


The article elucidates the myriad benefits of sequential study designs in expediting clinical research, emphasizing advantages such as:
These benefits are substantiated by evidence demonstrating how adaptive methodologies facilitate real-time adjustments based on interim findings. This ultimately culminates in more efficient trials and superior health outcomes for diverse patient populations.
The landscape of clinical research is evolving rapidly, driven by the imperative for efficiency and effectiveness in trial methodologies. Sequential study designs are emerging as a transformative approach, offering a multitude of benefits that streamline processes and enhance data quality.
However, what challenges do researchers face in implementing these innovative strategies? How can they leverage the unique advantages of sequential studies to improve outcomes?
This exploration delves into the ten key benefits of sequential study implementation, revealing how this approach not only accelerates clinical research but also fosters collaboration and adaptability in an ever-changing medical environment.
bioaccess® excels in accelerating research through innovative sequential study designs. These methodologies streamline processes, significantly reduce timelines, and enhance the overall quality of clinical trials. By allowing adaptive adjustments based on interim results, bioaccess® ensures that research remains relevant and effective throughout its duration. This focus on early-stage research bridges the gap between advanced medical technologies and regulatory standards, facilitating quicker approvals and patient enrollment. The effective implementation of sequential study methodologies not only improves trial timelines but also aligns with the evolving landscape of Medtech, Biopharma, and Radiopharma, ultimately enhancing patient care.
Regulatory Speed: Leverage Latin America's Fast-Track Approvals for Sequential Studies
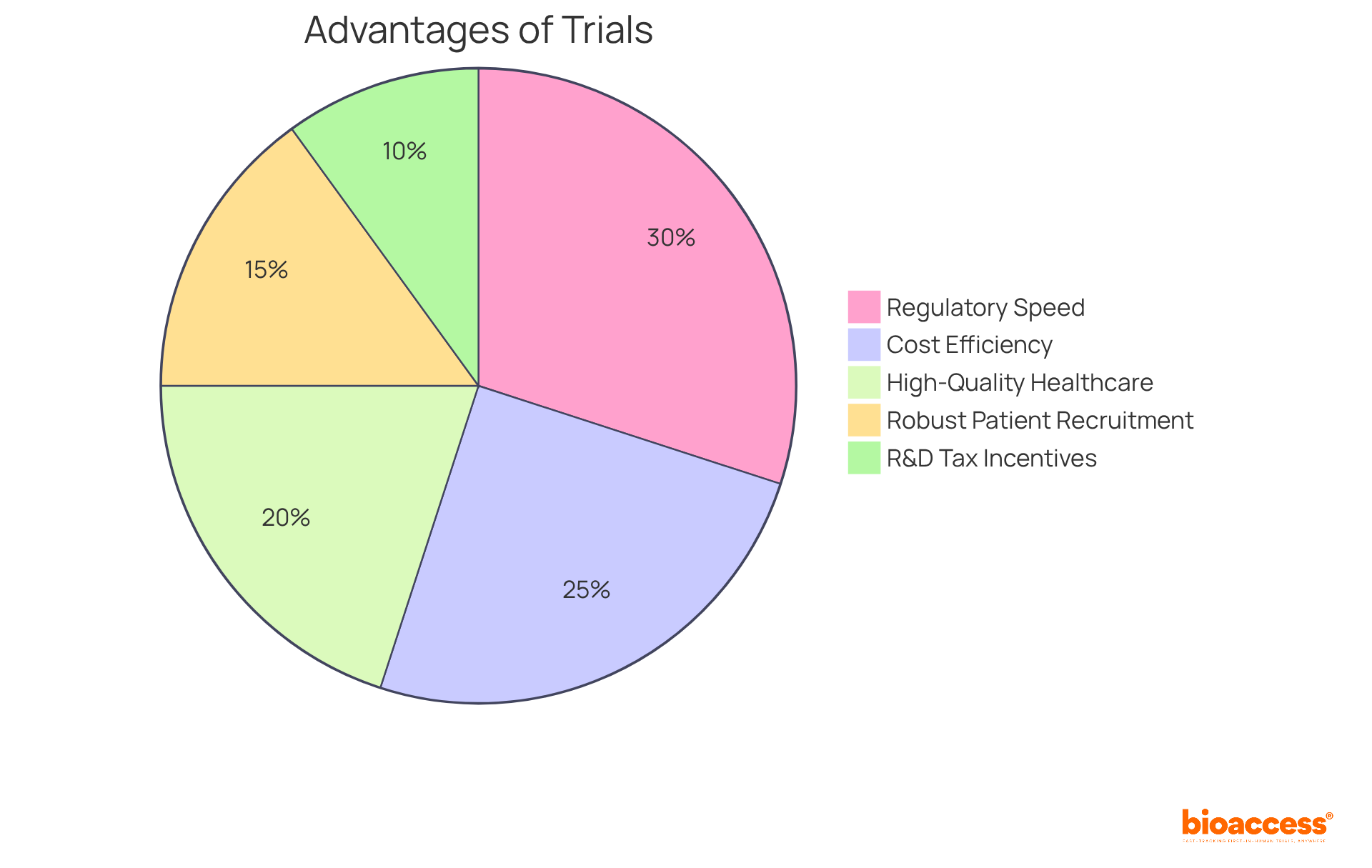
Latin America presents a distinctive regulatory environment that facilitates rapid approvals for clinical studies, particularly in Colombia, where the total IRB/EC and MoH (INVIMA) review process is completed in just 90-120 days. By capitalizing on this speed, bioaccess® can implement a sequential study design that allows for timely modifications based on interim results. This fast-tracking capability not only accelerates the research process but also amplifies the potential for successful outcomes. The ability to adapt research in real-time ensures that scientists can respond to new data, ultimately leading to more efficient and effective medical evaluations.
