

The article underscores ten significant benefits of clinical trial remote monitoring for research directors, asserting its efficiency in accelerating trials, enhancing patient safety, and improving data quality. These advantages are substantiated by compelling evidence indicating:
Collectively, these factors contribute to more effective and reliable clinical research outcomes.
The landscape of clinical trials is rapidly evolving, driven by the need for efficiency, cost-effectiveness, and enhanced patient safety. Remote monitoring has emerged as a transformative solution, offering research directors a multitude of benefits that streamline operations and improve outcomes. As organizations embrace this innovative approach, questions arise about how to effectively implement these technologies while ensuring compliance and participant engagement.
What are the key advantages of integrating remote monitoring into clinical trials?
How can it redefine the future of medical research?
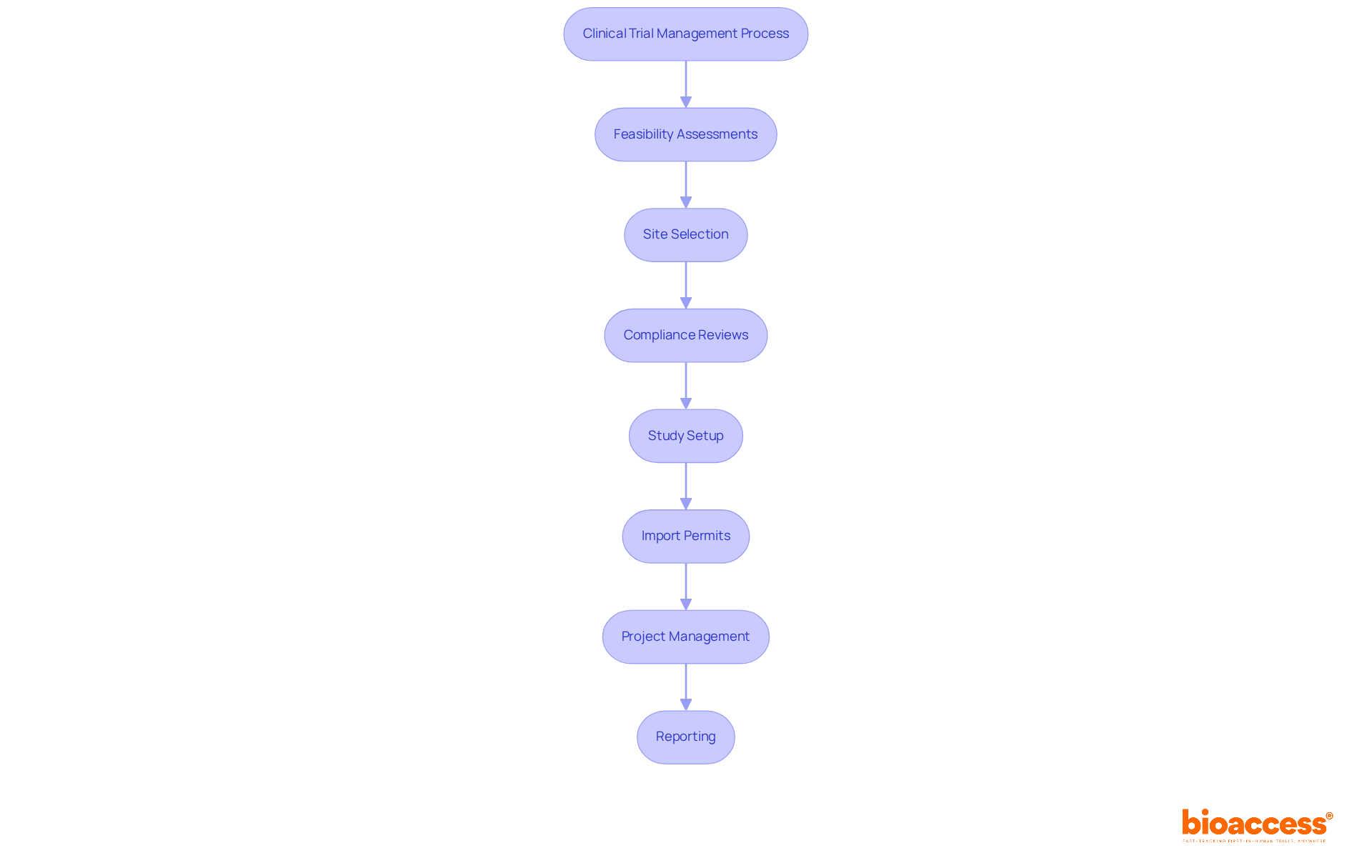
bioaccess® employs sophisticated systems for clinical trial remote monitoring that simplify clinical studies, significantly decreasing the time required for ethical approvals and patient enrollment. With a proven track record spanning over 20 years in Medtech, bioaccess® offers comprehensive clinical study management services, which include:
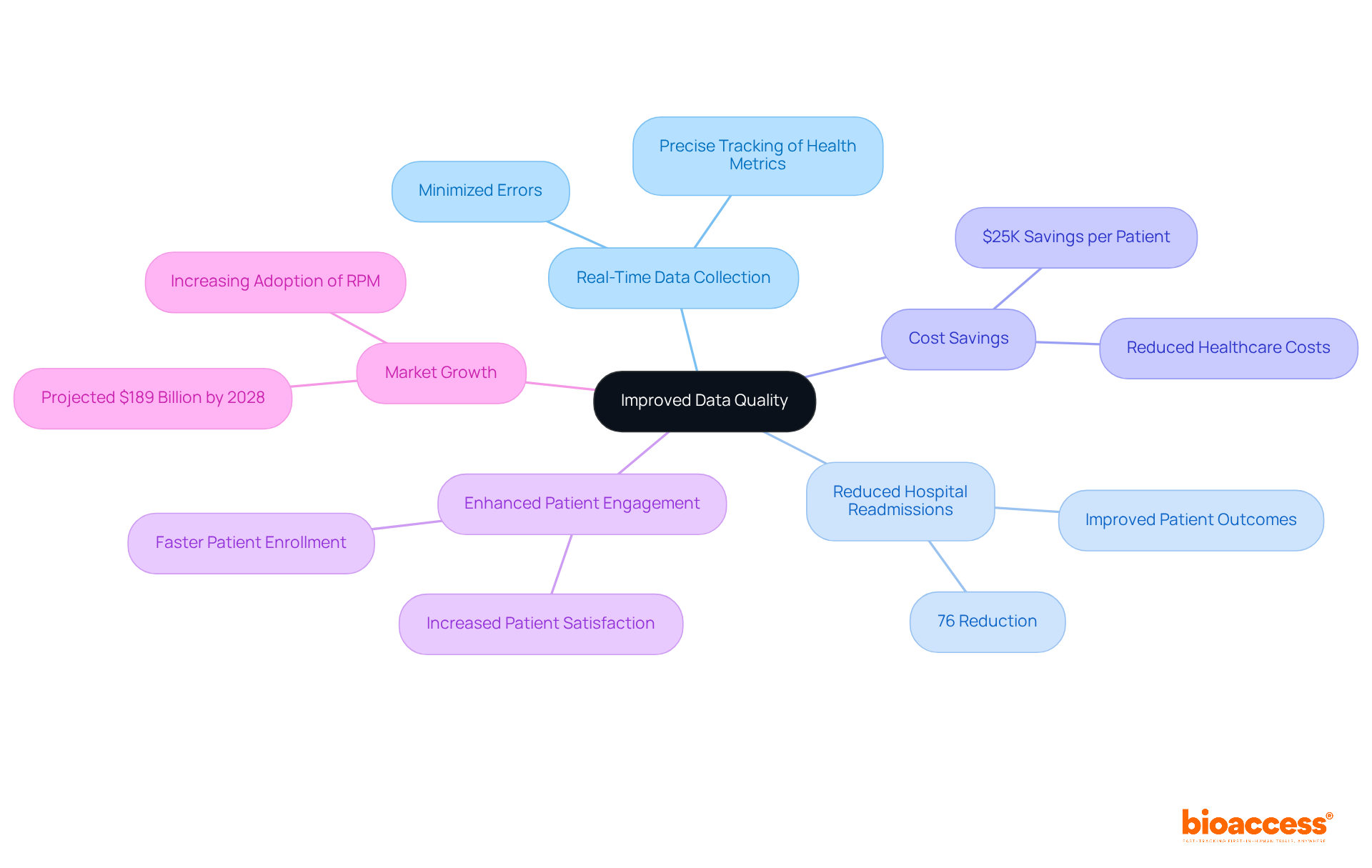
By integrating digital tools, bioaccess ensures that experiments are conducted efficiently, facilitating quicker data collection and analysis. This innovative approach to clinical trial remote monitoring accelerates the trial process—achieving patient enrollment 50% faster and saving $25K per patient with FDA-ready data—while enhancing the overall quality of research conducted in diverse regions such as Latin America, the Balkans, and Australia.
