


The article delineates ten significant benefits of rule-based medicine that enhance clinical research success. These benefits include:
Such advantages arise from the systematic and structured approach inherent in rule-based medicine, which optimizes decision-making and operational efficiency. Ultimately, this leads to more reliable study outcomes and expedited market access for medical innovations.
The landscape of clinical research is rapidly evolving, with innovative approaches reshaping how studies are conducted and outcomes are measured. Rule-based medicine emerges as a transformative framework, offering a systematic and data-driven methodology that enhances diagnostic accuracy, patient safety, and regulatory compliance.
As researchers and Medtech innovators seek to streamline processes and improve results, a critical question arises: how can the integration of rule-based systems elevate clinical research success in a landscape marked by complexity and diversity?
Exploring the myriad benefits of rule-based medicine reveals a pathway to not only optimize research efficiency but also to fundamentally enhance patient care.
bioaccess® stands at the forefront of medical research, particularly within the Medtech sector. By leveraging Colombia's regulatory efficiency—allowing ethical approvals in just 90-120 days—and the diverse participant groups found in the Balkans, alongside Australia's optimized pathways, bioaccess® accelerates clinical studies. This ensures that groundbreaking medical technologies reach the market more swiftly, ultimately benefitting individuals worldwide. The integration of rule based medicine within this framework fosters systematic decision-making, thereby enhancing study designs and patient recruitment processes.
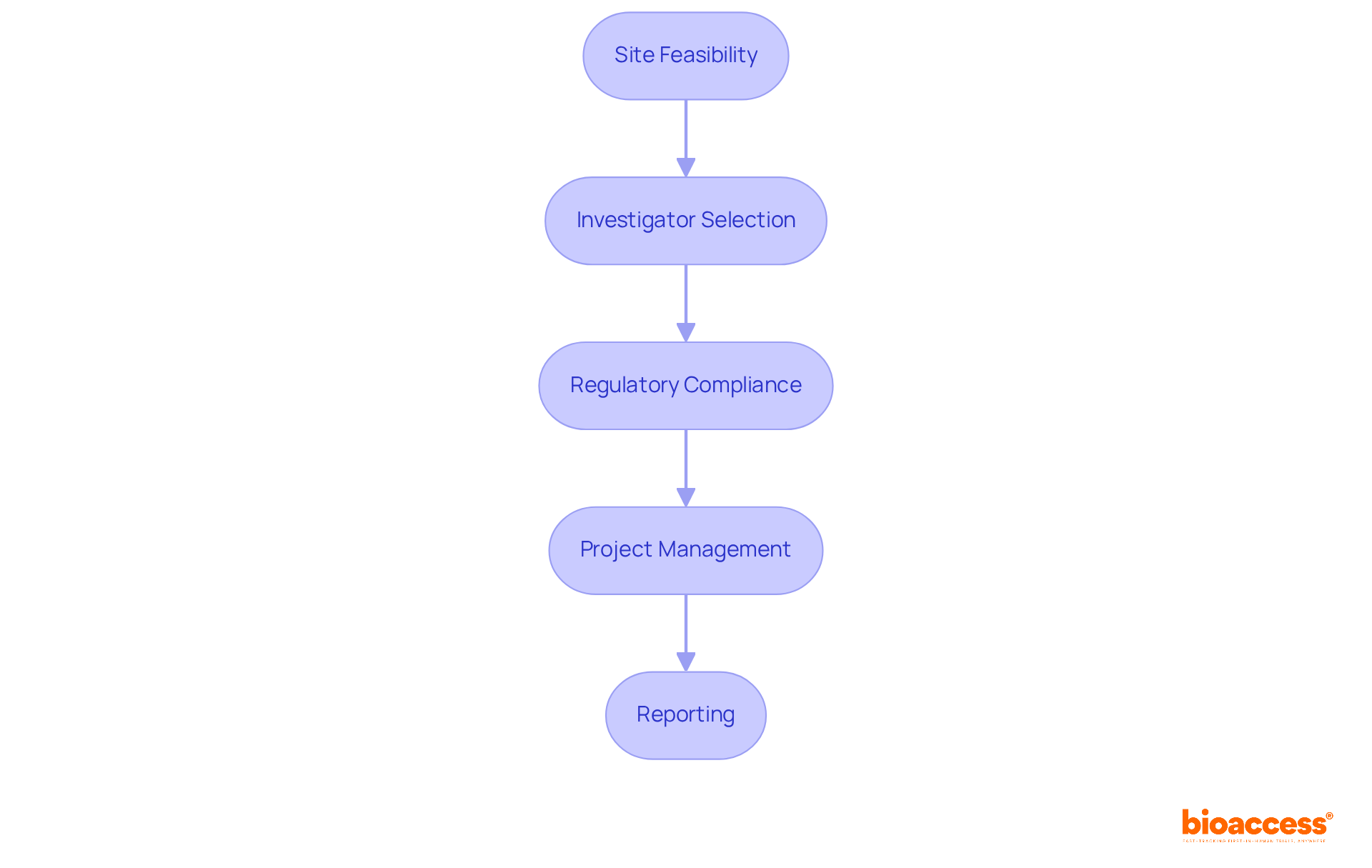
With over 15 years of experience, bioaccess® has established a comprehensive process for advancing medical device trials. This includes:
Such expertise is essential for Medtech innovators looking to expedite their research and development processes, positioning bioaccess® as a valuable ally in research endeavors. Furthermore, Colombia's competitive advantages—such as significant cost reductions exceeding 30% compared to North America and Western Europe, robust participant recruitment from a population of over 50 million, and attractive R&D tax incentives—solidify bioaccess®'s position as a leader in accelerating research studies throughout Latin America.
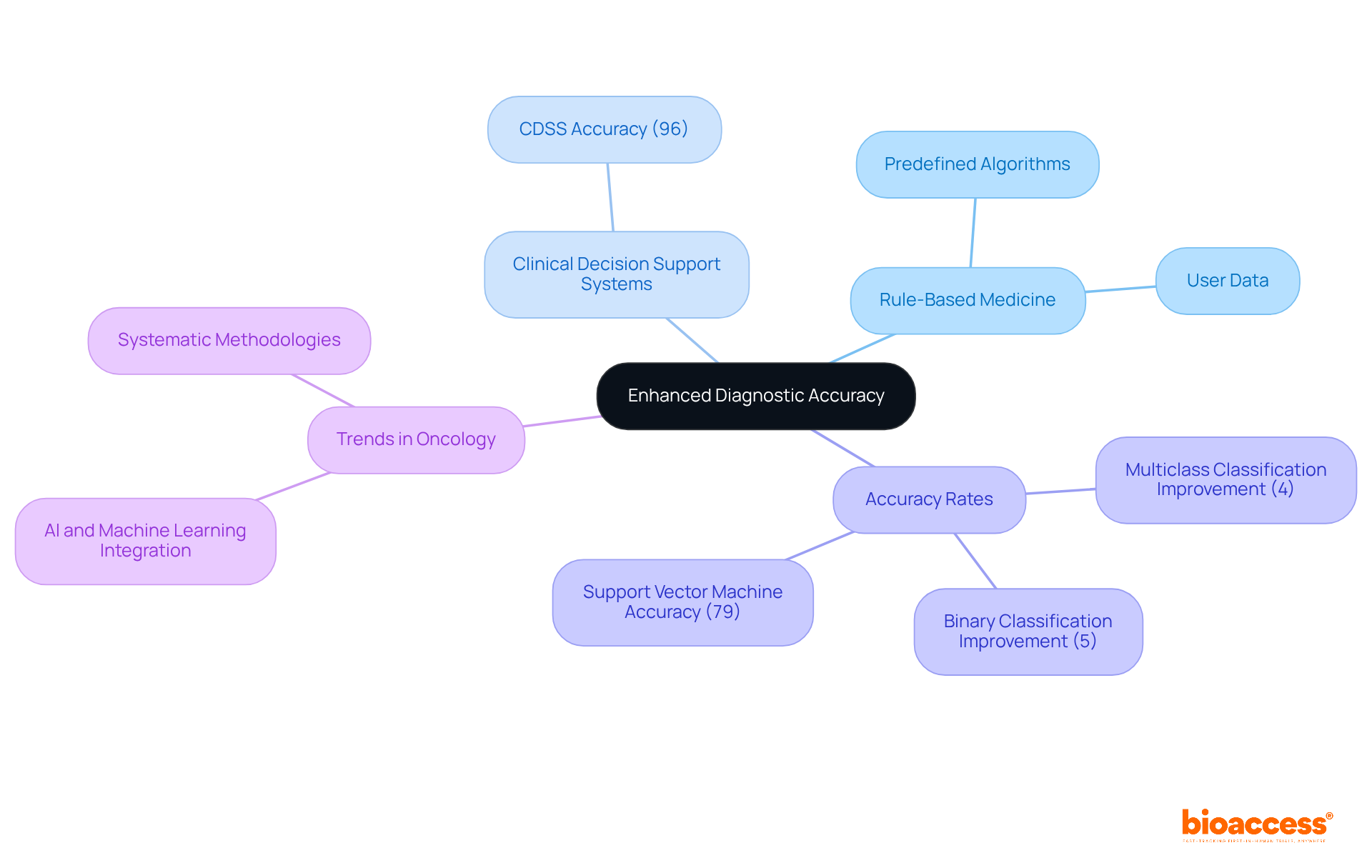
The implementation of predefined algorithms in rule based medicine significantly enhances diagnostic accuracy by guiding medical decision-making. These systems utilize established clinical guidelines and user data to provide consistent and reliable diagnostic outputs. For instance, in oncology, structured systems can automate the interpretation of complex data sets, ensuring clinicians make informed decisions grounded in the latest evidence.