Key Advantages of Conducting Clinical Trials in Colombia:
These factors collectively expedite medical trials, positioning Colombia as an ideal location for first-in-human research.
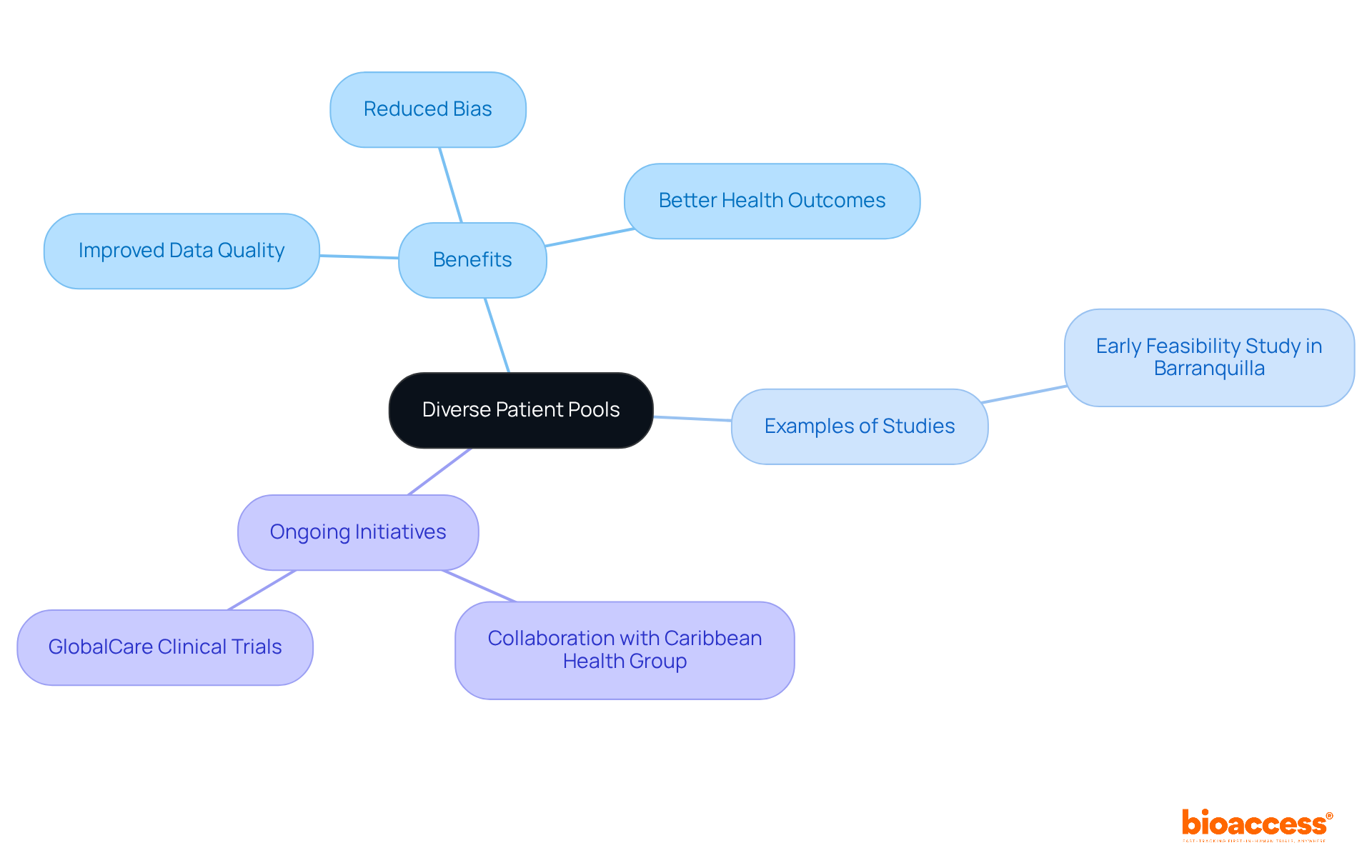
Incorporating diverse patient populations from the Balkans and Latin America significantly enhances the quality of information in a sequential study. Engaging these regions enables bioaccess® to perform a sequential study that captures a broad spectrum of demographics, which is essential for understanding varied responses to treatments. This variety not only strengthens the reliability of study findings but also fosters inclusiveness in medical investigations, ultimately resulting in better health outcomes for all patient groups.
Researchers have observed that varied demographics can reduce biases and improve the applicability of findings, making it essential to include participants from these areas in a sequential study. The distinctive traits of Balkan and Latin American groups provide important perspectives, ensuring that medical studies are representative of worldwide health requirements and issues.
The recent Early Feasibility Study conducted by ReGelTec in Barranquilla, Colombia, which successfully treated eleven patients with chronic low back pain using HYDRAFIL™, exemplifies the potential of such collaborations. Furthermore, the partnership between bioaccess® and Caribbean Health Group aims to establish Barranquilla as a premier location for medical trials in Latin America, supported by Colombia's Minister of Health.
This initiative, alongside GlobalCare Clinical Trials' efforts to enhance ambulatory services, has achieved over a 50% decrease in recruitment time and remarkable 95% retention rates, demonstrating the effectiveness of bioaccess's contributions to clinical studies.