Remote oversight through clinical trial remote monitoring can lead to substantial cost savings by minimizing the need for on-site staff and reducing travel expenses. By utilizing digital platforms for clinical trial remote monitoring and data gathering, organizations can reduce operational expenses by up to 46.2%. This financial efficiency enables sponsors to distribute resources more effectively, ultimately improving the project's return on investment. Furthermore, studies suggest that clinical trial remote monitoring can reduce yearly healthcare expenses for diabetic individuals by $5,000, demonstrating the wider financial advantages of incorporating such technologies into research.
Industry leaders stress that the progression towards intelligent, data-driven supervision, particularly through clinical trial remote monitoring, is vital for establishing a more robust and effective research environment. As Lifebit states, 'Through real-time data access, centralized oversight, and risk-based approaches, we are catching issues faster and generating more reliable data.' Additionally, clinical trial remote monitoring enhances patient involvement and retention in research studies, further aiding in better results. This approach aligns with bioaccess's comprehensive clinical trial management services, which include:
Ultimately supporting the goal of making Barranquilla a leading destination for clinical trials in Latin America.
Remote observation through clinical trial remote monitoring significantly enhances patient safety by enabling continuous oversight of participants' health data. This capability allows researchers to identify potential issues in real-time, facilitating prompt interventions when necessary. For instance, a study at the University of Pittsburgh Medical Center demonstrated that implementing remote patient monitoring led to a remarkable 76% reduction in hospital readmissions, while patient satisfaction scores soared above 90%. Furthermore, RPM adoption increased by roughly 1,300% in the U.S. from 2019 to 2022, emphasizing its rising significance in research studies.
By utilizing technology, clinical trial remote monitoring enables research studies to maintain high safety standards and lessen the burden on participants, who can take part in studies from the comfort of their homes. With almost 89% of patients indicating higher satisfaction when utilizing remote health tools, and 80% of Americans endorsing RPM, it is evident that this method not only boosts safety but also enriches the overall experience. Furthermore, the UC Davis Health remote blood pressure tracking program showcased significant improvements in patient outcomes, reinforcing the effectiveness of RPM in clinical settings.

