


The article titled "10 Best CROs to Work For in Clinical Research" seeks to identify and highlight the top Contract Research Organizations (CROs) that offer favorable working conditions and opportunities in the clinical research sector. This exploration is not only relevant but essential for professionals navigating the complexities of this field.
It is expected that the article will delve into the criteria that render these CROs desirable employers, emphasizing their commitment to:
By showcasing these attributes, the article aims to capture the attention of readers and establish the credibility of the highlighted organizations, ultimately generating interest and desire for collaboration within the clinical research community.
In the fast-evolving landscape of clinical research, the role of Contract Research Organizations (CROs) has become increasingly pivotal. As the demand for innovative medical solutions grows, identifying the best CROs to partner with can significantly impact the success of clinical trials. This article delves into the ten leading CROs that not only excel in operational efficiency but also prioritize employee satisfaction and innovative practices.
What sets these organizations apart? How can they help navigate the complexities of clinical research while ensuring rapid advancements in healthcare?
bioaccess® distinguishes itself in the CRO landscape by harnessing Latin America's regulatory efficiency, particularly in Colombia, where the total IRB/EC and MoH (INVIMA) review takes only 90-120 days. This strategic advantage allows bioaccess® to secure ethical approvals in a remarkable 4-6 weeks and achieve enrollment rates that are 50% faster than traditional markets. With a committed emphasis on initial-stage medical research, bioaccess® enables Medtech innovators to accelerate their product development, ensuring that groundbreaking technologies reach individuals more rapidly.
Colombia's healthcare system, ranked among the top five globally, coupled with a population of over 50 million and universal healthcare coverage for 95% of its citizens, provides a robust environment for patient recruitment. Moreover, the R&D tax incentives accessible in Colombia, featuring substantial tax deductions and grants, further improve the appeal of carrying out research in the region. Leveraging more than 20 years of experience, bioaccess® has a comprehensive grasp of the regulatory landscape, establishing itself as a vital ally for Medtech firms pursuing swift progress in their research studies.
The modernization of regulatory procedures in Latin America, especially in Colombia, further improves the pace and reliability of studies, making the region an increasingly appealing center for research. As Medtech innovators recognize the critical importance of early-phase research, bioaccess® stands ready to facilitate their breakthroughs.

