. Furthermore, the emphasis on long-term partnerships cultivates an environment ripe for continuous improvement and innovation, propelling advancements in medical technologies.
In light of these insights, it is clear that embracing collaborative approaches in clinical research is vital for driving progress in healthcare. Stakeholders are encouraged to actively seek out partnerships that align with their goals, as these relationships not only enhance the credibility and efficiency of research efforts but also contribute to the overall betterment of patient care. By prioritizing collaboration, the industry can ensure that innovative therapies reach those who need them most, fostering a healthier future for all.
What is bioaccess and what services does it provide?
Bioaccess is a company that specializes in navigating regulatory landscapes to secure ethical approvals for clinical trials in Latin America, the Balkans, and Australia. It offers expedited ethical approval processes, typically completing them in 4-6 weeks, which is crucial for Medtech, Biopharma, and Radiopharma innovators.
How does bioaccess enhance the ethical approval process?
With over 20 years of experience, bioaccess employs a tailored strategy to accelerate the approval process, which helps bring innovative therapies to market faster and increases patient access to essential medical advancements.
Why are rapid ethical approvals important in clinical research?
Rapid ethical approvals are significant because they allow for timely access to new therapies, which can profoundly influence patient outcomes and enable companies to respond quickly to patient needs and market demands.
How does bioaccess promote patient diversity in clinical trials?
Bioaccess engages in clinical research partnerships with healthcare institutions and community organizations to effectively reach diverse patient populations. This approach enhances data reliability and ensures treatments are effective across various demographics.
What are the benefits of the collaborations bioaccess is involved in?
Collaborations, such as those with the Caribbean Health Group and support from Colombia's Minister of Health, aim to position Barranquilla as a leading site for medical trials in Latin America, enhancing patient diversity and the reliability of study results.
How does bioaccess help Medtech and Biopharma companies manage their budgets?
Bioaccess provides cost-effective clinical research partnership solutions by identifying funding opportunities and improving resource distribution, allowing clients to conduct high-quality studies without exceeding financial limits.
Why is funding important for Medtech startups?
Funding is vital for Medtech startups to sustain innovation and advance medical breakthroughs, especially given the high costs of medical studies, which can range from $1 million to over $100 million depending on various factors.
What strategies should startups use to identify funding opportunities?
Startups should proactively engage with local funding agencies, explore partnerships with established organizations, and leverage resources from industry networks to effectively identify and secure funding opportunities.