By minimizing human errors and standardizing diagnostic processes, guideline-driven medicine not only elevates the precision of diagnoses but also improves the overall quality of care for patients. This is particularly beneficial in research studies, where accurate diagnostics are crucial for evaluating the efficacy of new therapies. Recent research indicates that the integration of structured methodologies in healthcare settings fosters a systematic and trustworthy diagnostic environment, ultimately leading to better outcomes for patients. For example, a Clinical Decision Support System (CDSS) achieved an impressive 96% overall accuracy in classifying health conditions, showcasing the potential of these systems to bolster diagnostic capabilities in oncology.
Moreover, incorporating contextual information has been demonstrated to enhance multiclass classification accuracy by 4%, further refining diagnostic processes. Current trends in oncology diagnostic systems reveal an increasing reliance on systematic methodologies, with advancements in artificial intelligence and machine learning further enhancing these processes. The support vector machine (SVM) has also shown effectiveness, achieving 79% accuracy for multiclass classification. As the field evolves, the role of rule based medicine is expected to be pivotal in shaping the future of oncology diagnostics, ensuring that care remains at the forefront of research.
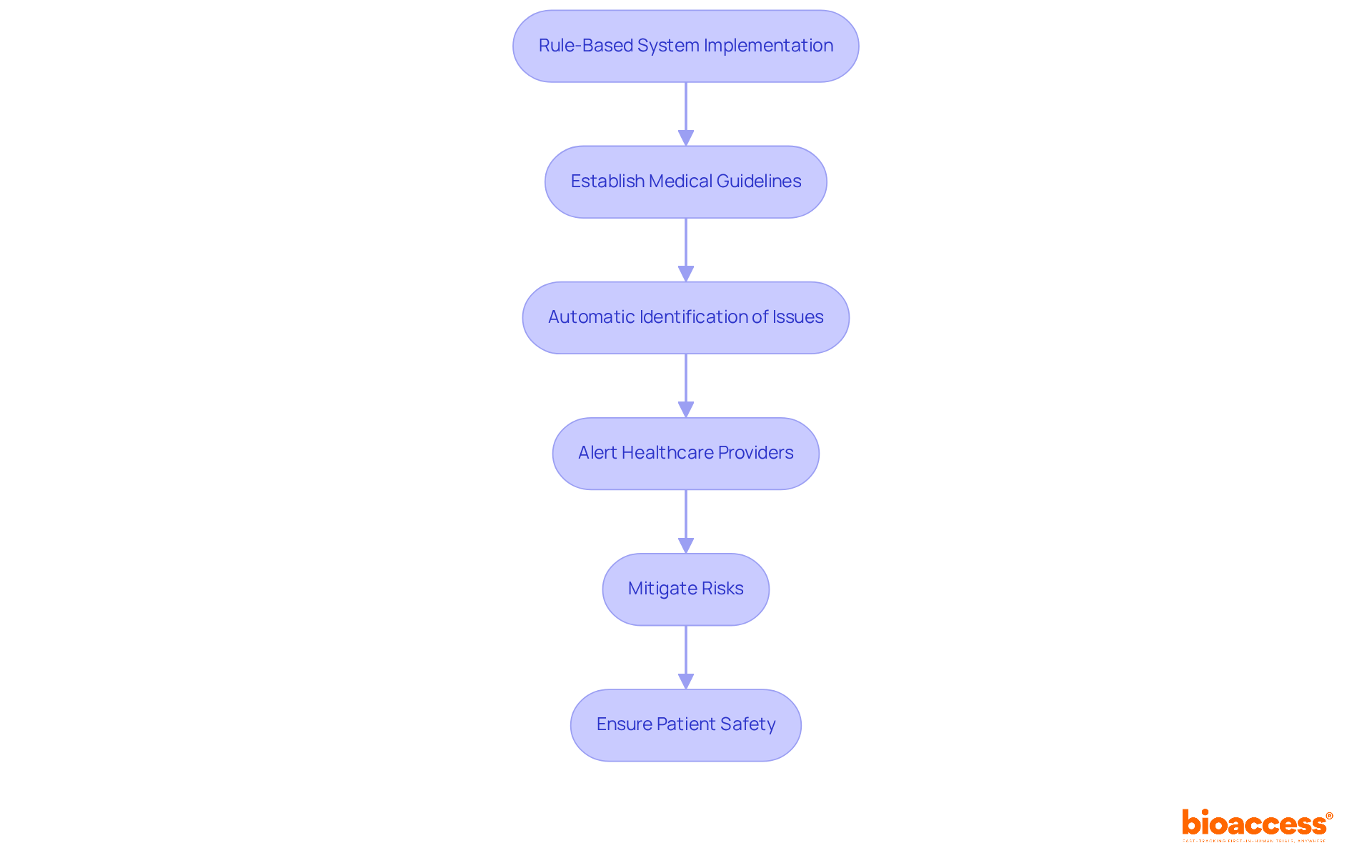
Rule-based medicine systems are essential in enhancing safety by implementing checks and balances throughout the research process. These systems automatically identify potential negative occurrences or contraindications based on established medical guidelines, ensuring that individuals receive appropriate care through rule-based medicine tailored to their specific needs.
