


The article titled "10 Clinical Research Organizations Transforming Medtech Innovation" identifies key organizations that are significantly impacting the field of medical technology through their clinical research efforts. It highlights various clinical research organizations (CROs) that enhance the efficiency and effectiveness of clinical trials. By leveraging advanced technologies, regulatory expertise, and diverse patient populations, these CROs accelerate medical innovations and improve patient outcomes.
The landscape of medical technology is undergoing a rapid evolution, propelled by the pressing need for innovation in clinical research. As the demand for effective and efficient trials intensifies, a select group of clinical research organizations (CROs) is stepping up to transform the Medtech sector. This article explores ten pioneering CROs that are not only enhancing research capabilities but also adeptly navigating the complexities of regulatory landscapes and patient recruitment.
What challenges do these organizations encounter in maintaining integrity and accelerating timelines? Furthermore, how are they reshaping the future of medical research?
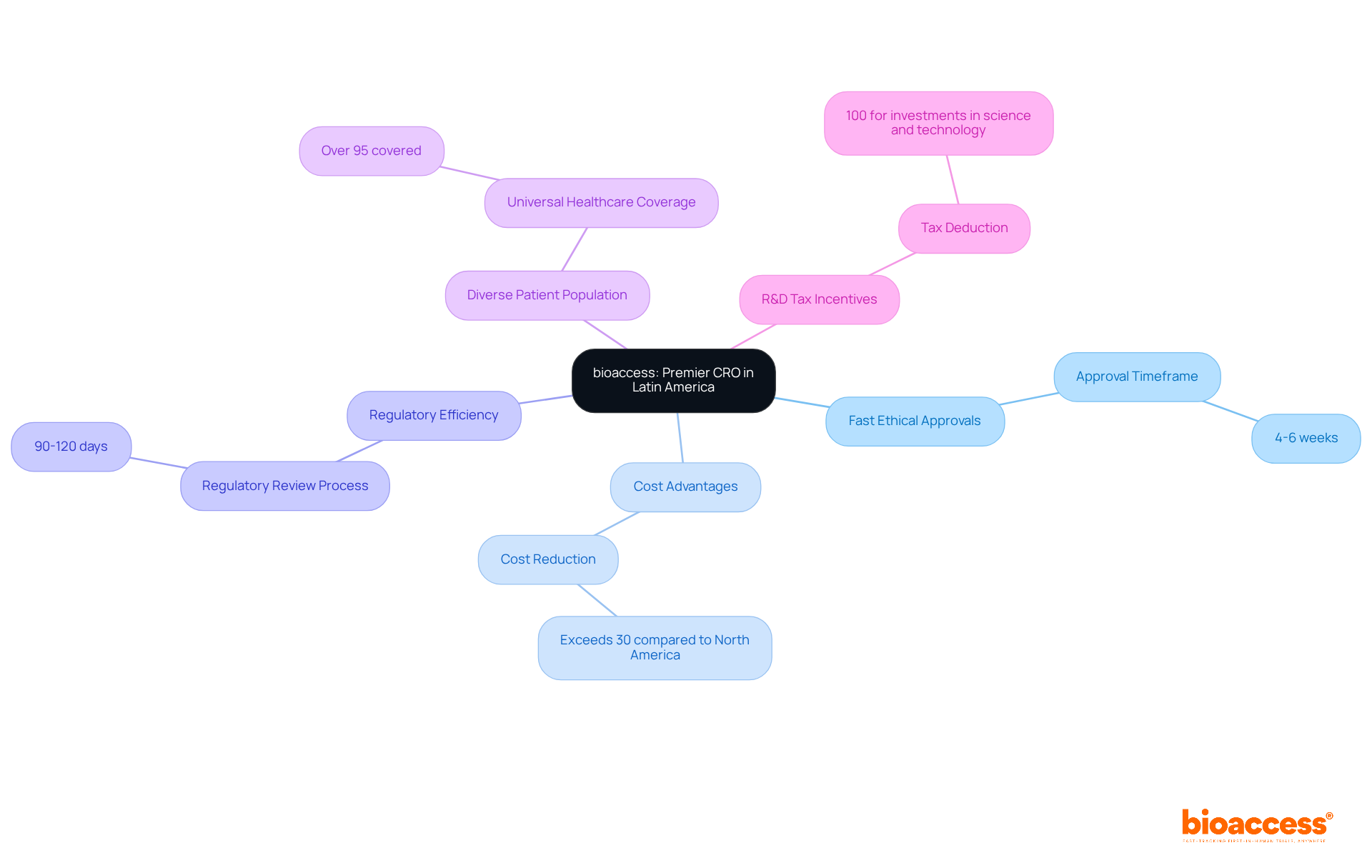
bioaccess® distinguishes itself as a premier contract research organization (CRO) in Latin America, recognized on the list of clinical research organizations for its capability to secure ethical approvals within a remarkable timeframe of just 4-6 weeks. By leveraging Colombia's competitive advantages—including cost reductions exceeding 30% compared to North America and Western Europe, a regulatory review process that spans only 90-120 days, and a high-quality healthcare system ranked among the best globally—bioaccess® utilizes a list of clinical research organizations to accelerate clinical trials.