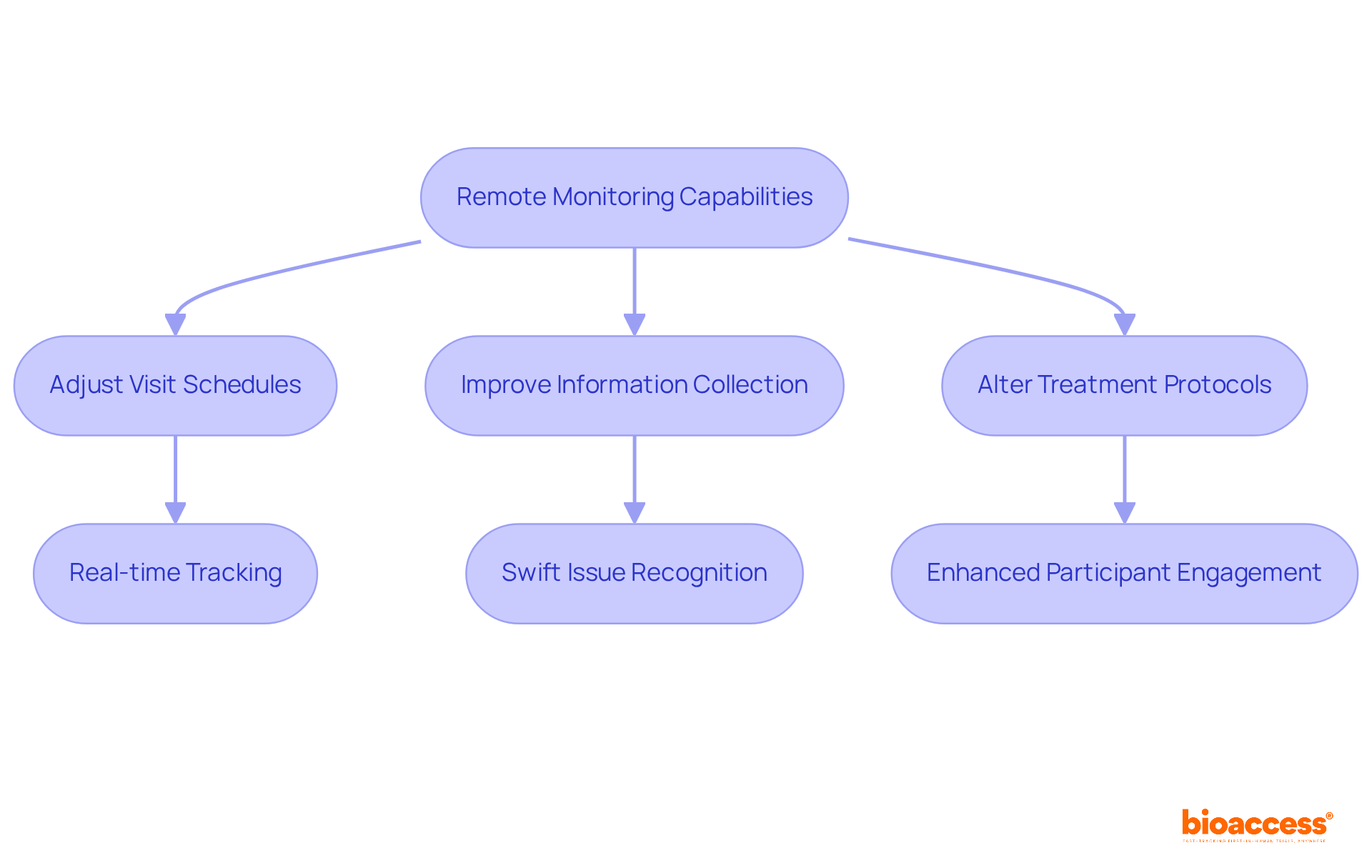
The flexibility of trial protocols is significantly enhanced by clinical trial remote monitoring, empowering researchers to swiftly adapt to evolving circumstances and participant needs. This adaptability encompasses various changes, such as:
For instance, the capability to track enrollment information in real-time enables study teams to recognize and tackle issues swiftly, thereby reducing delays and enhancing participant engagement. Medical researchers have observed that this real-time information access not only boosts participant satisfaction but also contributes to the overall success of the study. The incorporation of clinical trial remote monitoring technology fosters a more adaptive approach to clinical research, ensuring that trials can evolve in accordance with participant feedback and emerging information trends.
Remote observation significantly enhances information quality by facilitating real-time data collection and analysis. This immediacy minimizes errors typically associated with manual input, allowing for precise tracking of participant health metrics. Notably, the University of Pittsburgh Medical Center reported an impressive 76% reduction in hospital readmission rates following the implementation of remote patient monitoring (RPM). This underscores the effectiveness of accurate information gathering in improving patient outcomes. By ensuring that data is collected consistently and reliably, researchers can derive more robust conclusions from their studies.
