


The article delineates ten pivotal benefits of electronic Case Report Form (eCRF) clinical trials for research directors, highlighting significant enhancements in:
These advantages arise from eCRF's capacity to streamline data collection and management processes, which facilitates:
Ultimately, this leads to more effective and timely clinical research outcomes, underscoring the critical role of eCRF in advancing the field.
The landscape of clinical trials is evolving rapidly, driven by the imperative for efficiency, accuracy, and cost-effectiveness. As research directors navigate this intricate environment, the adoption of electronic Case Report Forms (eCRFs) emerges as a transformative opportunity. This article explores ten compelling benefits of eCRF clinical trials, illustrating how they:
What challenges do these innovations address, and how can research leaders leverage them to secure a competitive edge in their studies?
bioaccess® harnesses advanced electronic Case Report Form (eCRF) solutions to revolutionize eCRF clinical trials in Latin America, facilitating rapid ethical approvals and improving information management. By deploying eCRF clinical trials, bioaccess® markedly accelerates the speed and precision of data collection, allowing research directors to focus on the critical aspects of their studies. This innovative approach not only shortens timelines—achieving patient enrollment 50% faster than traditional Western sites for treatment-naive cardiology or neurology groups—but also elevates the overall quality of studies.
Research adhering to stringent protocols reveals up to 30% fewer regulatory inquiries during audits, underscoring the efficacy of medical studies. As a result, bioaccess® emerges as a preferred partner for Medtech, Biopharma, and Radiopharma innovators, delivering tangible benefits such as $25K savings per patient directly associated with eCRF clinical trials and providing FDA-ready information without the need for rework or delays.