The diverse patient population, with over 95% covered by universal healthcare, facilitates efficient patient recruitment, thereby enhancing the speed of research studies. Furthermore, Colombia offers significant R&D tax incentives, featuring a 100% tax deduction for investments in science and technology, which further strengthens medical research initiatives.
Their focus on early-phase studies and customized solutions positions them as an indispensable partner on the list of clinical research organizations for Medtech, Biopharma, and Radiopharma innovators aiming to navigate the complexities of research effectively. Successful partnerships, such as the collaboration with Welwaze Medical Inc. for the Celbrea® launch, exemplify bioaccess's proficiency in facilitating market access and ensuring regulatory compliance in Colombia.
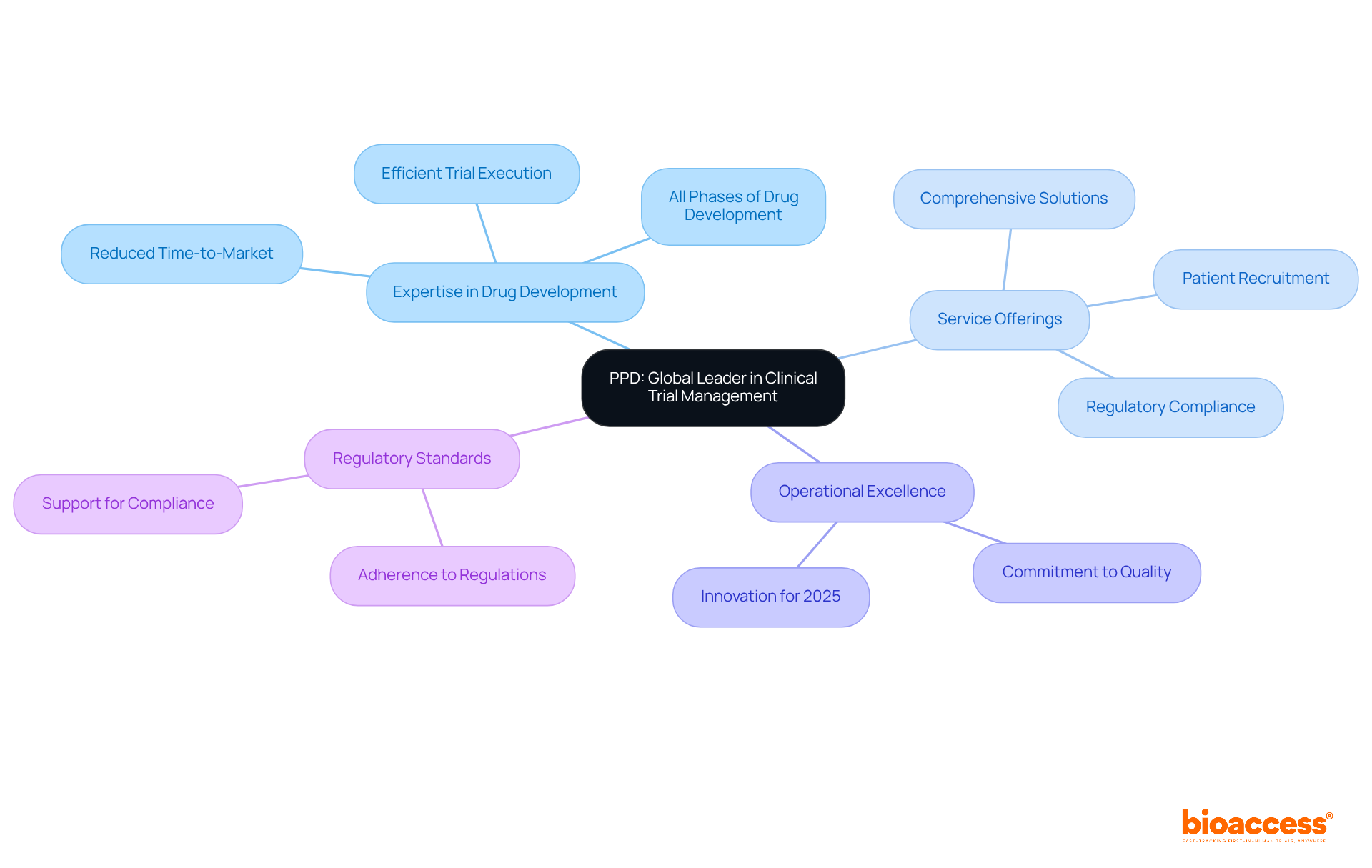
PPD, a division of Thermo Fisher Scientific, stands as a global leader in study management services, offering a comprehensive array of solutions tailored for pharmaceutical and biotechnology firms. Their profound expertise encompasses all phases of drug development, assuring sponsors of PPD's capacity for efficient and compliant trial execution. This reliability is further highlighted by an impressive track record, significantly reducing time-to-market for new therapies.
As we move into 2025, PPD continues to innovate, enhancing service offerings to address the evolving demands of the industry. Their unwavering commitment to operational excellence and strict adherence to regulatory standards positions them as a crucial partner in advancing medical innovations, ultimately facilitating faster access to life-saving treatments.
The Association of Clinical Study Professionals (ACRP) stands at the forefront of promoting medical investigations through education and certification. By providing essential resources and training programs, ACRP enhances the skills and knowledge of research professionals, thereby ensuring high standards of practice. Their certification programs serve as a testament to individual proficiency, fostering a culture of excellence and adherence within medical studies.
Meanwhile, organizations such as bioaccess are leading the charge by offering comprehensive research study management services. These include:
This vital synergy between education and practical application is essential for driving global health improvement and fostering international collaboration in Medtech innovation.