For instance, in medication management, systematic approaches can alert healthcare providers to potential drug interactions or allergies, significantly mitigating the risk of adverse reactions. This proactive method not only safeguards individuals but also fosters a culture of safety within research studies, where participant welfare is paramount. By integrating guideline-driven systems based on rule-based medicine into healthcare protocols, researchers can ensure that safety remains a primary concern, ultimately leading to more favorable research outcomes.
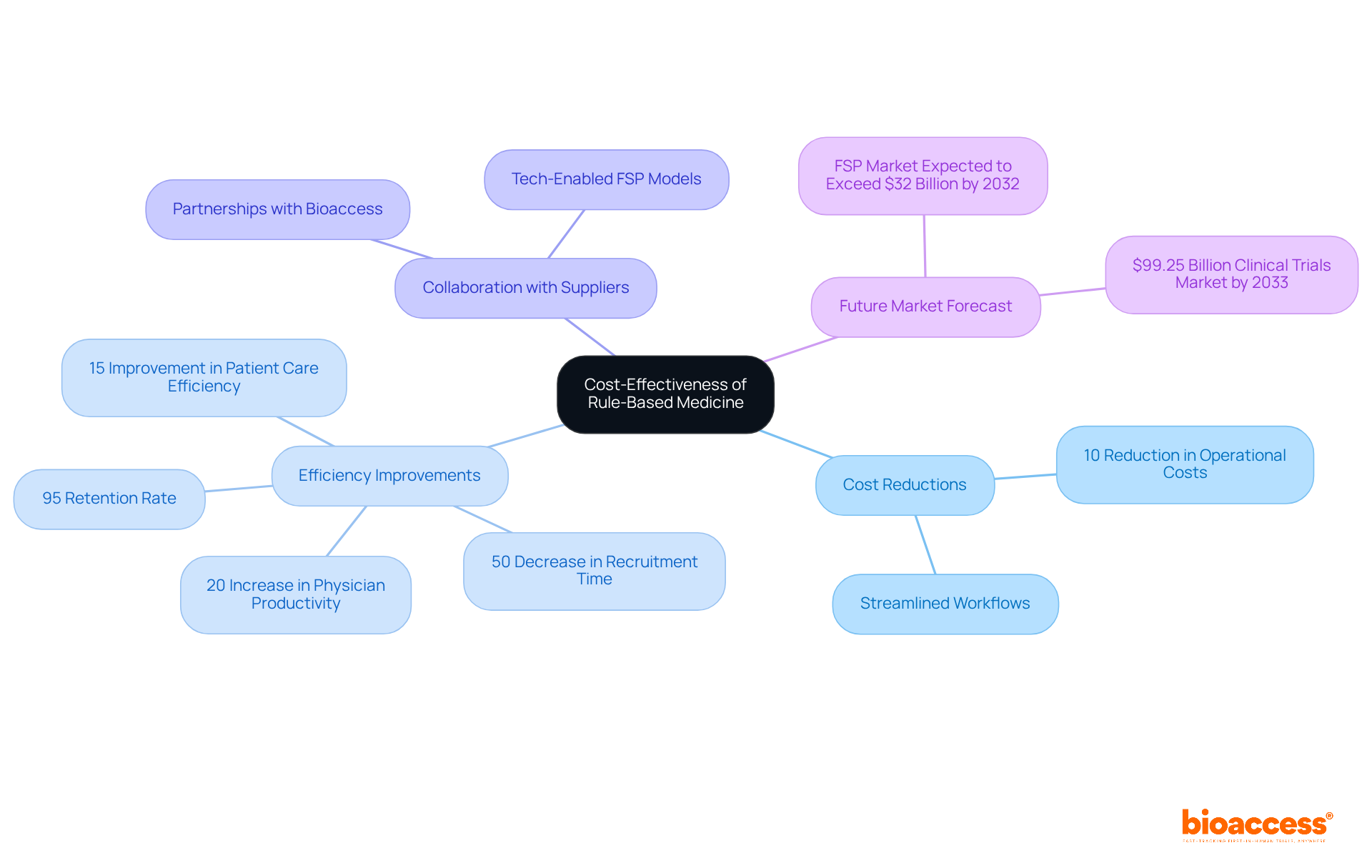
Utilizing rule based medicine in research studies can result in significant cost reductions by optimizing processes and minimizing the need for extensive manual supervision. These systems automate essential elements of study management, including patient recruitment and data analysis, which greatly lowers labor expenses and accelerates timelines. For instance, a collaboration between GlobalCare Clinical Studies and bioaccess resulted in an impressive 50% decrease in the duration of research subject recruitment and a retention rate exceeding 95%. This underscores the efficiency improvements attainable through automation and skilled oversight in research studies.
Moreover, tech-enabled Functional Service Provider (FSP) models are vital for cost optimization and operational flexibility in research studies. By enhancing diagnostic accuracy and improving patient safety, structured systems can diminish the frequency of costly adverse events and protocol deviations. This not only lowers the overall costs associated with research studies but also heightens the likelihood of favorable outcomes, making rule based medicine a financially prudent choice for Medtech innovators. As organizations strive to maximize their return on investment in research, the cost-effectiveness of systematic approaches becomes increasingly evident, with forecasts indicating that the research market could reach $99.25 billion by 2033. Collaboration with suppliers, such as bioaccess, is also essential for successful automation, ensuring that the implementation of these systems is both effective and sustainable.
