

The article delineates ten pivotal benefits of electronic data capture (EDC) for clinical trials, such as enhanced data quality, improved efficiency, and expedited participant recruitment. These advantages arise from EDC's capacity to:
This transformation is reshaping the landscape of clinical research, underscoring the critical role of EDC in addressing the challenges faced by the Medtech sector.
The clinical trial landscape is undergoing a significant transformation, propelled by the rapid adoption of electronic data capture (EDC) technologies. These innovations not only promise to enhance efficiency but also improve data quality and accelerate participant recruitment, establishing them as indispensable tools for researchers in Medtech, Biopharma, and Radiopharma. However, as organizations endeavor to harness these advantages, they must navigate the complexities of implementation and the challenges associated with ensuring data integrity.
What are the true advantages of EDC systems?
How can they reshape the future of clinical trials?
These questions are crucial as we explore the evolving dynamics of clinical research.
At bioaccess®, we harness the power of electronic data capture for clinical trials technologies to revolutionize clinical studies, ensuring that information is collected with unparalleled efficiency and precision. The integration of EDC into our workflows significantly reduces the time spent on data entry and management, enabling us to concentrate on expediting the overall study timeline. This strategic approach not only enhances operational efficiency but also aligns seamlessly with our commitment to delivering ethical approvals within 4-6 weeks and achieving enrollment rates that are 50% faster than traditional markets.