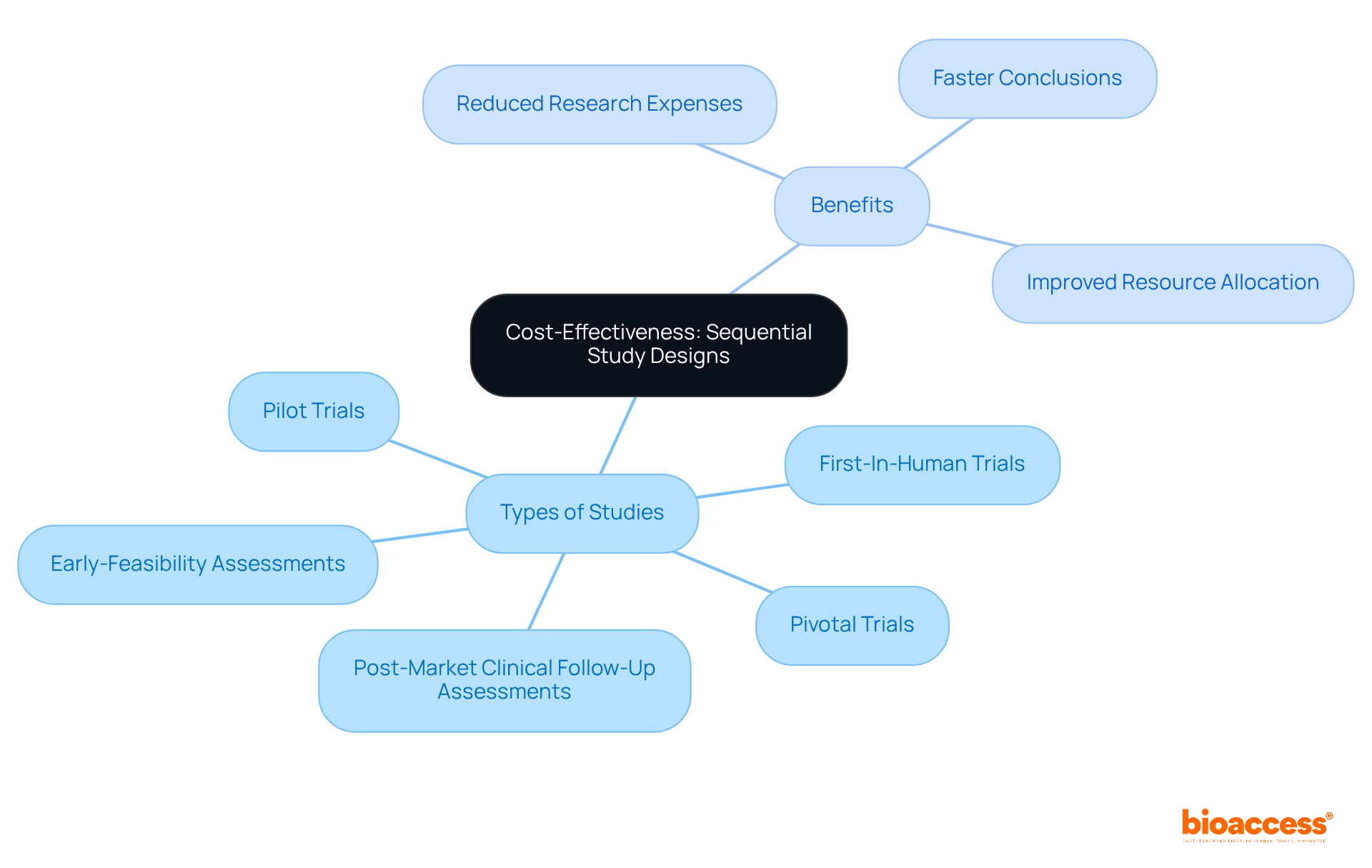
A sequential study approach presents a strategic advantage by significantly reducing expenses while amplifying impact. By facilitating early trial conclusions based on interim results, bioaccess® empowers clients to evade unnecessary costs associated with extended evaluations. This methodology not only conserves financial resources but also permits the reallocation of funds to critical study areas, thereby enhancing overall project efficiency and effectiveness.
With over 20 years of experience in Medtech, bioaccess® specializes in managing a diverse array of investigations, including:
These are crucial for accelerating medical exploration in Latin America. Notably, research indicates that adaptive designs can decrease the number of subjects required by 10 to 30, depending on the effect size, resulting in substantial cost savings. Additionally, the likelihood of halting studies for futility at the initial analysis can soar to 76.68%, enabling timely decisions that avert resource wastage.
As emphasized by industry experts, the ability to terminate studies prematurely when results indicate ineffectiveness is essential for both ethical and financial stewardship in medical research. By leveraging these methodologies, organizations can achieve significant reductions in testing expenditures while maintaining rigorous scientific integrity, all while benefiting from bioaccess®'s extensive management services for studies.