The integration of real-time information access further empowers research leaders to make swift, informed decisions, while the intuitive design of electronic case report forms enhances management efficiency. With a proven track record of over 20 years in Medtech, bioaccess® is committed to accelerating clinical studies through comprehensive management services, including:
This solidifies its status as a leader in the field.
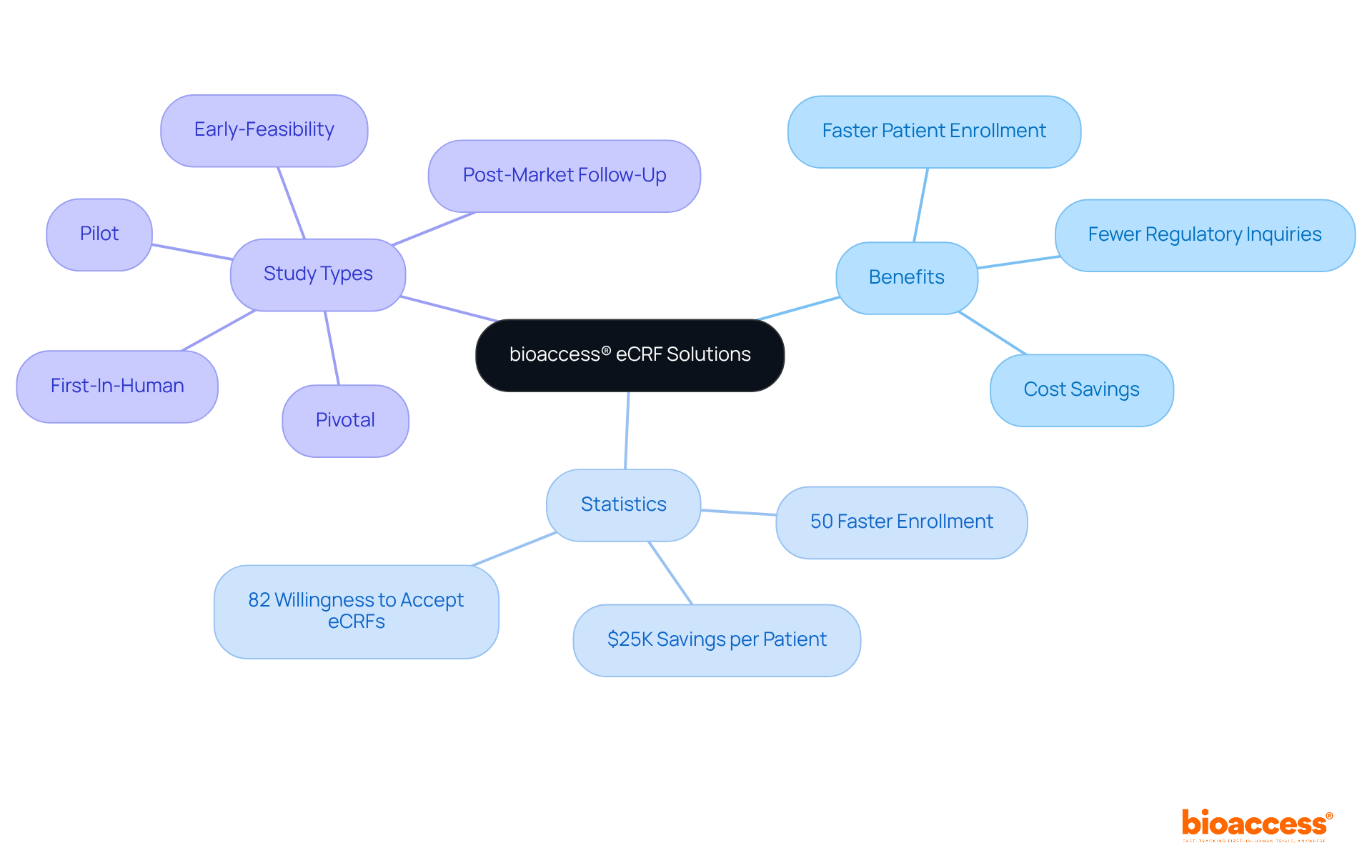
Electronic case report forms (eCRFs) significantly enhance information quality through integrated validation checks and real-time entry capabilities. These features reduce transcription mistakes and guarantee accuracy from the outset, which is crucial in clinical research. Studies indicate that experiments utilizing eCRFs demonstrate an average enhancement of 46% in consistency, with 83% of locations reporting improved quality metrics after adopting Statistical Data Monitoring (SDM). This proactive strategy for information management minimizes discrepancies, leading to more dependable outcomes and facilitating smoother regulatory submissions.
Furthermore, eCRFs enable early error identification through established validity standards during information entry. This ensures that the data is not only precise but also thorough, thereby strengthening the integrity of research outcomes. In conjunction with bioaccess's comprehensive clinical trial management services—including feasibility studies, compliance reviews, trial setup, import permits, project management, and reporting on study status—eCRF clinical trials play a crucial role in optimizing the clinical trial process and enhancing overall research efficiency. The integration of these elements underscores the importance of collaboration in navigating the complexities of clinical research.
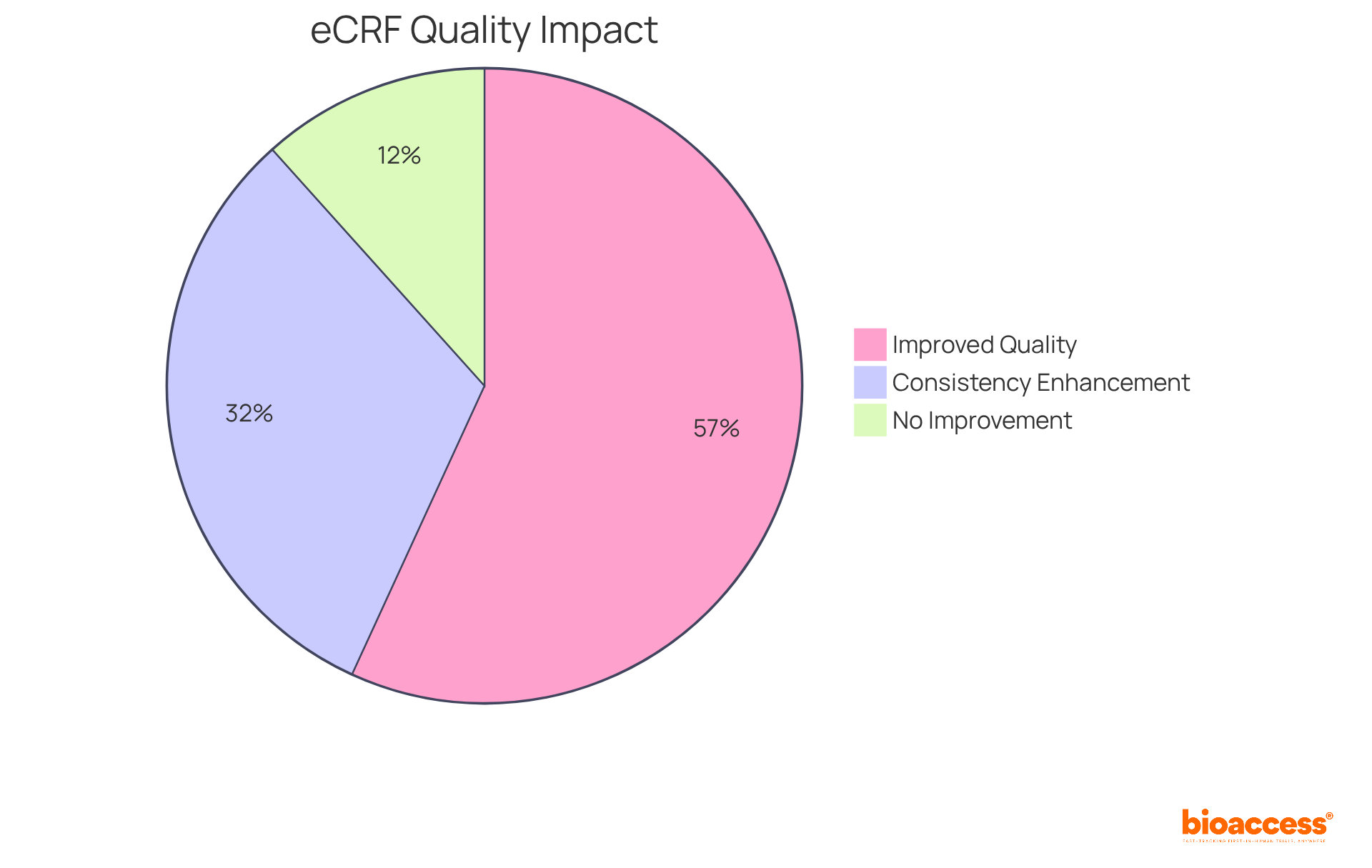