This capability not only leads to better patient outcomes but also enables research associates to focus on analysis and oversight, ultimately promoting higher-quality results in clinical studies. With bioaccess®'s innovative solutions, studies can achieve patient enrollment 50% faster and save $25K per patient through FDA-ready data, eliminating rework and delays. As a result, the integration of clinical trial remote monitoring technologies is becoming essential for modern medical research, with projections indicating that the clinical trial remote monitoring market will grow substantially, approaching $189 billion by 2028.
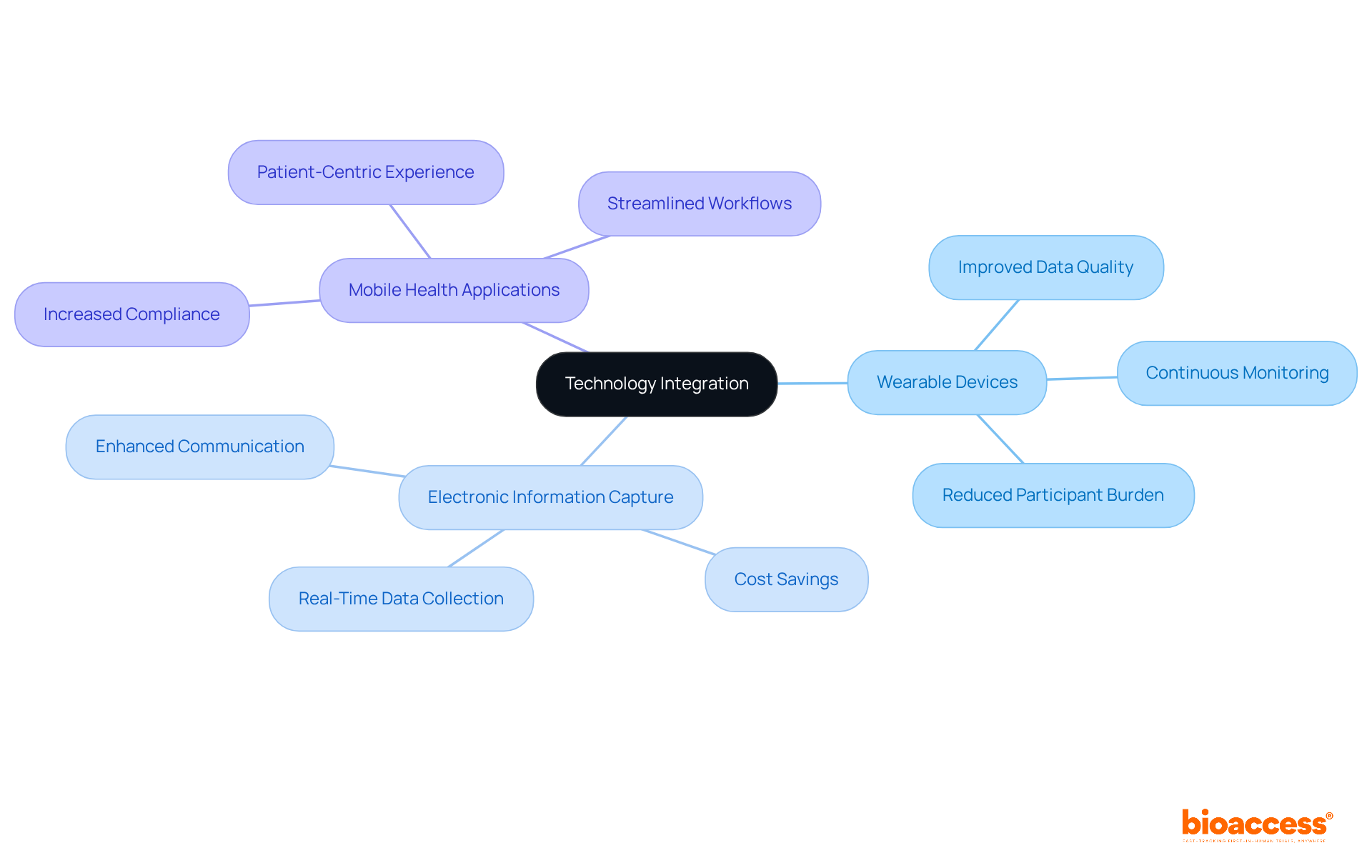
Incorporating digital tools into research oversight procedures is essential for enhancing efficiency and precision. Technologies such as electronic information capture systems, wearable devices, and mobile health applications simplify information collection and improve communication between researchers and participants. For instance, wearables are now employed in over 4,000 clinical studies, enabling clinical trial remote monitoring and providing objective health information that conventional methods frequently overlook. This shift not only reduces participant burden by eliminating the need for manual data entry but also enhances data quality by capturing real-time insights.