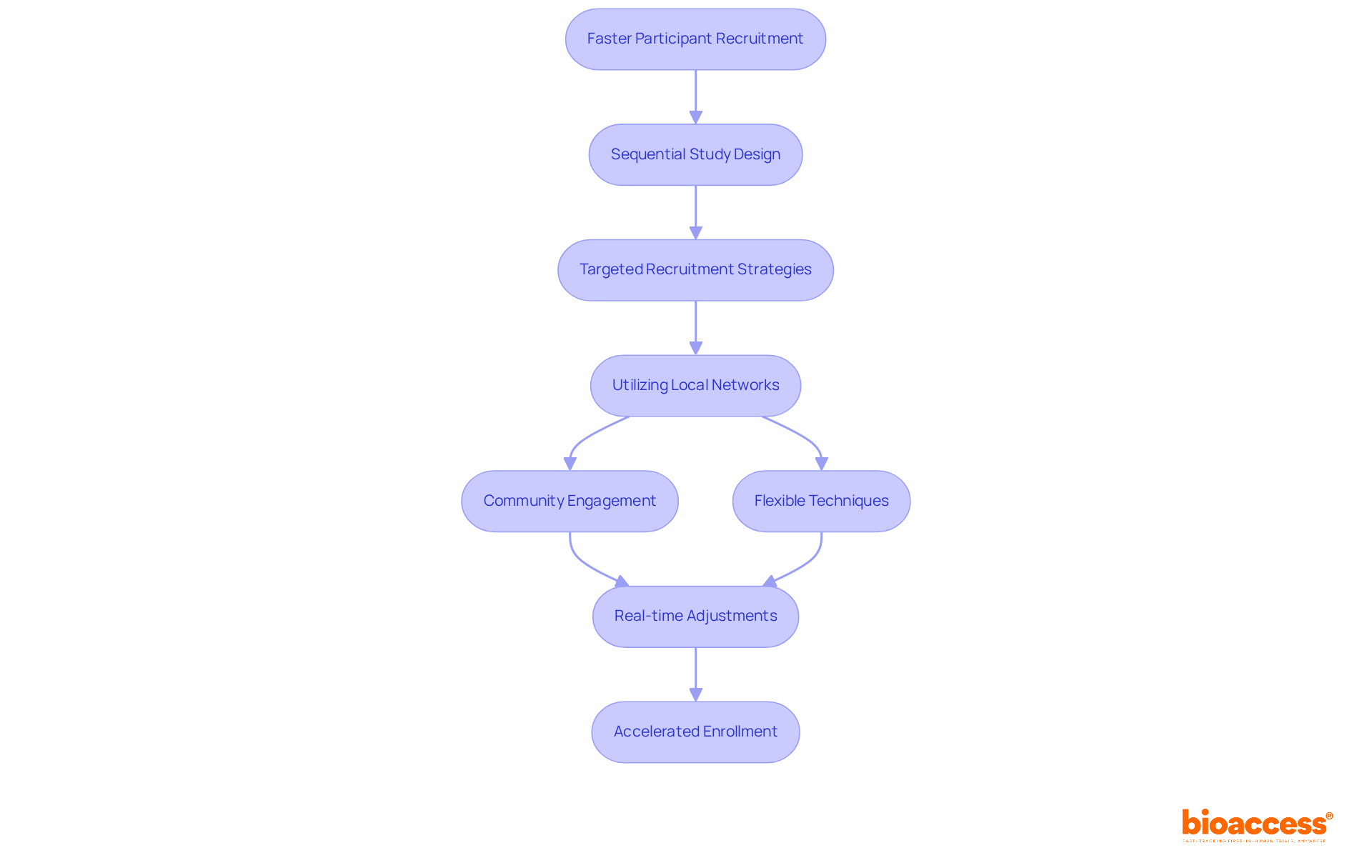
A sequential study design significantly enhances participant recruitment speed, which is a critical advantage in clinical trials. Bioaccess® employs targeted recruitment strategies that tap into local networks and foster community engagement, allowing for efficient participant attraction. By utilizing flexible techniques, the organization can adjust recruitment initiatives based on real-time information, guaranteeing that research is filled with the appropriate participants at the correct moment. This agility not only accelerates the enrollment process but also enhances the overall quality of the study.
According to leaders in medical trials, implementing a sequential study approach can shorten recruitment timelines by as much as 50%, establishing it as an essential tactic in today’s rapid-paced medical environment. Effective recruitment tactics, such as employing electronic health records and collaborating with patient advocacy groups, have demonstrated success in consecutive research. This further highlights the potential for swift enrollment and diverse participant inclusion, prompting stakeholders to consider how these strategies can address their own challenges in clinical research.