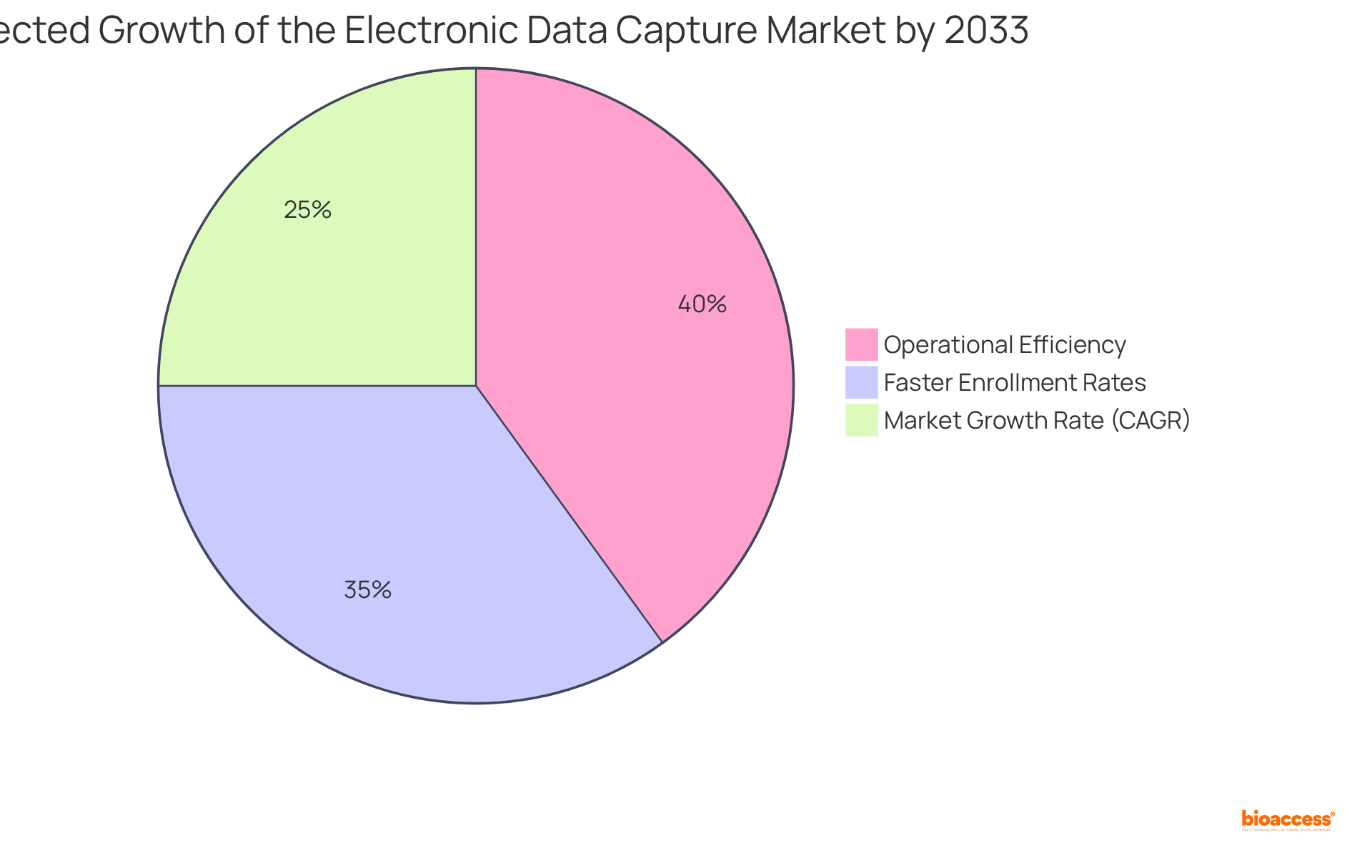
As the clinical research landscape evolves, the adoption of electronic data capture for clinical trials has become essential for Medtech, Biopharma, and Radiopharma innovators who are seeking to streamline processes and improve outcomes. The EDC market is projected to reach USD 14.8 billion by 2033, growing at a CAGR of 8.9% from 2025 to 2033, underscoring the increasing importance of these technologies. Industry leaders have noted, 'The effectiveness of EDC frameworks is revolutionizing clinical research processes, enabling real-time information access and enhanced decision-making.'
In summary, the collaboration between innovative technologies and clinical research practices is vital for addressing key challenges in the Medtech sector. As we continue to advance, the implementation of electronic data capture for clinical trials will play a pivotal role in shaping the future of clinical studies.
Electronic Information Capture (EIC) systems utilize electronic data capture for clinical trials, revolutionizing information gathering in clinical studies by enabling real-time input and significantly reducing reliance on traditional paper-based methods. This transformation not only minimizes manual input errors—where human error rates can fluctuate between 1% and 5%—but also ensures consistent data collection across all study locations.
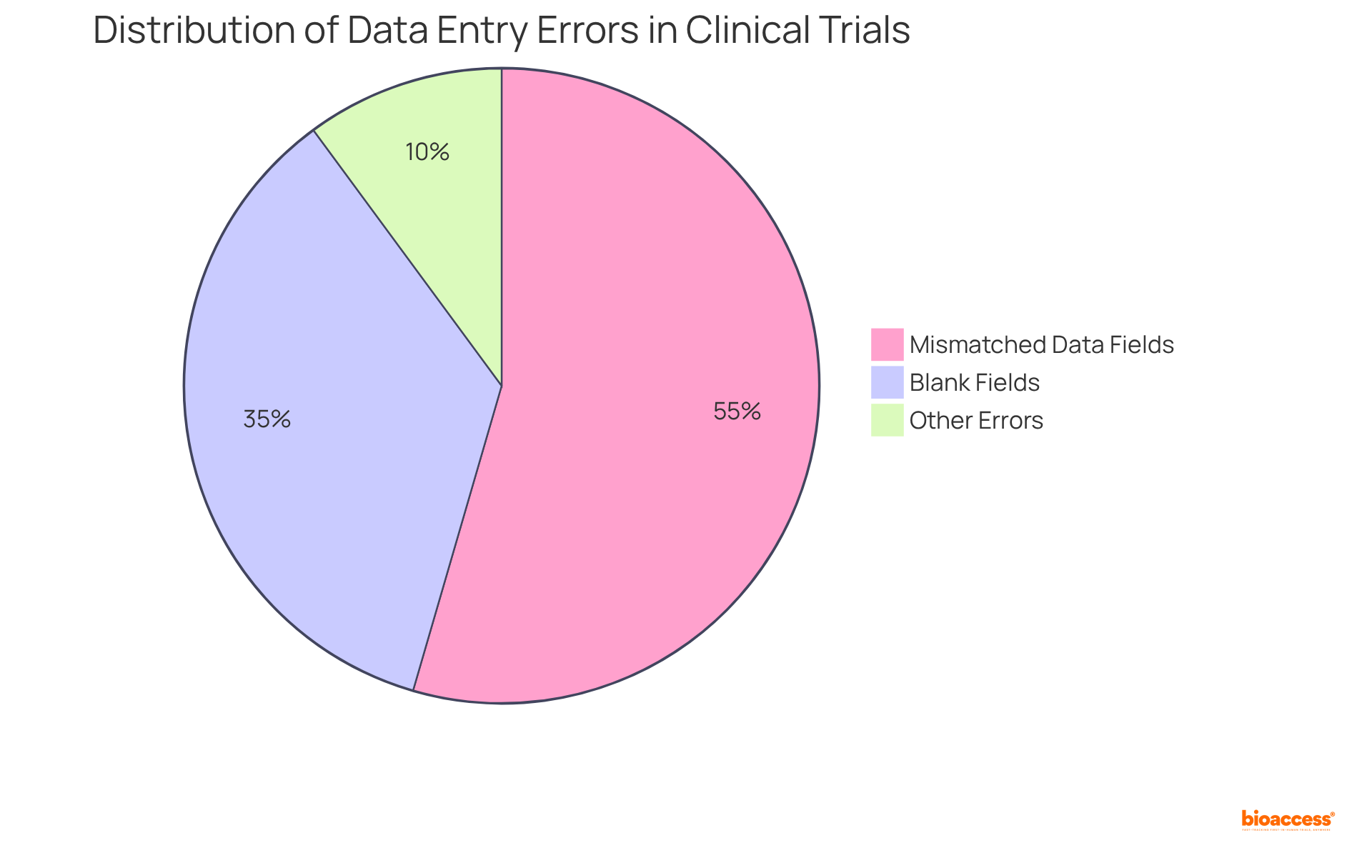