The adoption of electronic Case Report Forms (eCRFs) in eCRF clinical trials significantly enhances the information collection process by enabling instant entry and access. This transformation eliminates delays typically associated with paper forms, such as printing, shipping, and manual data entry. Consequently, research teams can concentrate on analysis rather than management in eCRF clinical trials, which fosters quicker decision-making and improved trial outcomes.
Research indicates that electronic case report forms can save an average of 2.25 minutes per entry compared to traditional paper forms, with overall completion times averaging 8.29 minutes for electronic forms versus 10.54 minutes for paper forms. Furthermore, the integration of mobile technology into electronic case report forms has proven to enhance information management efficiency, making them a preferred choice among research investigators.
As one study manager noted, the structure of a Case Report Form (CRF) is crucial, as it directly influences the accuracy and reliability of the information collected. Electronic case report forms not only reduce entry errors to an impressive 0% compared to a 5% error rate for paper documents but also improve the overall information-gathering process, enabling faster access and analysis.
Additionally, bioaccess®'s approach facilitates the enrollment of treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, achieving substantial cost savings of $25K per patient with FDA-ready data—no rework, no delays.
The case study titled 'Evaluation of Time Efficiency in Data Collection Methods' illustrates that data collection via electronic case report forms in eCRF clinical trials was significantly quicker, underscoring the efficiency enhancements from utilizing electronic case report forms in eCRF clinical trials and showcasing the effectiveness of bioaccess®'s solutions.
Overall, the efficiency gains from employing electronic case report forms in eCRF clinical trials are considerable, leading to more effective and timely research outcomes.