Rule based medicine offers significant scalability, making it well-suited for a variety of research designs, from early-phase studies to extensive multicenter trials. This adaptability allows structured systems to meet the unique needs of each study, ensuring consistent compliance with established protocols.
Such adaptability is critical in diverse medical environments, where differences in population groups and treatment guidelines can complicate study management. By utilizing systematic approaches, researchers can uphold a high level of consistency while addressing the distinct characteristics of each trial. This scalability not only improves operational efficiency but also facilitates the successful execution of complex research studies in rule based medicine, yielding more reliable data and better outcomes for individuals.
For instance, the application of structured systems has been shown to enhance protocol adherence, a vital factor in maintaining data integrity. As research studies increasingly embrace diversity in 2025, the ability to tailor structured systems to accommodate diverse patient populations will become essential. This ensures that trials are not only efficient but also inclusive, ultimately advancing the field of personalized medicine.
The incorporation of artificial intelligence (AI) with guideline-driven medicine marks a significant advancement in research methodologies within healthcare. By leveraging predictive analytics and machine learning, AI enhances structured systems, enabling more sophisticated decision-making informed by extensive datasets. For instance, AI algorithms can analyze historical study data to identify patterns that inform and refine study designs, while rule-based medicine ensures compliance with established medical guidelines throughout the research process. This integration not only boosts the effectiveness of studies but also sharpens participant selection and treatment protocols, leading to improved outcomes.