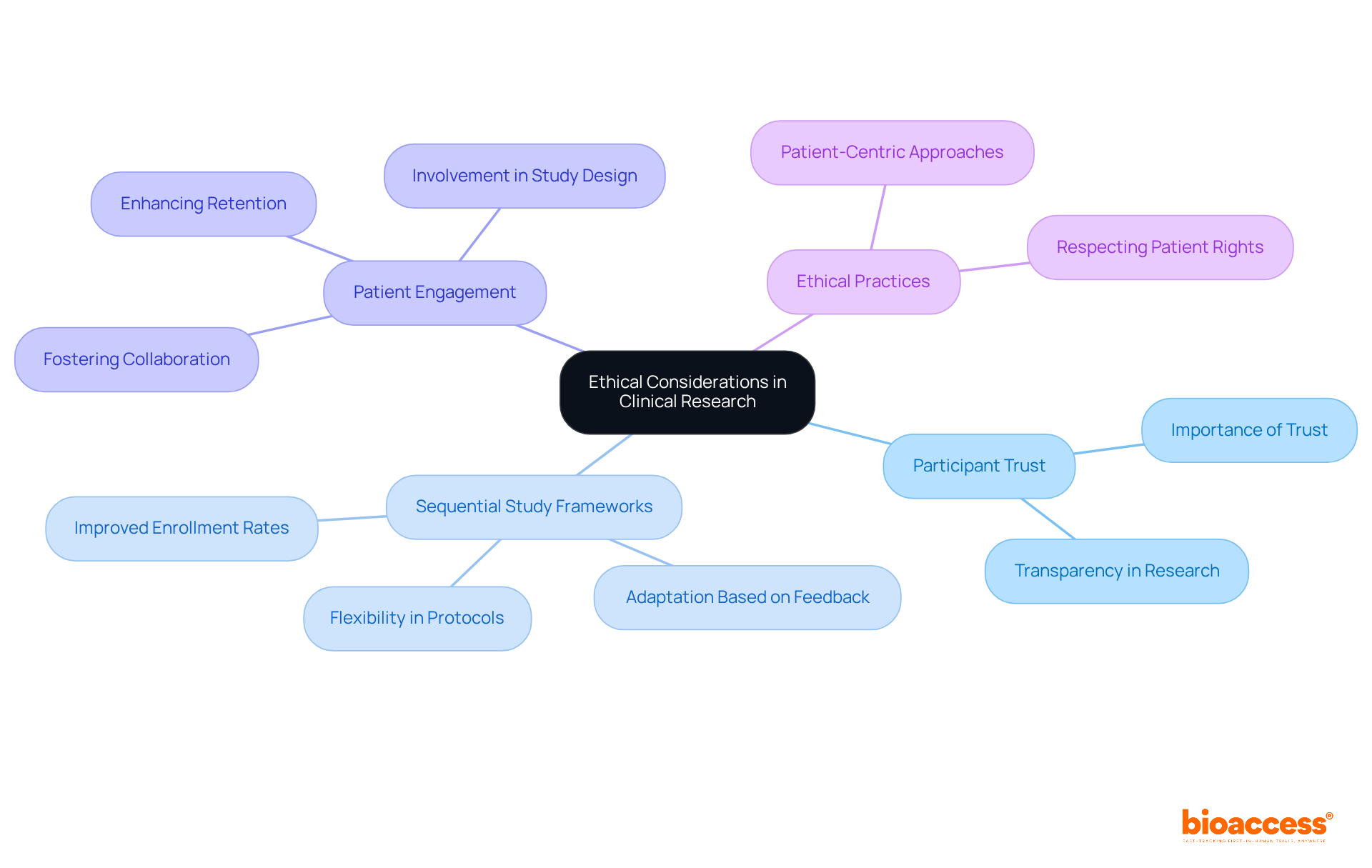
Incorporating ethical considerations into clinical research is essential for maintaining participant trust and ensuring the integrity of research findings. Sequential study frameworks facilitate a more patient-centric approach, enabling researchers to adapt protocols based on participant feedback and interim findings. This flexibility is crucial; research indicates that patient-focused experiments can enlist participants considerably quicker—frequently reaching enrollment targets in approximately four months versus the seven months common with conventional approaches.
At bioaccess®, ethical practices are prioritized by designing studies that focus on participant well-being, fostering a collaborative environment that respects patient rights and promotes transparency. By actively involving patients in the planning and design of studies, researchers can enhance engagement and retention, ultimately leading to more successful outcomes. As Daniel J Herron highlights, 'Patient centricity should be a fundamental approach in research studies because it leads to better engagement, retention, and overall success of the study.'
This dedication to patient focus not only enhances the experience of the examination but also aligns with the increasing trend of integrating various viewpoints in health investigations, ensuring that evaluations are both ethical and effective.
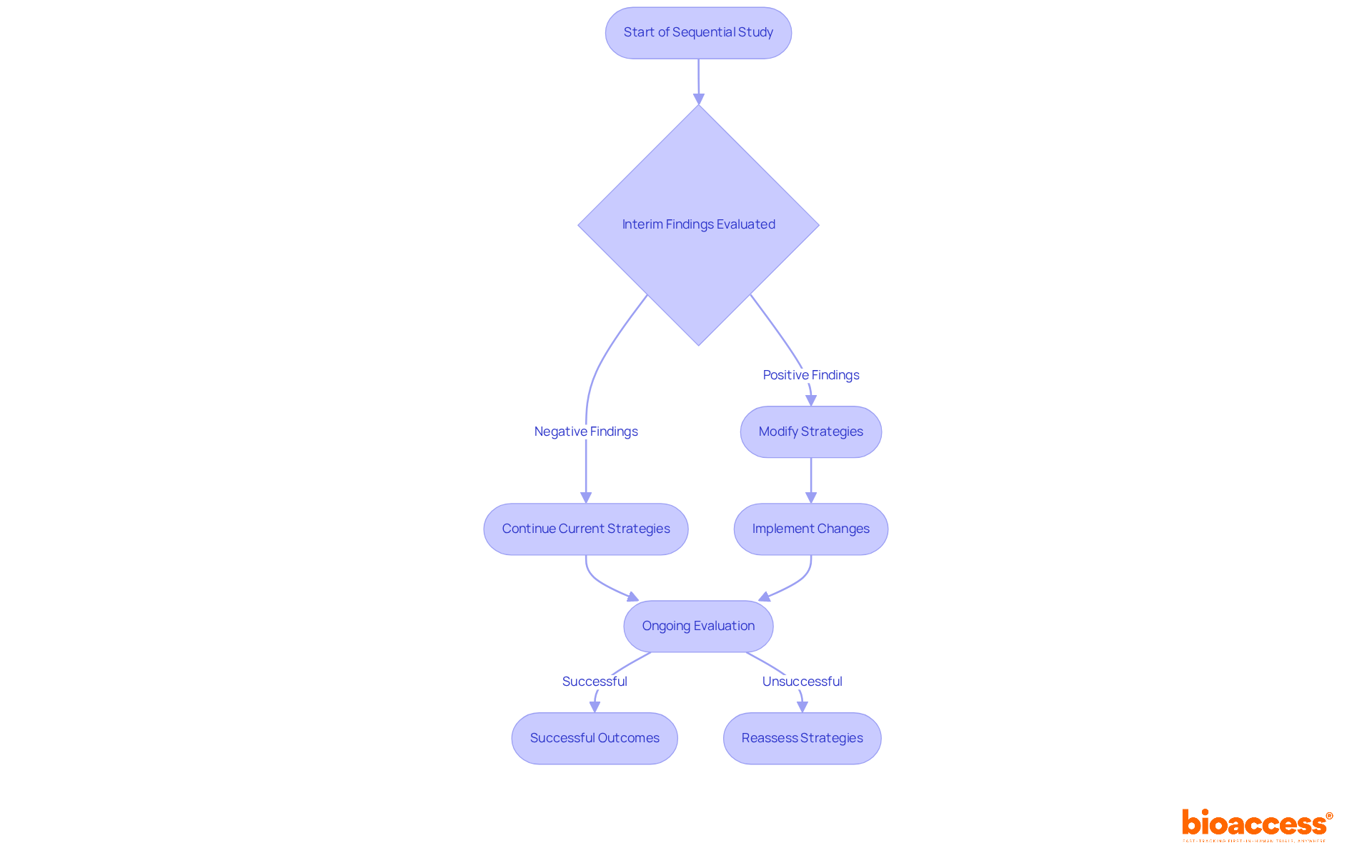
Sequential study exemplifies adaptability, enabling researchers to modify strategies based on interim findings. This flexibility is particularly advantageous for bioaccess®, a leading contract organization that supports medical device clinical studies in Latin America. With