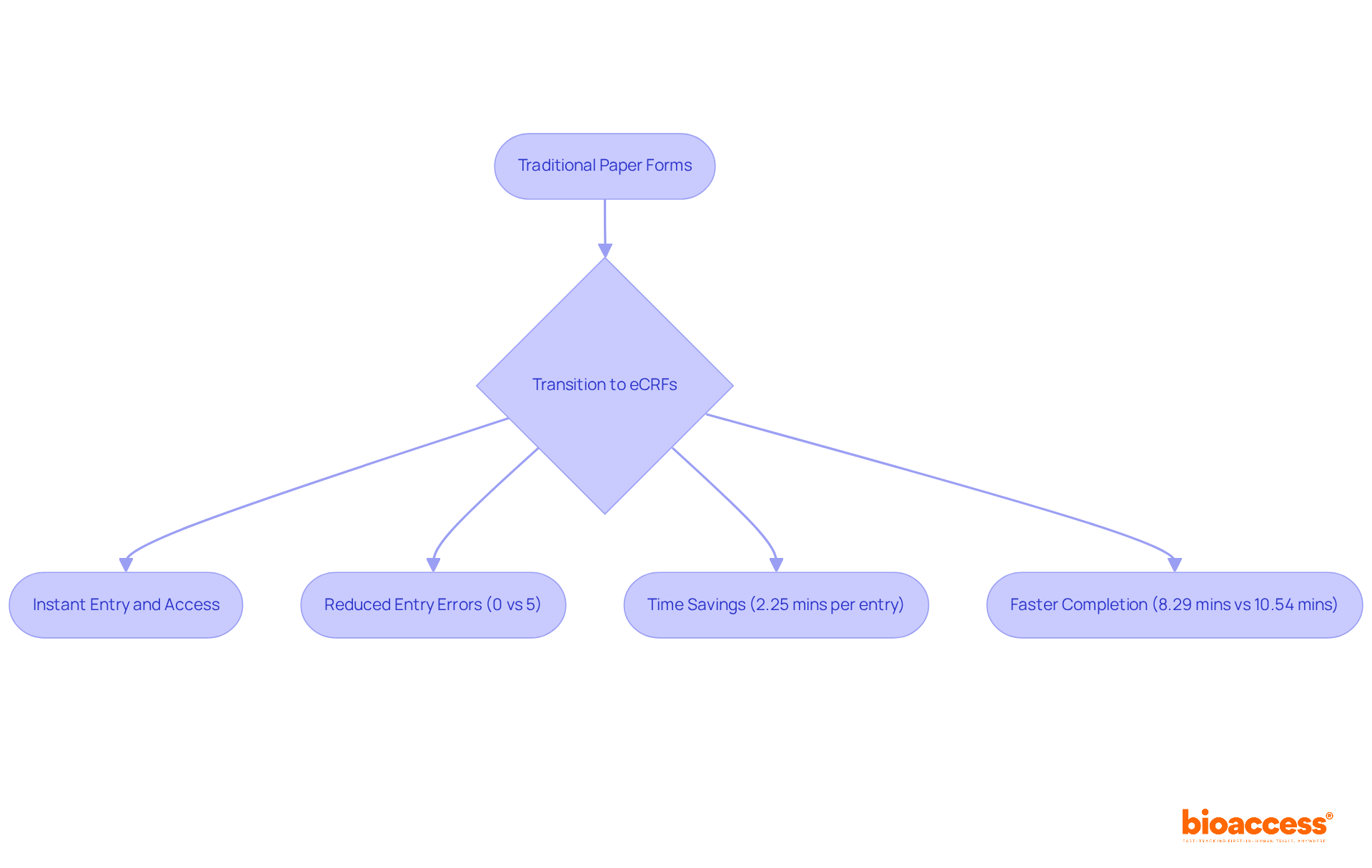
The implementation of electronic Case Report Forms (eCRFs) in eCRF clinical trials significantly reduces clinical study expenses while enhancing the management of clinical research. By eliminating the need for physical resources and streamlining information entry and management processes, eCRFs contribute to lower operational costs.
Research demonstrates that eCRF clinical trials can achieve savings of up to 62% in data management costs when compared to traditional paper-based methods. Moreover, the total cost per patient in eCRF clinical trials is substantially reduced, amounting to €374 versus €1,135 for paper CRFs, thus offering a more comprehensive financial perspective.
This transition not only boosts efficiency but also positions eCRF clinical trials as a financially advantageous option for research directors. Financial analysts emphasize that, despite the initial investment in eCRF clinical trials systems, the considerable long-term savings make them an appealing choice for optimizing research budgets.