This company is emerging as a formidable player in the clinical research sector, offering a comprehensive suite of services essential for medical device development. Their capabilities encompass:
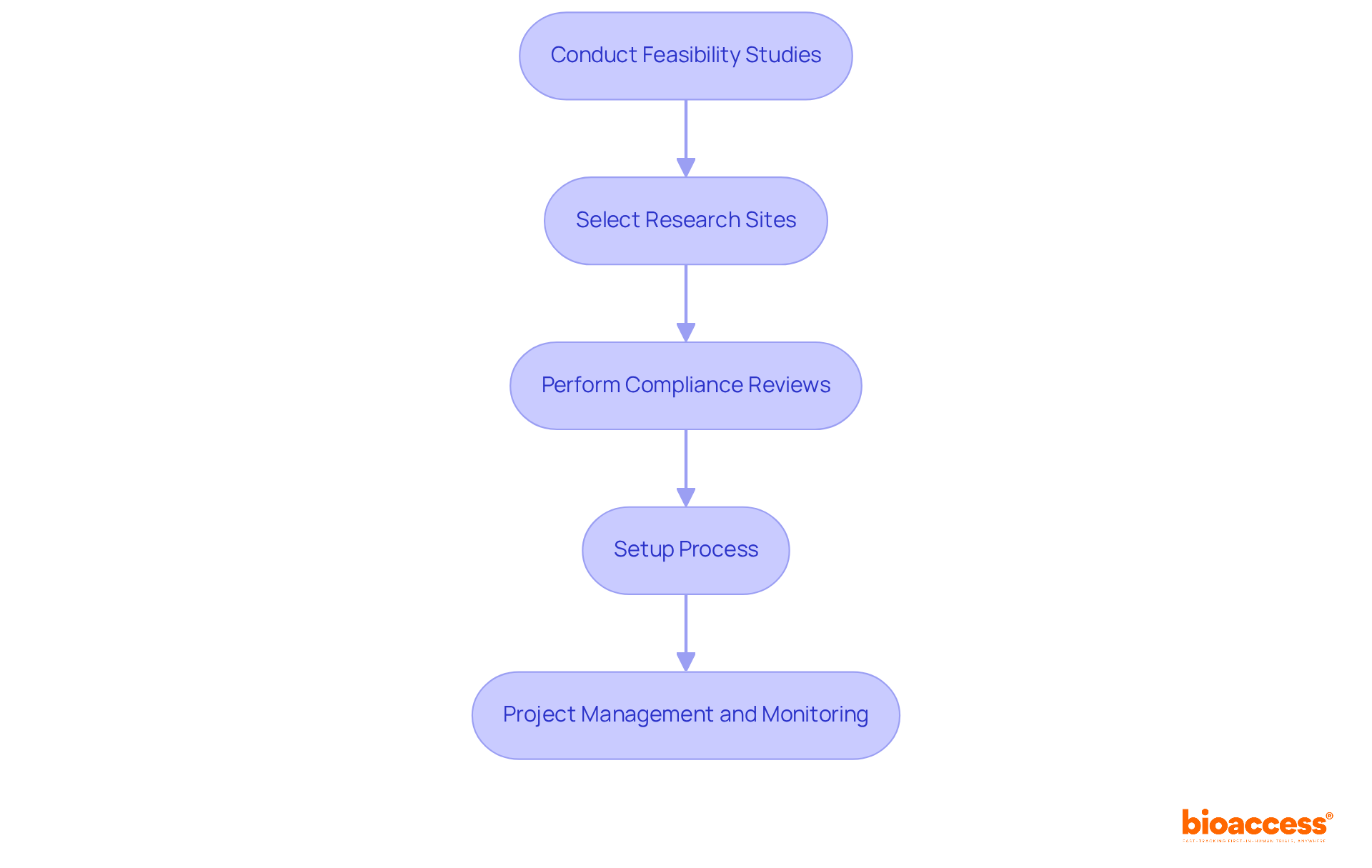
They ensure effective setup of the process, including obtaining ethics committee and health ministry approvals. By enabling import permits and the nationalization of investigational devices, the organization guarantees that studies are conducted smoothly and in compliance with regulatory requirements.
Their project management and monitoring services provide real-time insights into study status, inventory, and adverse events—elements that are crucial for improving patient outcomes and shortening study timelines. With the worldwide research market expected to expand considerably, reaching US$ 143.46 Billion by 2033, the company is ideally positioned to foster innovation and efficiency in this field. For Directors of Clinical Research, collaborating with Bioaccess means leveraging their expertise to navigate the complexities of research studies effectively, ultimately contributing to enhanced global health outcomes.
bioaccess is a leader in accelerating clinical studies, enabling treatment-naive cardiology or neurology groups to enroll 50% faster than traditional Western locations. This remarkable efficiency translates into significant cost savings of $25K per individual, thanks to their FDA-ready data that eliminates rework and delays. Their comprehensive service capabilities encompass:
By leveraging these strengths, the company effectively addresses the common challenges faced by Medtech and Biopharma startups in patient recruitment and study execution.
As the demand for innovative solutions in medical research continues to rise, bioaccess emerges as a premier choice for sponsors aiming to expedite their development programs. Their commitment to driving global health improvement through international collaboration and innovation positions them at the forefront of the industry. By choosing bioaccess, sponsors can navigate the complexities of clinical research with confidence, ensuring a streamlined approach to their projects.
LabCorp distinguishes itself as a leading provider of laboratory services crucial for biopharmaceutical research. Their vast network of laboratories ensures that studies are supported by high-quality data and reliable testing services. With offerings that include:
LabCorp plays a pivotal role in the success of research studies. Recent trends indicate a growing focus on bioanalytical testing, which enhances study outcomes by ensuring accurate biomarker analysis and compliance with evolving regulatory standards. LabCorp's commitment to data integrity is underscored by executive perspectives that emphasize the necessity of robust validation procedures to maintain high-quality standards in research. By partnering with LabCorp, sponsors can leverage streamlined processes and enhanced data integrity, ultimately achieving quicker and more effective study results.