bioaccess®, researchers can enroll treatment-naive cardiology or neurology cohorts 50% faster than their Western counterparts, achieving significant cost savings of $25K per patient through FDA-ready data—effectively eliminating rework and delays. Ongoing evaluation of outcomes empowers bioaccess® to refine study strategies, ensuring that experiments remain relevant and efficient throughout their duration. This iterative approach not only enhances the quality of data collected but also significantly increases the likelihood of successful outcomes.
For example, in a recent study involving blinatumomab, interim analyses prompted early termination due to substantial improvements in event-free survival, with children receiving blinatumomab demonstrating markedly better outcomes compared to those undergoing standard chemotherapy. Such modifications based on interim findings underscore the critical role of adaptive designs in sequential studies, where timely decisions can safeguard patient safety and enhance treatment effectiveness.
Researchers assert that rigorous planning and transparency are vital to minimizing operational bias. As Philip Pallmann noted, "The optimal approach to reduce operational bias is through thorough planning and openness," highlighting the necessity of adaptive methodologies in contemporary trials.
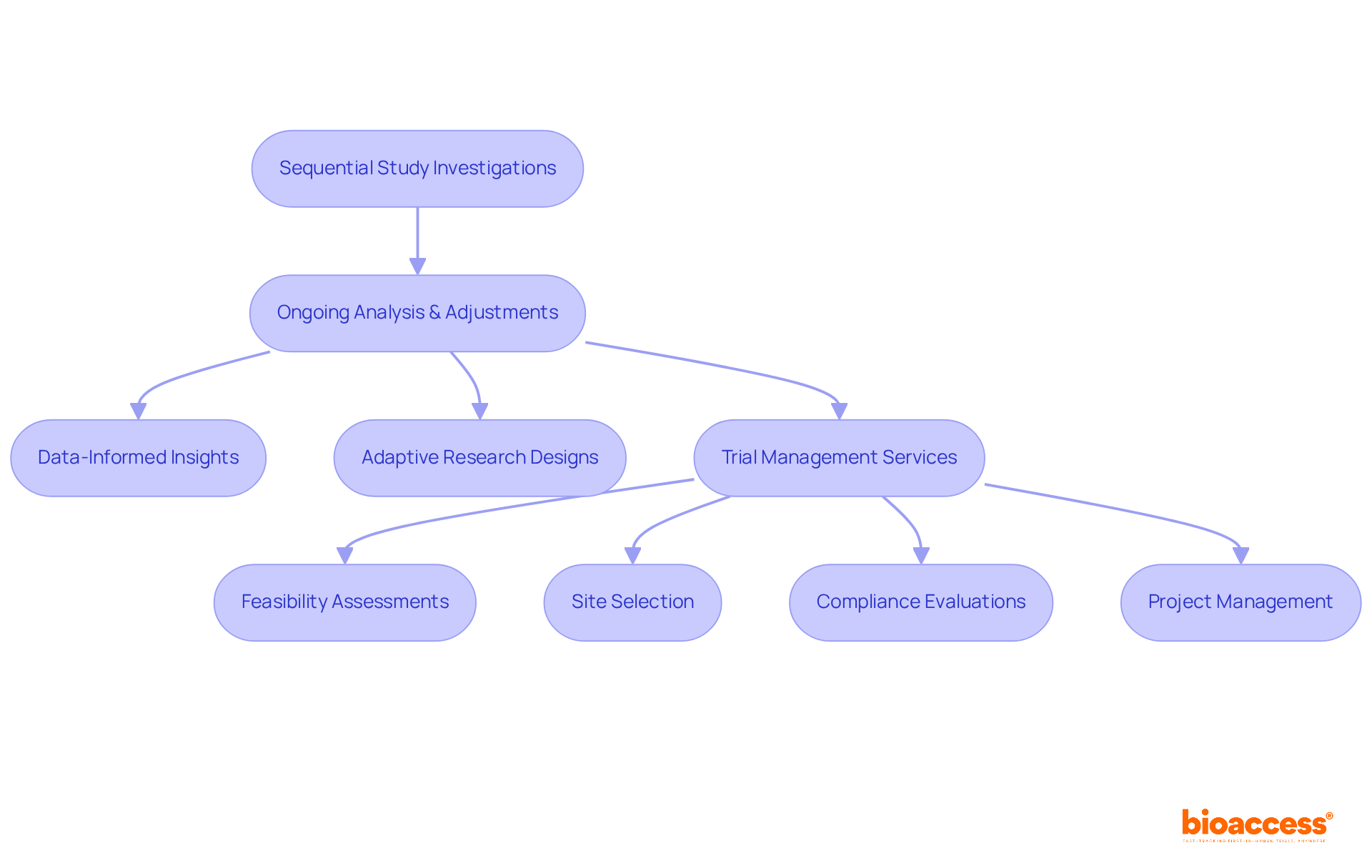
The repetitive aspect of sequential study investigations is pivotal in enhancing medical outcomes. By facilitating ongoing analysis and adjustments, bioaccess® continuously refines research protocols, which leads to improved research quality. This method not only yields more reliable outcomes but also cultivates a culture of continuous improvement within sequential studies.