Notably, bioaccess® enables treatment-naive cardiology or neurology groups to be enrolled 50% faster than traditional Western sites, resulting in substantial savings of $25K per individual with FDA-ready data—no rework, no delays. This capability directly addresses the challenges of participant recruitment in early-stage studies, particularly for Medtech and biopharma startups. Furthermore, bioaccess® offers comprehensive trial management services, including:
These services streamline the approval processes for startups, overcoming regulatory hurdles that frequently impede progress.
As AI technology continues to evolve, its collaboration with conventional medicine is poised to foster innovative approaches in medical research, ultimately enhancing patient care and treatment efficacy.
Rule based medicine significantly enhances regulatory compliance in clinical research by establishing clear guidelines and automated checks that ensure adherence to established protocols. These systems can be tailored to meet specific regulatory requirements, effectively reducing the risk of non-compliance and the associated penalties.
By automating documentation and reporting processes, systematized frameworks alleviate the administrative burden on research teams, allowing them to concentrate on the scientific aspects of their studies. This shift not only enhances the efficiency of case management but also cultivates a culture of compliance, where adherence to regulations becomes a fundamental aspect of daily operations. As the regulatory environment continues to evolve, the flexibility of rule-based systems will be crucial for ensuring compliance and safeguarding the integrity of research.
Recent advancements in automated compliance systems have demonstrated promising outcomes in medical trials, with organizations increasingly recognizing the importance of these technologies. For instance, automated checks can ensure that all medical protocols are followed meticulously, thereby enhancing the overall quality of research outcomes. As the industry progresses toward more automated solutions, the incorporation of systems based on rule based medicine will play a pivotal role in shaping the future of clinical research compliance.

Rule based medicine significantly enhances client engagement by providing clear, actionable guidelines that empower individuals to actively participate in their healthcare. By implementing rule based medicine, healthcare providers can create personalized treatment plans tailored to each individual's unique needs and preferences. This customization fosters a sense of ownership and involvement in their health journey.
Furthermore, these systems promote efficient interaction between individuals and healthcare professionals, ensuring that individuals are well-informed about their treatment choices and the research process. Such clarity not only fosters confidence but also encourages increased involvement from individuals, which is crucial for the success of clinical studies. Prioritizing participant engagement through rule-based medicine approaches can lead to improved recruitment and retention rates, ultimately resulting in more successful trial outcomes.
Data suggests that organizations prioritizing client engagement achieve up to 40% improved recruitment and 30% enhanced retention rates. Moreover, personalized treatment plans have been shown to enhance patient involvement, making them more likely to adhere to study protocols and share accurate data. By utilizing structured systems, researchers can develop a more inclusive and efficient research environment.
Rule based medicine enhances data-driven decision-making by establishing a structured framework for analyzing health data. These systems efficiently aggregate and interpret extensive datasets, empowering researchers to make informed decisions grounded in empirical evidence rather than relying solely on intuition. This method is crucial in the clinical research landscape, as it ensures that decisions are firmly rooted in strong data, ultimately resulting in enhanced care and more effective treatment strategies.
In research studies, structured systems systematically evaluate individual outcomes and treatment reactions, recognizing patterns that guide protocol enhancement. This data-focused approach not only enhances the dependability of study outcomes but also promotes ongoing improvement in healthcare practices. By utilizing the advantages of guideline-driven medicine, researchers can significantly bolster the quality of their findings, leading to a more reliable healthcare system.
Furthermore, as the intricacy of medical studies grows, the incorporation of sophisticated analytics and immediate data gathering becomes crucial. By employing these technologies, rule based medicine can enhance the effectiveness of testing protocols and participant recruitment, ensuring that research remains adaptable to changing challenges. With bioaccess®'s expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, trials can achieve 50% quicker enrollment and $25K savings through FDA-ready data, effectively addressing common recruitment challenges encountered by Medtech and biopharma startups. This alignment with current trends in data-driven strategies is essential for achieving successful results in medical research.
In summary, the integration of rule-based medicine is crucial for improving clinical research outcomes. Collaboration among researchers, supported by robust data and analytics, is vital for navigating the complexities of modern medical studies. As the field continues to evolve, embracing these advancements will be key to overcoming challenges and achieving impactful results.