The organization distinguishes itself through its extensive expertise in research management services, which includes:
A pivotal aspect of their service involves reviewing and providing feedback on study documents to ensure compliance with country requirements. With a global presence, bioaccess offers a comprehensive range of services designed to ensure that research studies adhere to regulatory standards while effectively engaging patients. Their innovative approaches significantly enhance enrollment rates and promote diversity within research studies.
Research indicates that nearly 90% of potential applicants for research studies never achieve enrollment, underscoring the importance of proactive strategies in addressing this challenge. The research study sector invests over $44 billion annually, highlighting the financial implications of recruitment inefficiencies. By prioritizing adherence and participant engagement, the organization positions itself as a trusted partner for biopharmaceutical firms navigating the complexities of clinical research.
The organization’s commitment to leveraging advanced analytics and tailored communication strategies further demonstrates its dedication to improving recruitment outcomes, ensuring that studies not only comply with regulatory requirements but also resonate with diverse populations. Moreover, the organization acknowledges the obstacles faced by patients, particularly those from low-income areas who often encounter travel challenges, and actively seeks to address these disparities.

Bioaccess is a leading entity in clinical development, recognized for its extensive clinical study management services that simplify the research process. Their offerings encompass:
This comprehensive range of services enhances efficiency in testing and accelerates timelines, making bioaccess a formidable partner in the Medtech landscape.
The partnership with Caribbean Health Group aims to establish Barranquilla as a prominent hub for medical studies in Latin America, with the backing of Colombia's Minister of Health. This collaboration has already yielded promising results, including a notable reduction in recruitment time and high retention rates. Such achievements ensure that sponsors receive high-quality data and insights, reinforcing the value of bioaccess's expertise in clinical research.
By leveraging global collaborations and innovative solutions, bioaccess continues to be a preferred ally for numerous entities within the biopharmaceutical sector. Their authoritative presence in the field not only addresses key challenges in clinical research but also fosters a collaborative environment that is essential for advancing medical studies.
The organization is steadfast in its commitment to delivering patient-focused solutions that significantly enhance the clinical research experience. By prioritizing participant involvement and support, bioaccess ensures that studies are designed with the individual’s needs in mind. Their innovative strategies enable the enrollment of treatment-naive cardiology or neurology cohorts at a pace 50% faster than Western sites, yielding a remarkable $25K savings per patient with FDA-ready data—no rework, no delays. This unwavering dedication to patient-centricity not only increases enrollment rates but also elevates the overall quality of data collected during studies. Research indicates that studies incorporating patient-focused designs can witness retention rates rise by as much as 30%. Thus, bioaccess emerges as an essential ally for biopharmaceutical firms, aligning research processes with the evolving landscape of patient engagement in medical studies.
Moreover, the organization offers a comprehensive suite of research management services, including:
This holistic approach effectively addresses recruitment challenges and ensures successful outcomes. By partnering with bioaccess, research directors can streamline their processes and enhance the overall effectiveness of their studies.
Fortrea stands out as a leader in providing tailored services for early-stage research, specifically addressing the unique challenges of first-in-human studies. Their advanced facilities and expert teams are committed to conducting trials with both efficiency and precision. By leveraging innovative methodologies and a steadfast commitment to quality, Fortrea adeptly navigates the complexities inherent in early-phase research. This positions them as a reliable collaborator for biopharmaceutical firms aiming to transition their products from concept to testing, ensuring a seamless pathway to market.