For instance, a longitudinal study conducted within an Australian regional Drug and Alcohol Service revealed that mismatched data fields constituted 54.5% of errors, while blank fields accounted for 35.4%, highlighting the urgent need for precise data entry processes.
By utilizing electronic data capture for clinical trials, researchers gain rapid access to current information, which facilitates timely decision-making and necessary modifications to trial protocols. The implementation of EDC technologies has proven to stabilize error rates; one study noted a reduction to below 7.3% within 1.5 years post-implementation, with the quarterly error rate decreasing to 6.6 in Year 2 Quarter 2.
This efficiency not only improves data quality but also bolsters the overall integrity of clinical research, ultimately enhancing patient outcomes. As underscored in a report by McKinsey & Company, automation significantly elevates information quality and reduces errors, further validating the advantages of electronic data capture for clinical trials.
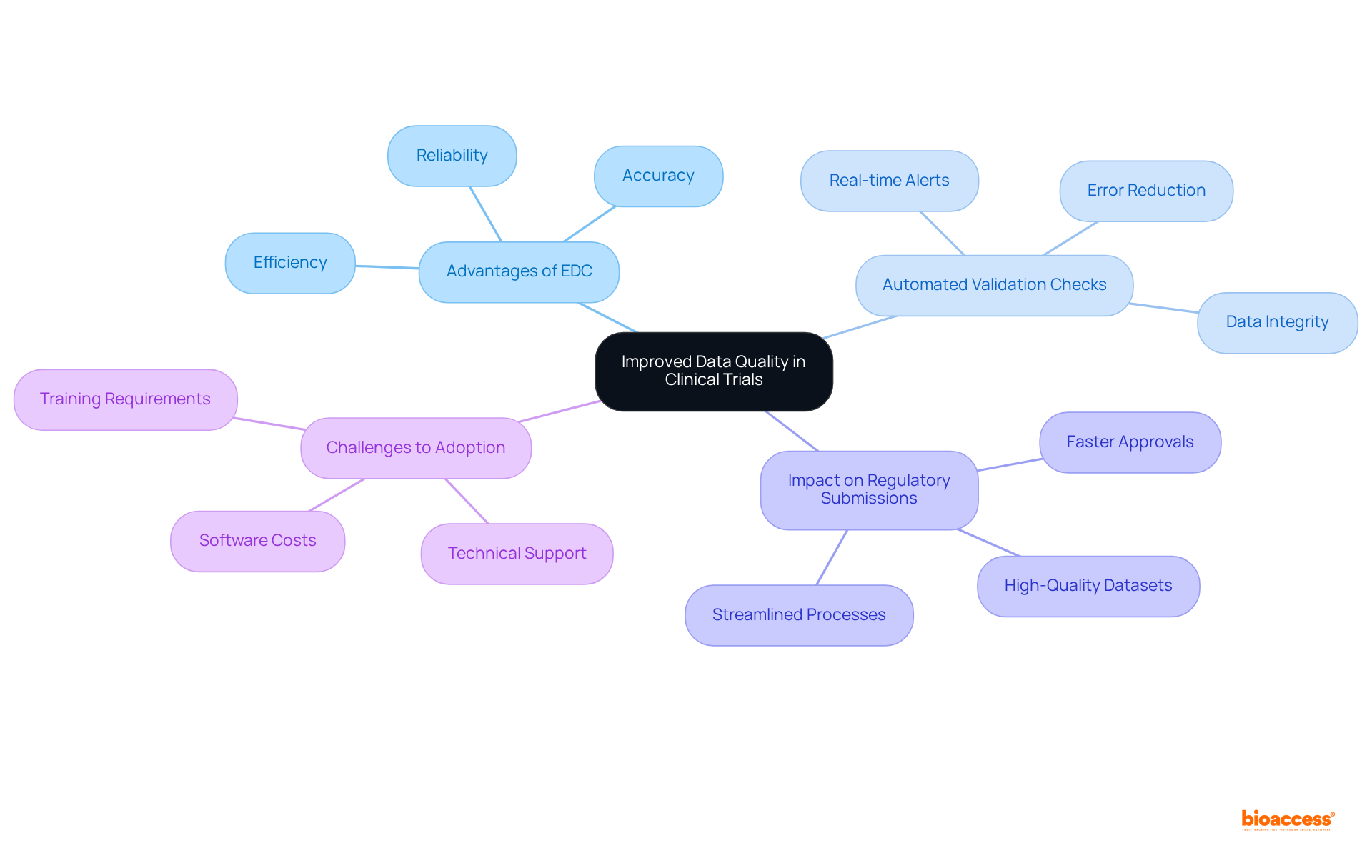
A key advantage of electronic data capture for clinical trials lies in their ability to significantly enhance information quality through automated validation checks. These checks operate in real-time, designed to identify discrepancies and errors early in the information collection process. For example, built-in validation rules alert users to inconsistencies or missing information, ensuring that only accurate data is captured. This proactive approach not only bolsters the reliability of the information but also facilitates regulatory submissions by providing high-quality datasets that comply with established standards.
In 2025, the integration of automated validation checks has become increasingly essential in electronic data capture for clinical trials. Researchers have observed that these checks can drastically minimize the risk of transcription errors and missing data, common challenges in traditional data collection methods. By utilizing electronic data capture for clinical trials, cleaner datasets can be achieved, leading to expedited regulatory submissions and a reduction in delays associated with data-related issues. Ethical approvals can now be secured in just 4-6 weeks, underscoring the effectiveness of EDC tools in streamlining the regulatory process.
Moreover, the implementation of automated validation checks has been shown to improve research accuracy. A recent study highlighted that frameworks for electronic data capture for clinical trials featuring robust validation capabilities led to a notable enhancement in information integrity, ultimately facilitating smoother interactions with regulatory bodies. As Santam Chakraborty noted, "EDC frameworks can be arranged to include alert mechanisms which can lessen the occurrence of missed information." This capability is critical for maintaining the trustworthiness of research outcomes, ensuring that the information analyzed is both accurate and reliable, thus supporting the overall success of clinical trials. However, it is important to recognize that 63.0% of respondents identified a lack of technical support as a barrier to EDC adoption, and software costs remain a significant challenge for many organizations.