To fully leverage the benefits of eCRF clinical trials, research directors should consider integrating these systems into their study budgeting processes.
In addition to cost reductions, bioaccess offers extensive management services for research studies, including:
These services not only streamline the clinical research process but also stimulate local economies through job creation and healthcare improvements, fostering international collaboration in medtech clinical studies.
Electronic case report forms are meticulously crafted to adhere to stringent regulatory standards, ensuring that all collected data meets industry requirements. Key features, such as:
significantly enhance the integrity and security of research data. Furthermore, these forms improve information quality and expedite trial timelines, facilitating quicker market entry for new medical devices. By employing electronic case report forms, research directors can adeptly navigate the complex regulatory landscape, effectively mitigating the risk of non-compliance. This adherence not only streamlines the regulatory submission process but also elevates the credibility of research studies, fostering trust among stakeholders.
Compliance with vital regulatory standards, such as 21 CFR Part 11, is essential, and expert insights reveal that eCRF clinical trials allow for enrollment 50% faster than traditional methods, underscoring their efficiency advantages in research. Moreover, leveraging bioaccess®'s FDA-ready information can yield $25K savings per patient, further amplifying the value of eCRF clinical trials in accelerating processes for Medtech and Biopharma startups.
eCRF clinical trials significantly enhance collaboration among research teams by providing a centralized system for information entry and sharing. This structure allows team members to access real-time data, facilitating effective communication and swift decision-making. Research indicates that streamlined communication through real-time access can reduce delays in clinical studies, leading to improved efficiency and outcomes.
For instance, studies show that organizations utilizing electronic case report forms in eCRF clinical trials can initiate worldwide studies 40% faster, underscoring the importance of these platforms in fostering a unified research environment. Furthermore, insights from research groups highlight that the user-friendly designs of electronic case report forms reduce confusion and errors, ultimately bolstering collaboration and information integrity.
By integrating eCRF clinical trials into their processes, research teams can cultivate a more cooperative atmosphere, leading to enhanced study outcomes and advancing medical knowledge.
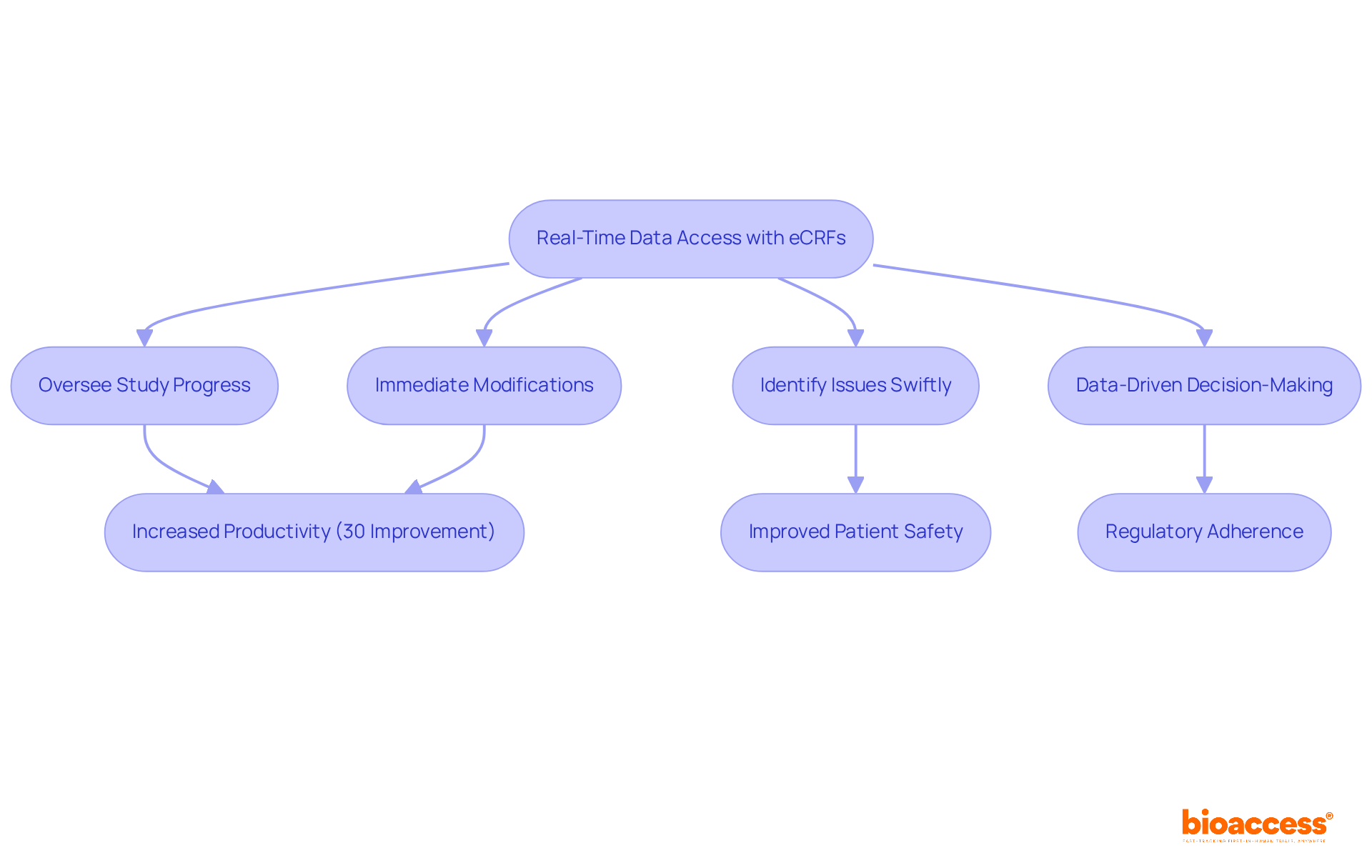
A key benefit of eCRF clinical trials is their provision of real-time information access, empowering research directors to oversee study progress and swiftly identify issues. This capability facilitates immediate, data-driven decision-making, significantly enhancing the responsiveness of research teams.