Research outcomes are significantly shaped by rule based medicine, which enhances diagnostic accuracy, improves individual safety, and streamlines regulatory compliance. By integrating guideline-driven systems into medical studies, researchers can achieve more reliable outcomes, optimize resource allocation, and reduce costs. Notably, bioaccess® has shown its capability to enroll treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, resulting in substantial savings of $25K per patient with FDA-ready data—effectively eliminating rework and delays.
The scalability and adaptability of structured approaches make them indispensable tools for Medtech innovators across diverse clinical environments. Furthermore, the implementation of rule based medicine systems has been linked to improved health outcomes, as evidenced by case studies such as the collaboration between Welwaze Medical Inc. and bioaccess™ for the introduction of the Celbrea® medical device in Colombia. This partnership exemplifies how regulatory access and market entry strategies can be streamlined, ultimately enhancing patient management and shortening trial timelines. Organizations that adopt systematized methodologies have reported a reduction in time to market by 3-5 years, highlighting the concrete benefits of these practices.
As the clinical research landscape evolves, rule-based medicine will play a pivotal role in advancing patient care and treatment efficacy, ultimately leading to improved health outcomes for patients globally.

The integration of rule-based medicine within clinical research signifies a transformative approach that enhances the overall efficacy and efficiency of medical studies. By leveraging structured systems, researchers can achieve significant improvements in diagnostic accuracy, patient safety, and regulatory compliance, ultimately leading to better health outcomes for patients. This methodology not only streamlines processes but also fosters a culture of data-driven decision-making, ensuring that clinical trials are grounded in robust evidence.
Key insights from the article underscore the multifaceted benefits of rule-based medicine. From reducing costs and improving patient engagement to enhancing scalability across diverse clinical trials, the advantages are evident. Furthermore, the collaboration between innovative platforms like bioaccess® and rule-based systems illustrates how these frameworks can expedite research timelines and optimize resource allocation, culminating in a faster path to market for critical medical technologies.
As the landscape of clinical research continues to evolve, embracing rule-based medicine will be essential for advancing patient care and achieving impactful results. Organizations are urged to adopt these methodologies not only to improve their research outcomes but also to contribute to a more reliable and effective healthcare system. By prioritizing systematic approaches, the medical community can ensure that clinical research remains at the forefront of innovation, ultimately benefiting patients worldwide.
What is bioaccess® and what role does it play in clinical research for Medtech innovations?
bioaccess® is a leader in medical research, particularly in the Medtech sector, that accelerates clinical studies by leveraging Colombia's regulatory efficiency, diverse participant groups in the Balkans, and optimized pathways in Australia. This approach helps bring groundbreaking medical technologies to market more swiftly.
How does bioaccess® enhance the clinical research process?
bioaccess® enhances the clinical research process through a comprehensive approach that includes site feasibility, investigator selection, regulatory compliance, project management, and reporting. Their expertise supports Medtech innovators in expediting research and development.
What are the advantages of conducting clinical research in Colombia?
Colombia offers significant advantages for clinical research, including regulatory approvals in 90-120 days, cost reductions exceeding 30% compared to North America and Western Europe, a population of over 50 million for participant recruitment, and attractive R&D tax incentives.
What is rule-based medicine and how does it improve diagnostic accuracy?
Rule-based medicine involves the use of predefined algorithms that guide medical decision-making, enhancing diagnostic accuracy. It utilizes established clinical guidelines and user data to provide reliable diagnostic outputs, minimizing human errors and standardizing processes.
How does rule-based medicine benefit oncology diagnostics specifically?
In oncology, rule-based medicine automates the interpretation of complex data sets, allowing clinicians to make informed decisions based on the latest evidence. This leads to improved diagnostic precision and overall quality of care for patients.
What impact do structured methodologies have on healthcare diagnostics?
The integration of structured methodologies in healthcare diagnostics fosters a systematic and trustworthy environment, leading to better patient outcomes. For example, a Clinical Decision Support System (CDSS) achieved 96% accuracy in classifying health conditions.
How do rule-based systems enhance patient safety in clinical research?
Rule-based systems enhance patient safety by implementing checks and balances that identify potential negative occurrences or contraindications based on established medical guidelines, ensuring appropriate care and mitigating risks of adverse reactions.
What role do rule-based medicine systems play in medication management?
In medication management, rule-based systems can alert healthcare providers to potential drug interactions or allergies, significantly reducing the risk of adverse reactions and promoting a culture of safety in research studies.