Industry leaders emphasize the transformative potential of these technologies. One researcher noted, "Wearables will see continued investment led by site networks and governments because they deliver the best patient and clinician results." Furthermore, the application of digital tools has been shown to significantly improve recruitment and retention rates in studies, with research indicating that digitally-enhanced studies surpass conventional methods in achieving enrollment goals.
Moreover, the adoption of a 'Bring Your Own Device' (BYOD) approach allows participants to use their own devices, leading to increased compliance and cost savings. This model not only fosters a more patient-centric experience but also aligns with the growing trend of integrating technology into everyday healthcare practices. As the landscape of clinical research evolves, the implementation of clinical trial remote monitoring and digital tools will be crucial for ensuring that all stakeholders have immediate access to vital information, ultimately promoting the success of clinical studies.
Remote oversight practices significantly enhance regulatory compliance by facilitating transparent and traceable data collection methods. By leveraging digital platforms that align with regulatory standards, sponsors can ensure that all study activities are meticulously documented and readily accessible for audits. This enhanced level of compliance not only safeguards participant safety but also fosters trust with regulatory bodies.
As Andrea Bastek, a prominent authority in research studies, points out, the incorporation of specially designed tools for clinical trial remote monitoring can significantly enhance adherence, enabling real-time oversight and prompt recognition of compliance patterns. Moreover, bioaccess provides extensive management services for studies, including:
These services are vital for maneuvering through the intricacies of regulatory requirements.
Statistics suggest that distant patient observation can decrease hospital readmissions by as much as 66% and enhance patient satisfaction ratings to over 90%, highlighting its efficacy in research trials. However, it is crucial to recognize possible regulatory hurdles, such as HIPAA compliance and state laws, which must be navigated to fully achieve the advantages of clinical trial remote monitoring in clinical research.

Remote monitoring in clinical trial remote monitoring significantly enhances participant engagement by empowering individuals to take an active role in managing their health. Digital platforms facilitate easy access to health data, which supports clinical trial remote monitoring by enabling participants to communicate seamlessly with researchers and receive timely reminders for assessments. This proactive involvement not only enhances participant satisfaction but also results in better retention rates during the clinical trial remote monitoring.
Thorough research study management services provided by bioaccess™, encompassing feasibility assessments, site selection, and compliance evaluations, guarantee that studies are carried out efficiently and effectively. The partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for medical research in Latin America, backed by Colombia's Minister of Health. This initiative not only boosts participant involvement but also aids local economic development, job creation, and better healthcare results, ultimately resulting in higher retention rates and successful study completions.

Remote monitoring in clinical trial remote monitoring empowers organizations to enhance their clinical research operations with exceptional efficiency. By leveraging centralized digital platforms, research teams can effectively manage multiple studies across diverse locations, significantly reducing the need for extensive on-site visits. This scalability not only optimizes resource allocation but also accelerates the overall testing process, facilitating quicker results and a faster time-to-market for groundbreaking therapies.