Data-informed insights empower bioaccess® to design research that prioritizes efficacy and safety, ultimately benefiting both participants and stakeholders. Research indicates that adaptive research designs correlate positively with success rates, underscoring the importance of flexibility in medical investigations. Experts in the field emphasize that such methodologies can significantly reduce delays, with some studies suggesting a duration reduction of up to 50%.
Furthermore, bioaccess® offers comprehensive trial management services, including:
thereby enhancing the trial landscape in Barranquilla. By embracing these iterative processes, bioaccess® positions itself at the forefront of clinical innovation, ensuring that each trial is optimized for success.
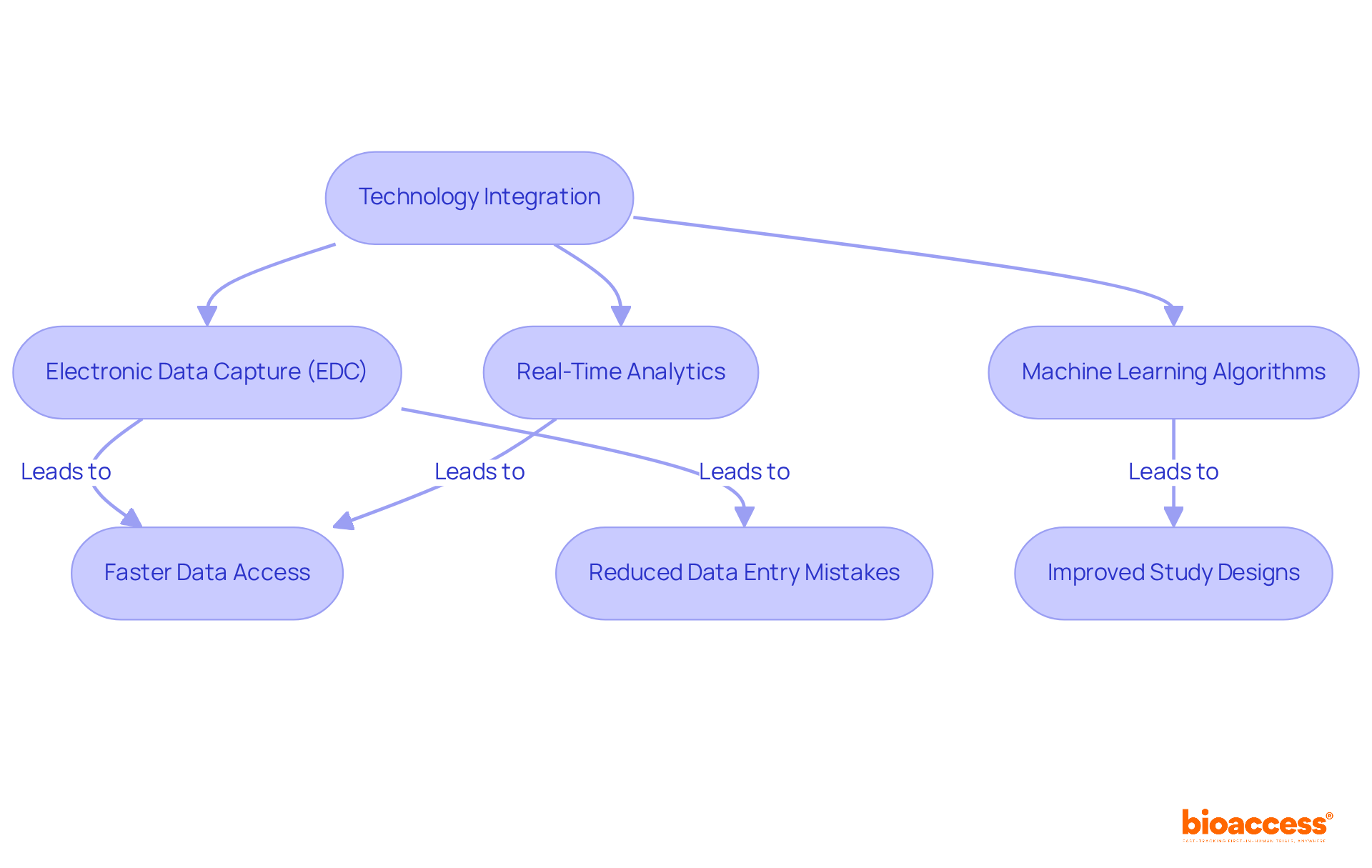
The incorporation of advanced technology is essential to bioaccess®'s approach in sequential study analyses. By leveraging state-of-the-art tools for data collection and analysis, the organization significantly enhances the accuracy and reliability of research findings. For instance, electronic data capture (EDC) systems facilitate real-time data access, allowing researchers to gain insights sooner in the research process. This capability not only shortens the total length of clinical evaluations but also decreases data entry mistakes, with research suggesting a possible decrease of up to 30% in data entry time.