EDC frameworks are equipped with sophisticated security features designed to protect sensitive clinical information. These frameworks utilize:
This ensures that only authorized individuals can access and modify data. This robust security architecture not only fulfills regulatory compliance but also fosters trust among participants and stakeholders in the research process. Given that healthcare information breaches have escalated to alarming levels—averaging 364,571 records compromised daily in 2023, alongside 1.99 healthcare breaches of 500 or more records reported each day—the significance of EDC in safeguarding patient information is more critical than ever. Furthermore, with 70% of clinical studies reporting some form of information breach, the implementation of EDC frameworks is essential in mitigating risks associated with data exposure. By prioritizing information protection through EDC, organizations can bolster the integrity of their clinical trials and sustain participant confidence.
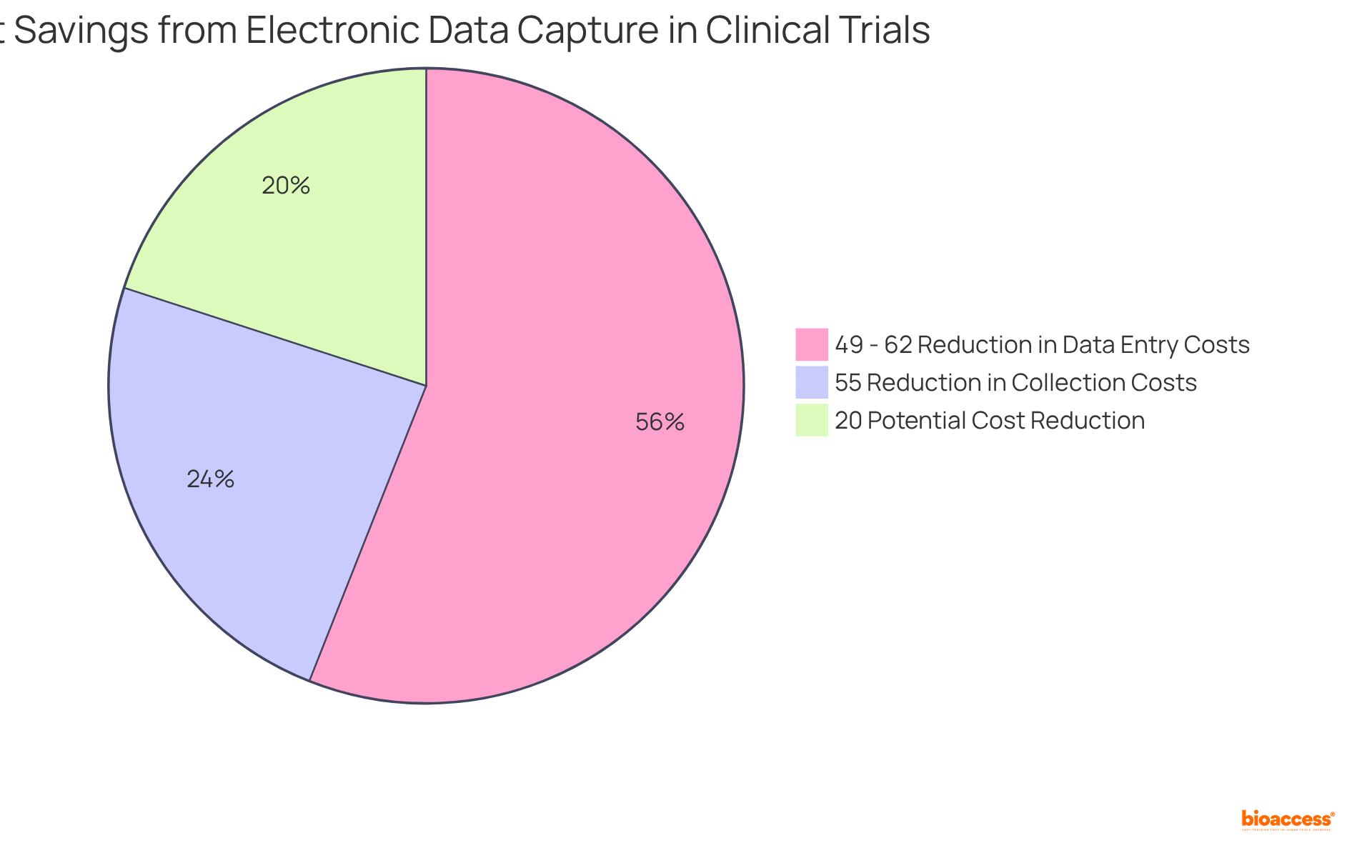
The adoption of electronic data capture for clinical trials technologies represents a pivotal advancement in clinical research operations, significantly reducing costs. By transitioning from traditional paper documentation to digital solutions, organizations can markedly decrease expenses associated with data entry and management. EDC solutions streamline processes, facilitating real-time access to information and minimizing validation time, which can lead to savings ranging from 49% to 62% compared to conventional methods. Additionally, the potential for cost reduction in utilizing electronic data capture for clinical trials platforms is estimated at approximately 20%, providing a broader perspective on their financial impact.