For instance, real-time data entry allows access to current information, enabling directors to make informed modifications that keep projects on schedule and within budget. Statistics reveal that eCRF clinical trials can achieve up to 30% productivity improvements, a remarkable figure considering the pharmaceutical sector incurs expenses ranging from $3.7 to $12 billion for each new medication.
Furthermore, research indicates that nearly one-third of FDA-approved drugs from 2001 to 2010 encountered major safety issues post-approval, underscoring the critical role of timely decision-making in mitigating risks. By leveraging real-time insights in eCRF clinical trials, such as tracking patient enrollment and adjusting strategies, research directors can enhance study outcomes and ensure adherence to regulatory standards, ultimately resulting in improved patient safety and study efficiency.
With bioaccess®'s innovative approach, trials can enroll treatment-naive cardiology or neurology groups 50% faster than Western sites, achieving significant savings of $25K per patient through FDA-ready data—no rework, no delays. As Andrés Escallón, VP of eCOA Practice, asserts, "Access to high-quality information powered by fit-for-purpose technology is crucial for making real-time decisions.
Electronic Case Report Forms (eCRFs) can be seamlessly integrated with various clinical tools used in eCRF clinical trials, including:
This integration facilitates a smooth flow of information across platforms, significantly reducing redundancy and enhancing consistency. For instance, when eCRFs are linked with EDC systems, researchers can attain real-time information synchronization, which reduces errors and enhances the precision of patient details. This streamlined method not only accelerates the information gathering process but also improves the overall effectiveness of eCRF clinical trials.
IT specialists emphasize that such integration fosters collaboration among research teams in eCRF clinical trials, allowing for quicker decision-making and improved patient safety. By leveraging bioaccess®'s FDA-ready data, research directors can achieve patient enrollment 50% faster, resulting in significant cost savings of $25K per patient. Moreover, bioaccess® provides extensive management services that guarantee a unified and efficient research environment, ultimately leading to quicker product approvals and improved patient outcomes.
Notably, eCRF clinical trials help lower entry mistakes to under 1%, in contrast to a 5% error rate for conventional paper forms, with the typical completion duration for eCRF clinical trials being 8.29 minutes—considerably shorter than the 10.54 minutes needed for paper-based case report forms. Additionally, EDC integration allows healthcare providers to quickly identify patient allergies, further enhancing patient safety. With bioaccess®'s expertise in Latin America, research directors can navigate the complexities of clinical trials in this region with confidence.