Furthermore, the application of machine learning algorithms and real-time analytics enables flexible study designs, which are crucial in sequential study investigations. These technologies empower researchers to make informed decisions based on robust data insights, ultimately leading to improved data quality and operational efficiency. Trials utilizing integrated, automated data management workflows have been shown to complete patient enrollment 30% faster and achieve database lock in 45% less time compared to those using fragmented systems.
Experts in the field highlight the transformative impact of these advanced tools. As observed by industry leaders, the adoption of EDC technologies is reshaping the landscape of medical studies, enhancing data integrity and adherence to regulatory standards. By embracing these innovations, bioaccess® not only simplifies its processes but also positions itself at the forefront of research excellence.
Collaboration is essential for the success of sequential studies, and bioaccess® prioritizes fostering teamwork among researchers, stakeholders, and participants involved in these studies. By creating a welcoming atmosphere that encourages open dialogue and shared objectives, bioaccess® significantly enhances the efficiency of its research studies. This collaborative approach not only improves the quality of studies but also strengthens relationships with stakeholders, ensuring alignment in goals and expectations.
Notably, collaborations with nearby healthcare organizations, such as Welwaze Medical Inc. for the introduction of the Celbrea® medical device, have proven successful in increasing patient enrollment, particularly for rare illnesses, thereby diversifying the pool of trial participants. Furthermore, leveraging varied expertise and perspectives fosters innovation, leading to improved outcomes in medical studies.
As emphasized by industry leaders like Bill Andrews, Ph.D., promoting collaboration is crucial in a sequential study, as it transforms obstacles into opportunities for growth and success in research. By implementing structured collaboration measures, bioaccess® exemplifies how strategic teamwork can expedite the clinical research process, ultimately benefiting all involved parties.
To maximize the impact of collaboration, continuous engagement with stakeholders and adaptive strategies based on feedback and outcomes are essential.

The implementation of sequential study designs signifies a transformative shift in clinical research, markedly enhancing efficiency and effectiveness across multiple domains. By facilitating adaptive modifications based on interim results, these methodologies not only streamline processes but also substantially decrease timelines and costs, ultimately resulting in improved patient outcomes. The emphasis on early-stage research and the capacity to respond dynamically to emerging data positions sequential studies as a crucial element in the ever-evolving landscape of medical research.
Throughout this article, the key benefits of sequential studies have been underscored, including:
Each of these components contributes to the overarching objective of accelerating clinical trials and enhancing healthcare solutions.
In light of these insights, it is imperative for stakeholders in the clinical research arena to contemplate the adoption of sequential study methodologies. By embracing these practices, researchers can not only elevate the quality and speed of their investigations but also cultivate a collaborative environment that prioritizes patient welfare. The future of clinical research hinges on the ability to adapt, innovate, and engage diverse populations, positioning sequential studies as a pivotal strategy for achieving successful outcomes in medical advancements.
What is bioaccess® and how does it contribute to clinical research?
bioaccess® accelerates clinical research through innovative sequential study designs that streamline processes, reduce timelines, and enhance trial quality. It allows for adaptive adjustments based on interim results, ensuring research remains relevant and effective.
How does bioaccess® utilize sequential study methodologies?
The implementation of sequential study methodologies by bioaccess® improves trial timelines and aligns with the evolving landscape of Medtech, Biopharma, and Radiopharma, ultimately enhancing patient care.
What advantages does Latin America offer for conducting clinical trials?
Latin America, particularly Colombia, offers rapid approvals for clinical studies, with a total IRB/EC and MoH (INVIMA) review process taking only 90-120 days. This regulatory speed allows for timely modifications based on interim results.
What are the cost benefits of conducting clinical trials in Colombia?
Conducting clinical trials in Colombia can save over 30% compared to tests in North America or Western Europe, making it a cost-effective option for research.
How does Colombia's healthcare system support clinical trials?
Colombia's healthcare system is ranked among the best globally, with a robust patient recruitment pool of over 50 million people, 95% of whom are covered by universal healthcare.
What tax incentives are available for R&D in Colombia?
Investments in science and technology projects in Colombia benefit from significant tax advantages, including a 100% tax deduction and various credits.
Why is it important to include diverse patient populations in sequential studies?
Incorporating diverse patient populations from the Balkans and Latin America enhances the quality of data, reduces biases, and improves the applicability of findings, leading to better health outcomes for all patient groups.
Can you provide an example of successful clinical research collaboration in Latin America?
An example is the Early Feasibility Study conducted by ReGelTec in Barranquilla, Colombia, which successfully treated eleven patients with chronic low back pain using HYDRAFIL™. This collaboration highlights the potential of establishing Barranquilla as a premier location for medical trials.
What results have been achieved through bioaccess®'s initiatives in clinical studies?
bioaccess®'s initiatives have led to over a 50% decrease in recruitment time and remarkable 95% retention rates in clinical studies, demonstrating their effectiveness in enhancing research outcomes.