Moreover, the expedited turnaround times enabled by EDC contribute to faster study completions, thereby enhancing overall cost efficiency. Research indicates that EDC frameworks can reduce collection costs by as much as 55%, allowing organizations to allocate resources more effectively. With 70% of EDC users reporting improved information quality, the financial implications extend beyond mere savings, fostering a more reliable and efficient research environment. As noted by Santam Chakraborty, information quality is a multifaceted concept, underscoring its importance within the context of EDC frameworks.
As the global electronic data capture for clinical trials market is projected to reach USD 2 billion by 2025, the trend towards adopting these solutions is unmistakable. The anticipated growth of the mobile EDC solutions market at an annual rate of 16% further underscores this increasing adoption. The financial benefits of electronic data capture for clinical trials are emphasized by the fact that 85% of large organizations utilize EDC frameworks to ensure data accuracy and regulatory compliance, thereby reinforcing their role in reducing operational costs in clinical studies.
In the realm of clinical trials, ensuring compliance with industry regulations is paramount. Solutions for electronic data capture for clinical trials are meticulously crafted to uphold these standards, particularly Good Clinical Practice (GCP) and the FDA's 21 CFR Part 11. Key attributes such as electronic signatures, thorough audit trails, and strong integrity checks are essential to these frameworks, ensuring that all information is gathered and maintained in strict compliance with regulatory standards. This level of compliance not only safeguards the integrity of the research but also significantly enhances the credibility of the findings.
As pointed out by Remington-Davis, 'The execution of electronic data capture for clinical trials frameworks simplifies compliance procedures, enabling real-time observation and documentation, which is crucial for upholding high standards in clinical studies.' Moreover, the use of electronic data capture for clinical trials frameworks enhances compliance with GCP by automating data gathering and validation procedures, minimizing the likelihood of human error.
By leveraging these advanced features, organizations can effectively navigate the complexities of regulatory requirements, ensuring that their clinical trials meet the highest ethical and scientific standards. To maximize the advantages of EDC tools, clinical research teams should emphasize training on these resources and frequently assess compliance protocols to adapt to changing regulations.
The use of electronic data capture for clinical trials significantly enhances accessibility by allowing authorized individuals to retrieve information from any location at any time. This capability is particularly advantageous for multi-site studies, where researchers and stakeholders collaborate seamlessly across diverse locations. By facilitating real-time information sharing, electronic data capture for clinical trials platforms empower teams to exchange insights and make informed decisions swiftly—an essential factor in improving trial outcomes.
Research indicates that efficient information sharing can lead to a 15% variation in quality measure computations when longitudinal records are included, highlighting the importance of comprehensive access provided by electronic data capture for clinical trials. Furthermore, organizations that implement electronic data capture for clinical trials often report increased participant diversity and improved patient recruitment, as this approach streamlines the process of collecting and analyzing data from varied populations.
This collaborative approach not only elevates the quality of research but also fosters trust among participants, ultimately resulting in better health outcomes and more effective treatment options.
A key advantage of electronic data capture for clinical trials lies in its ability to facilitate the reuse of collected data for future studies. By establishing a centralized repository of clinical study information, researchers gain streamlined access to historical records, allowing them to effortlessly explore new research inquiries or conduct comparative studies. This method not only conserves time and resources but also significantly propels the advancement of medical knowledge.
For instance, leveraging historical clinical research data can inform the design of new studies, enabling researchers to build upon earlier discoveries and refine methodologies. Furthermore, centralized databases support the identification of trends and patterns across multiple studies, ultimately leading to more robust conclusions and improved patient outcomes.