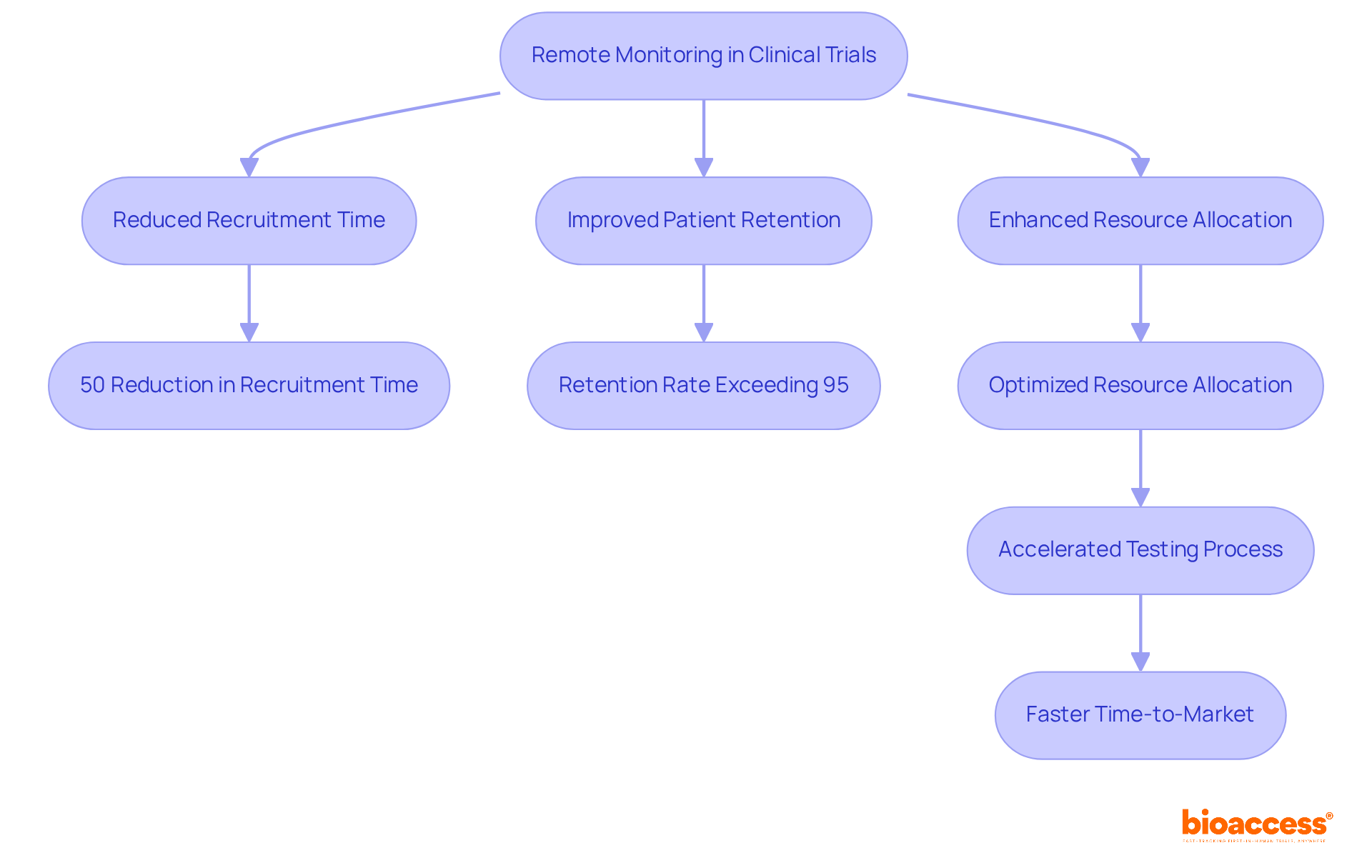
For example, GlobalCare Clinical Trials, in collaboration with bioaccess™, has successfully expanded its ambulatory services in Colombia, utilizing bioaccess's extensive presence in the region to support global pharmaceutical clients. This partnership has achieved over a 50% reduction in clinical study subject recruitment time, alongside a retention rate exceeding 95%. Such efficiencies are vital in an industry where timely patient access to new treatments can profoundly impact health outcomes. Research indicates that hybrid evaluation models can enhance the number of patient visits assessed by 34%, while simultaneously reducing review costs by as much as 46.2%. Moreover, these models have demonstrated a reduction in total monitoring duration by 13.8%, further enhancing timelines.