The implementation of electronic case report forms in eCRF clinical trials significantly enhances patient safety by ensuring accurate and secure data collection. Features such as real-time monitoring and automated alerts for adverse events enable research teams to respond swiftly to potential safety issues. This proactive strategy not only prioritizes patient welfare but also aligns with ethical standards and regulatory requirements, thereby fostering trust among participants and stakeholders.
Experts in ethics emphasize that the moral implications of utilizing electronic case report forms in eCRF clinical trials extend beyond mere compliance; they are essential for safeguarding participant rights and enhancing the integrity of research studies. By facilitating clear communication and efficient information management, electronic case report forms contribute to a more ethical research environment, ultimately improving patient outcomes and safety.
By simplifying data gathering and enhancing collaboration, electronic case report forms (eCRFs) facilitate quicker study completions compared to conventional methods. Research indicates that studies utilizing eCRFs can shorten overall research timelines by as much as 30%. This acceleration not only benefits research directors by enabling faster market entry for new therapies but also enhances the potential for improved patient outcomes.
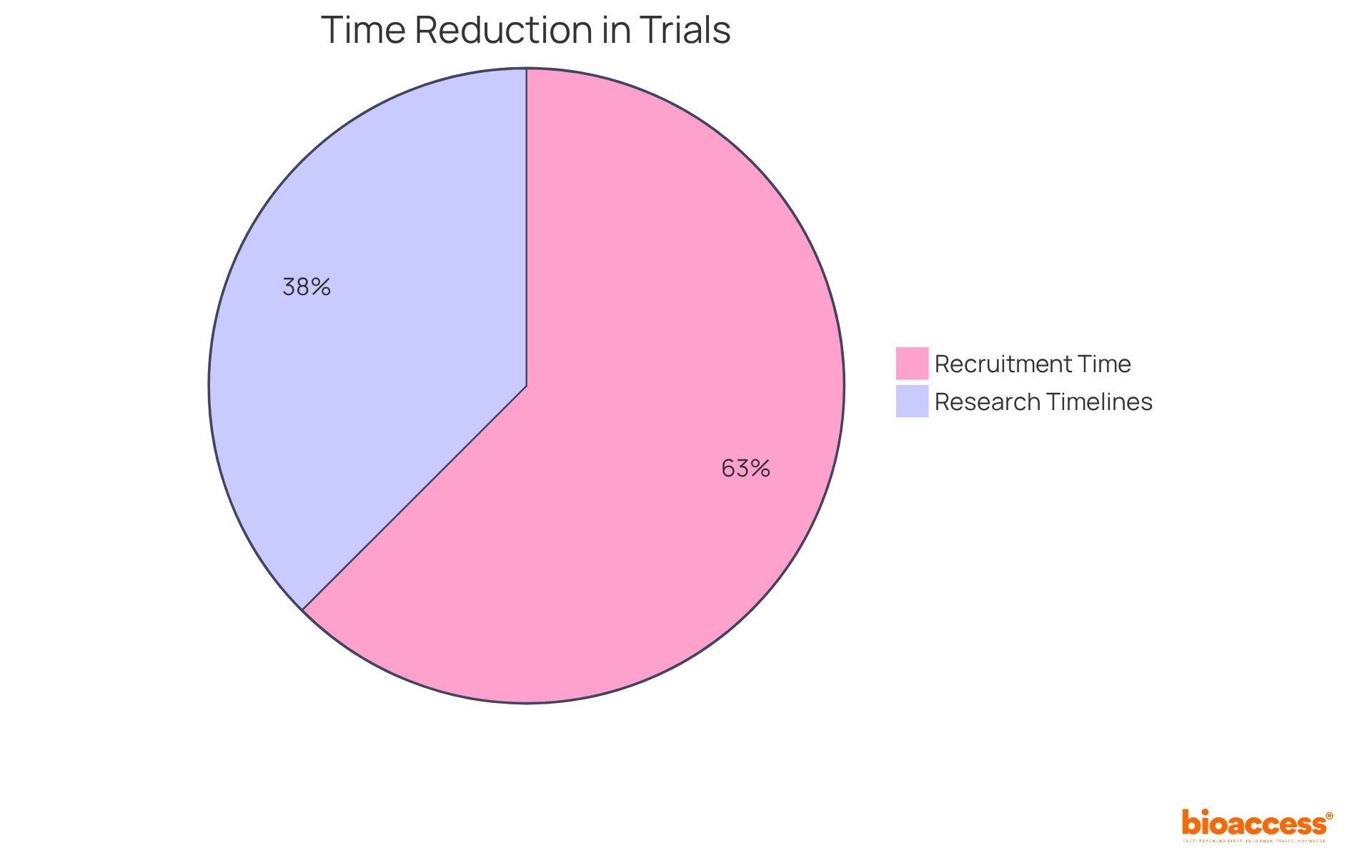
Furthermore, initiatives such as the partnership between bioaccess™ and Caribbean Health Group aim to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health, Juan Pablo Uribe. With bioaccess®'s expert services, Medtech, Biopharma, and Radiopharma startups can achieve over a 50% reduction in recruitment time while maintaining 95% retention rates. This underscores the advantages of adopting eCRF clinical trials in the field of clinical research.