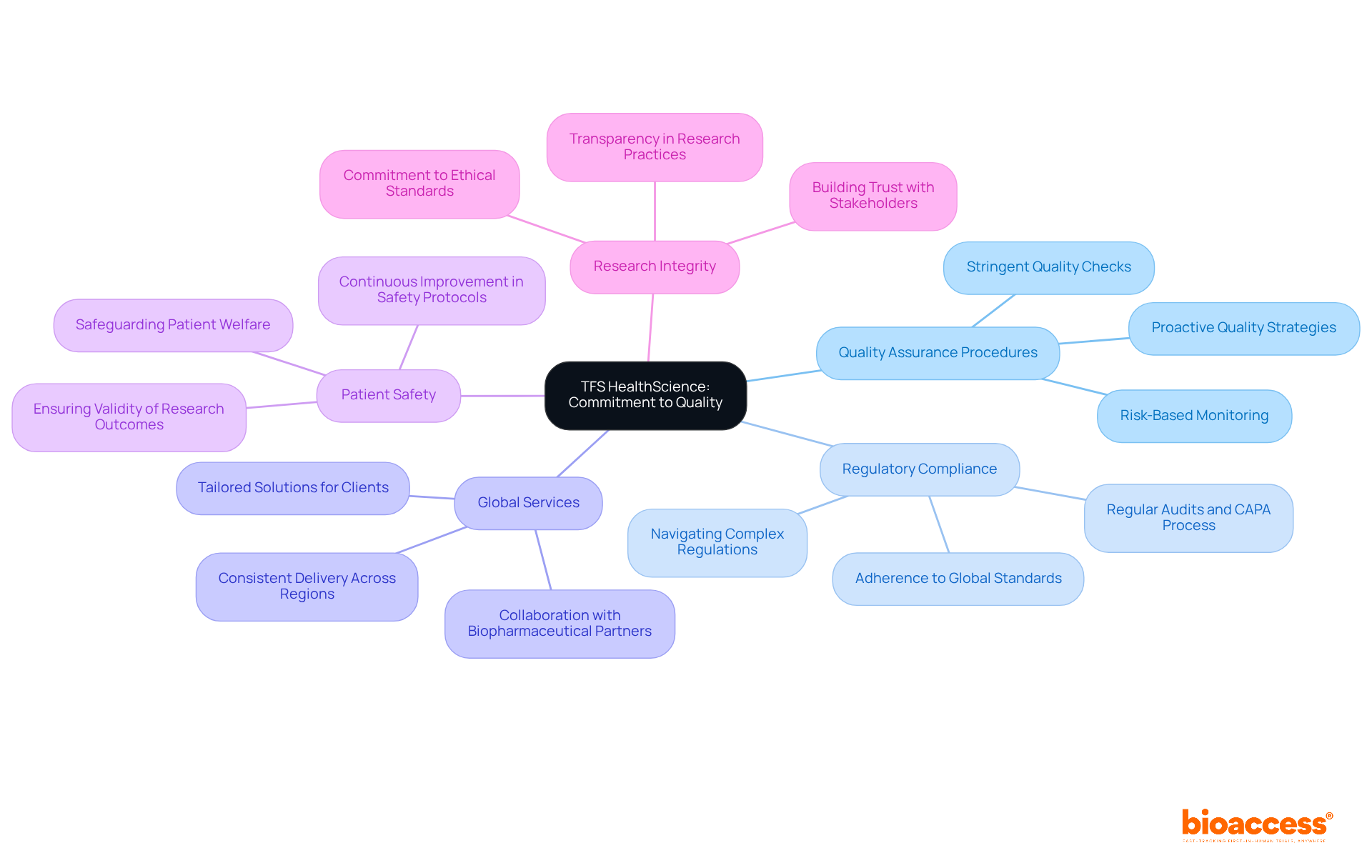
TFS HealthScience exemplifies a steadfast commitment to upholding the highest standards of quality in medical research. Their thorough strategy includes stringent quality assurance procedures and a strong commitment to regulatory compliance, which is vital for the integrity of research studies.
With a global footprint, TFS ensures consistent delivery of high-quality services across diverse regions, fostering reliability and trust among biopharmaceutical partners. This commitment to quality not only improves the success of research trials but also aligns with current trends highlighting the significance of compliance in the changing regulatory environment.
As TFS leaders assert, maintaining high standards is essential for safeguarding patient safety and ensuring the validity of research outcomes. By prioritizing these principles, TFS HealthScience establishes itself as one of the best cros to work for while assisting companies in navigating the complexities of clinical research.
The company stands as a prominent worldwide supplier of integrated services that facilitate the entire drug development process, particularly in medtech, biopharma, and radiopharma. Their innovative approach enables patient enrollment in treatment-naive cardiology or neurology cohorts to occur 50% faster than traditional Western sites, yielding significant cost savings of $25K per patient with FDA-ready data—effectively eliminating rework and delays.