Real-time dashboards and Key Risk Indicators (KRIs) facilitate early detection of trends and outliers, leading to swifter decision-making and improved management. Furthermore, bioaccess® brings over 20 years of experience in overseeing a variety of research studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring comprehensive support for research initiatives. Embracing clinical trial remote monitoring not only optimizes operations but also allows organizations to swiftly adapt to the evolving landscape of medical studies.
Remote monitoring, specifically clinical trial remote monitoring, plays a crucial role in expediting participant recruitment by broadening access to clinical studies. This innovative approach allows individuals to participate from the comfort of their homes, thereby attracting a wider range of participants, particularly from distant or underserved areas. Such enhanced accessibility not only boosts recruitment rates but also fosters more representative study populations. Notably, studies indicate that digital recruitment methods can increase enrollment rates by up to 21%, while patient-centric strategies can enhance these rates by as much as 25%.
Moreover, the incorporation of mobile health applications facilitates real-time communication, simplifying the interaction process between potential participants and study coordinators. Consequently, the overall quality and generalizability of research outcomes are greatly enhanced, addressing the essential demand for diversity in medical studies. Clinical study recruiters have observed that the use of clinical trial remote monitoring strategies can lead to a more effective enrollment process, ultimately benefiting both participants and researchers alike.
In partnership with Caribbean Health Group, bioaccess™ is actively striving to establish Barranquilla as a premier location for medical studies in Latin America, with backing from Colombia's Minister of Health. This initiative aims to enhance clinical trial ambulatory services, achieving a remarkable 50% reduction in recruitment time and 95% retention rates. Such outcomes showcase the effectiveness of accelerated clinical trials through innovative strategies.

The integration of clinical trial remote monitoring signifies a transformative shift in research methodologies, streamlining processes and enhancing outcomes for both researchers and participants. By leveraging advanced technologies and digital tools, this innovative approach not only accelerates patient enrollment but also ensures the collection of high-quality data, ultimately leading to more efficient clinical trials.
Throughout this discussion, key benefits of remote monitoring have been underscored, including:
Organizations are achieving faster recruitment rates and enhanced participant engagement, illustrating the potential for remote monitoring to revolutionize clinical research. The emphasis on regulatory compliance and the ability to manage multiple trials efficiently further underscores the importance of adopting these practices in modern medical research.
As the landscape of clinical trials evolves, embracing clinical trial remote monitoring is essential for research directors aiming to enhance efficiency and effectiveness. The call to action is unequivocal: organizations must invest in these technologies to not only bolster their research capabilities but also to ensure they remain at the forefront of innovation in the healthcare sector. By doing so, they can contribute to a more robust and responsive clinical research environment that prioritizes patient safety and accelerates the development of groundbreaking therapies.
What is bioaccess and what services does it offer for clinical trials?
bioaccess® is a company that employs advanced systems for remote monitoring in clinical trials, simplifying the process and reducing the time for ethical approvals and patient enrollment. Its services include feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting.
How does bioaccess improve the efficiency of clinical trials?
By integrating digital tools, bioaccess enhances the efficiency of clinical trials, facilitating quicker data collection and analysis. This approach allows for patient enrollment to be achieved 50% faster and saves $25K per patient with FDA-ready data.
What are the cost savings associated with clinical trial remote monitoring?
Clinical trial remote monitoring can reduce operational expenses by up to 46.2% by minimizing the need for on-site staff and lowering travel costs. This financial efficiency helps sponsors allocate resources more effectively, improving the return on investment for projects.
How does remote monitoring impact patient safety in clinical trials?
Remote monitoring significantly enhances patient safety by allowing continuous oversight of participants' health data, enabling researchers to identify issues in real-time and intervene promptly. This has led to a 76% reduction in hospital readmissions in some studies.
What are the patient satisfaction rates associated with remote patient monitoring?
Approximately 89% of patients report higher satisfaction when using remote health tools, and 80% of Americans support the use of remote patient monitoring, indicating its positive impact on the patient experience.
What regions does bioaccess focus on for conducting clinical trials?
bioaccess conducts clinical trials in diverse regions, including Latin America, the Balkans, and Australia, with the goal of making Barranquilla a leading destination for clinical trials in Latin America.