As one clinical research director noted, 'The speed of eCRF clinical trials allows us to respond to patient needs more rapidly, ultimately benefiting everyone involved in the eCRF clinical trials.
The transition to electronic Case Report Forms (eCRFs) signifies a pivotal advancement in the domain of clinical trials, particularly for research directors. By embracing eCRF solutions, organizations can streamline processes, enhance data quality, and reduce operational costs, culminating in more efficient and effective research outcomes.
Throughout this discussion, several critical benefits of eCRF clinical trials have been underscored. These encompass:
Furthermore, eCRFs promote collaboration among research teams and facilitate real-time data access, which is essential for informed decision-making. The capability to integrate eCRFs with other clinical tools further amplifies their effectiveness, ensuring a seamless flow of information that enhances overall study management.
In conclusion, the advantages of implementing eCRF clinical trials transcend mere operational enhancements; they represent a transformative shift in clinical research methodologies. By adopting these advanced solutions, research directors can not only accelerate timelines and reduce costs but also uphold ethical standards and enhance patient safety. The call to action is unequivocal: leveraging eCRFs is imperative for any research organization striving to excel in today’s competitive landscape and deliver impactful medical advancements.
What is bioaccess® and how does it improve clinical trials?
bioaccess® utilizes advanced electronic Case Report Form (eCRF) solutions to enhance clinical trials in Latin America by facilitating rapid ethical approvals and improving information management, resulting in faster patient enrollment and higher quality studies.
How much faster does bioaccess® achieve patient enrollment compared to traditional methods?
bioaccess® achieves patient enrollment 50% faster than traditional Western sites for treatment-naive cardiology or neurology groups.
What financial benefits are associated with using bioaccess®'s eCRF clinical trials?
Utilizing bioaccess®'s eCRF clinical trials can result in savings of $25K per patient and provides FDA-ready information without the need for rework or delays.
How does the use of eCRFs enhance data quality in clinical trials?
eCRFs improve data quality through integrated validation checks and real-time entry capabilities, reducing transcription errors and enhancing consistency by an average of 46%.
What are some of the comprehensive management services provided by bioaccess®?
bioaccess® offers services including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies to support clinical trials.
How do eCRFs streamline the data collection process?
eCRFs enable instant data entry and access, eliminating delays associated with paper forms, which allows research teams to focus more on analysis rather than management tasks.
What is the average time saved per entry when using eCRFs compared to paper forms?
eCRFs save an average of 2.25 minutes per entry, with overall completion times averaging 8.29 minutes for electronic forms versus 10.54 minutes for paper forms.
What impact does mobile technology have on eCRFs?
The integration of mobile technology into eCRFs enhances information management efficiency, making them a preferred choice among research investigators.
How does bioaccess® ensure the integrity of research outcomes?
By enabling early error identification and establishing validity standards during data entry, bioaccess® strengthens the integrity of research outcomes through thorough and precise data collection.
What evidence supports the efficiency of eCRFs in clinical trials?
A case study titled 'Evaluation of Time Efficiency in Data Collection Methods' demonstrated that data collection via eCRFs was significantly quicker, highlighting the efficiency gains from using bioaccess®'s solutions.