The extensive range of services provided includes:
This commitment to accelerating clinical trials and enhancing global health through international collaboration establishes bioaccess as a valuable partner for biopharmaceutical companies navigating the complexities of drug development.
In a landscape where efficiency and reliability are paramount, bioaccess's expertise not only addresses key challenges but also sets a new standard for clinical research. Their ability to streamline processes and deliver actionable insights positions them as an indispensable ally in the quest for innovative medical solutions. The importance of collaboration in this endeavor cannot be overstated; as the industry evolves, partnerships that foster knowledge sharing and resource optimization will be crucial for success.

The landscape of clinical research is rapidly evolving, with the organizations highlighted in this article exemplifying best practices and innovations in the field. Each of these top Contract Research Organizations (CROs) demonstrates a commitment to accelerating medical advancements through efficient processes, regulatory expertise, and a patient-centric approach. From bioaccess's impressive enrollment rates and regulatory efficiencies in Colombia to LabCorp's unwavering focus on data integrity, these CROs are paving the way for more effective and timely clinical trials.
Key insights from the article reveal the diverse strengths of these leading CROs. Companies like IQVIA and ICON plc offer comprehensive data analytics and project management capabilities, while Parexel and TFS HealthScience emphasize regulatory compliance and quality assurance. Furthermore, the innovative methodologies employed by Fortrea and WuXi AppTec highlight the importance of tailored services in early-phase trials and the integration of comprehensive drug development solutions. Together, these organizations not only enhance operational efficiencies but also contribute significantly to the advancement of global health outcomes.
As the clinical research sector continues to expand, the importance of selecting the right CRO cannot be overstated. For biopharmaceutical firms and Medtech innovators, partnering with these top-tier organizations can lead to faster and more reliable study outcomes. Emphasizing collaboration, regulatory knowledge, and patient engagement will be crucial in navigating the complexities of clinical trials. By fostering these partnerships, the industry can ensure that groundbreaking medical solutions reach those in need, ultimately improving health and well-being on a global scale.
What distinguishes bioaccess® in the CRO landscape?
bioaccess® stands out by leveraging Latin America's regulatory efficiency, particularly in Colombia, where ethical approvals can be secured in 4-6 weeks, and total IRB/EC and MoH (INVIMA) reviews take only 90-120 days.
How does bioaccess® improve enrollment rates for clinical trials?
bioaccess® achieves enrollment rates that are 50% faster than traditional markets, allowing Medtech innovators to accelerate their product development.
What advantages does Colombia offer for clinical research?
Colombia's healthcare system is ranked among the top five globally, has a population of over 50 million, and provides universal healthcare coverage for 95% of its citizens, creating a robust environment for patient recruitment.
Are there financial incentives for conducting research in Colombia?
Yes, Colombia offers R&D tax incentives, including substantial tax deductions and grants, which enhance the appeal of conducting research in the region.
What experience does bioaccess® have in the clinical research field?
bioaccess® has over 20 years of experience and a comprehensive understanding of the regulatory landscape, making it a vital ally for Medtech firms.
How does bioaccess® contribute to the modernization of regulatory procedures?
The modernization of regulatory procedures in Latin America, particularly in Colombia, improves the pace and reliability of studies, making the region more appealing for research.
What services does IQVIA provide in clinical research?
IQVIA offers a comprehensive suite of services, including conducting feasibility studies, selecting research sites and principal investigators, and performing compliance reviews of study documents.
How does IQVIA ensure compliance during studies?
IQVIA ensures compliance by obtaining ethics committee and health ministry approvals, enabling import permits, and nationalizing investigational devices.
What is the projected growth of the global research market?
The worldwide research market is expected to expand significantly, reaching US$ 143.46 billion by 2033.
What benefits does bioaccess offer for Medtech and Biopharma startups?
bioaccess helps address common challenges in patient recruitment and study execution, enabling treatment-naive cardiology or neurology groups to enroll 50% faster and save significant costs.
How does bioaccess® support sponsors in clinical research?
By choosing bioaccess®, sponsors can navigate the complexities of clinical research with confidence, ensuring a streamlined approach to their projects and contributing to global health improvement.