As specialists emphasize, the strategic repurposing of information is crucial for fostering innovation and efficiency in clinical research, with electronic data capture for clinical trials marking it as an essential component of modern trial design.
Electronic Data Capture (EDC) systems play a pivotal role in expediting participant recruitment by streamlining screening and enrollment processes. By leveraging features such as automated eligibility checks and real-time data updates, researchers can swiftly identify and enroll suitable participants. This efficiency not only decreases the time to start but also significantly improves the overall success of the study.
Notably, almost 80% of studies do not achieve their initial recruitment objectives, underscoring the essential requirement for efficient solutions like EDC. A compelling case study demonstrated that utilizing EDC allowed a client to automate patient screening and enrollment, achieving enrollment targets 50% faster while minimizing administrative burdens on staff.
Consequently, the execution of EDC not only accelerates participant recruitment but also enhances patient involvement and satisfaction, ultimately aiding in the success of clinical studies. As one clinical research leader observed, "We managed to streamline and accelerate patient screening and enrollment, and probably patient retention as well."
Furthermore, the average cost of screen failure rates across the industry is roughly $1,200 per failure, highlighting the financial benefits of efficient recruitment processes.
The adaptability of electronic data capture for clinical trials frameworks allows researchers to customize information collection formats and processes to meet the specific needs of each study. This adaptability is essential for accommodating diverse study designs, therapeutic areas, and regulatory requirements. By aligning electronic data capture for clinical trials with the unique demands of a trial, researchers can significantly optimize data collection and enhance the overall efficiency of the study.
The integration of electronic data capture (EDC) in clinical trials signifies a transformative shift in the methodologies of data collection, management, and analysis. By harnessing advanced technologies, EDC not only enhances efficiency but also improves accuracy and security within clinical research processes—ultimately resulting in better outcomes and expedited timelines for study completion.
Throughout this discussion, several pivotal benefits of EDC have been underscored. These encompass:
Furthermore, EDC systems facilitate regulatory compliance, enhance accessibility for real-time collaboration, and encourage the reusability of data for future research initiatives, all while accelerating participant recruitment.
In conclusion, the advantages of electronic data capture for clinical trials are not only extensive but also essential for the progression of clinical research. Embracing EDC technologies is imperative for organizations striving to maintain competitiveness and efficacy in the continuously evolving landscape of clinical studies. By implementing these solutions, researchers can bolster the integrity of their trials, cultivate participant trust, and ultimately contribute to the acceleration of medical advancements.
What is the purpose of electronic data capture (EDC) in clinical trials?
Electronic data capture (EDC) is used to revolutionize clinical studies by ensuring that information is collected with unparalleled efficiency and precision, significantly reducing the time spent on data entry and management.
How does EDC improve the efficiency of clinical trials?
EDC enhances operational efficiency by allowing real-time data input, minimizing manual input errors, and ensuring consistent data collection across study locations, which helps expedite the overall study timeline.
What are the projected growth statistics for the EDC market?
The EDC market is projected to reach USD 14.8 billion by 2033, growing at a compound annual growth rate (CAGR) of 8.9% from 2025 to 2033.
How does electronic data capture affect data quality in clinical trials?
EDC significantly enhances information quality through automated validation checks that identify discrepancies and errors in real-time, leading to cleaner datasets and improved accuracy in research.
What are the benefits of automated validation checks in EDC?
Automated validation checks minimize the risk of transcription errors and missing data, facilitate expedited regulatory submissions, and improve research accuracy, ultimately supporting smoother interactions with regulatory bodies.
What are the average error rates associated with traditional data collection methods compared to EDC?
Traditional methods can have human error rates fluctuating between 1% and 5%, while EDC has shown to stabilize error rates, reducing them to below 7.3% within 1.5 years post-implementation.
How quickly can ethical approvals be secured using EDC tools?
Ethical approvals can now be secured in just 4-6 weeks when utilizing electronic data capture tools.
What challenges do organizations face when adopting EDC?
A significant barrier to EDC adoption is the lack of technical support, and software costs remain a challenge for many organizations.