Clinical Trials Arena serves as a premier platform that delivers critical insights and trends within the medical investigation landscape. By meticulously examining data and reporting on significant advancements, it equips stakeholders with the knowledge required to navigate the complexities of research studies effectively. The platform underscores emerging innovations, regulatory changes, and shifting market dynamics, establishing itself as an indispensable resource for professionals striving to make informed decisions in an increasingly complex environment. As the medical research field progresses, staying abreast of these insights is essential for enhancing study outcomes and elevating the overall efficacy of Medtech innovations.
A Contract Research Organization (CRO) serves as a crucial ally for the pharmaceutical, biotechnology, and medical device sectors by focusing on the oversight of clinical studies, which often involves referring to a list of clinical research organizations and associated services. By providing expertise in study design, patient recruitment, data management, and regulatory compliance, CROs significantly streamline the drug development process. This collaboration empowers sponsors to concentrate on their core competencies while ensuring efficient trial execution.
In 2025, the global CRO market is projected to reach USD 139.42 billion, reflecting an increasing reliance on these organizations to enhance research portfolios and optimize clinical study designs. Contract research organizations not only expedite drug development but also offer scalability, enabling companies to adjust resources according to project requirements without the challenges associated with maintaining extensive in-house capabilities.
Current trends indicate that a list of clinical research organizations are increasingly adopting advanced technologies such as artificial intelligence (AI) and machine learning (ML) to improve data analysis and operational efficiency. This tech-driven approach enhances decision-making and patient stratification, ultimately optimizing clinical study designs and outcomes.
A list of clinical research organizations highlights substantial contributions to drug development, exemplified by Catalyst Clinical Research, which recently received the Excellence in Clinical Study Management award for its strategic recommendations that led to successful study outcomes. Such accomplishments underscore the pivotal role CROs play in advancing life sciences studies and improving patient outcomes, rendering them essential in the evolving landscape of medical innovation.
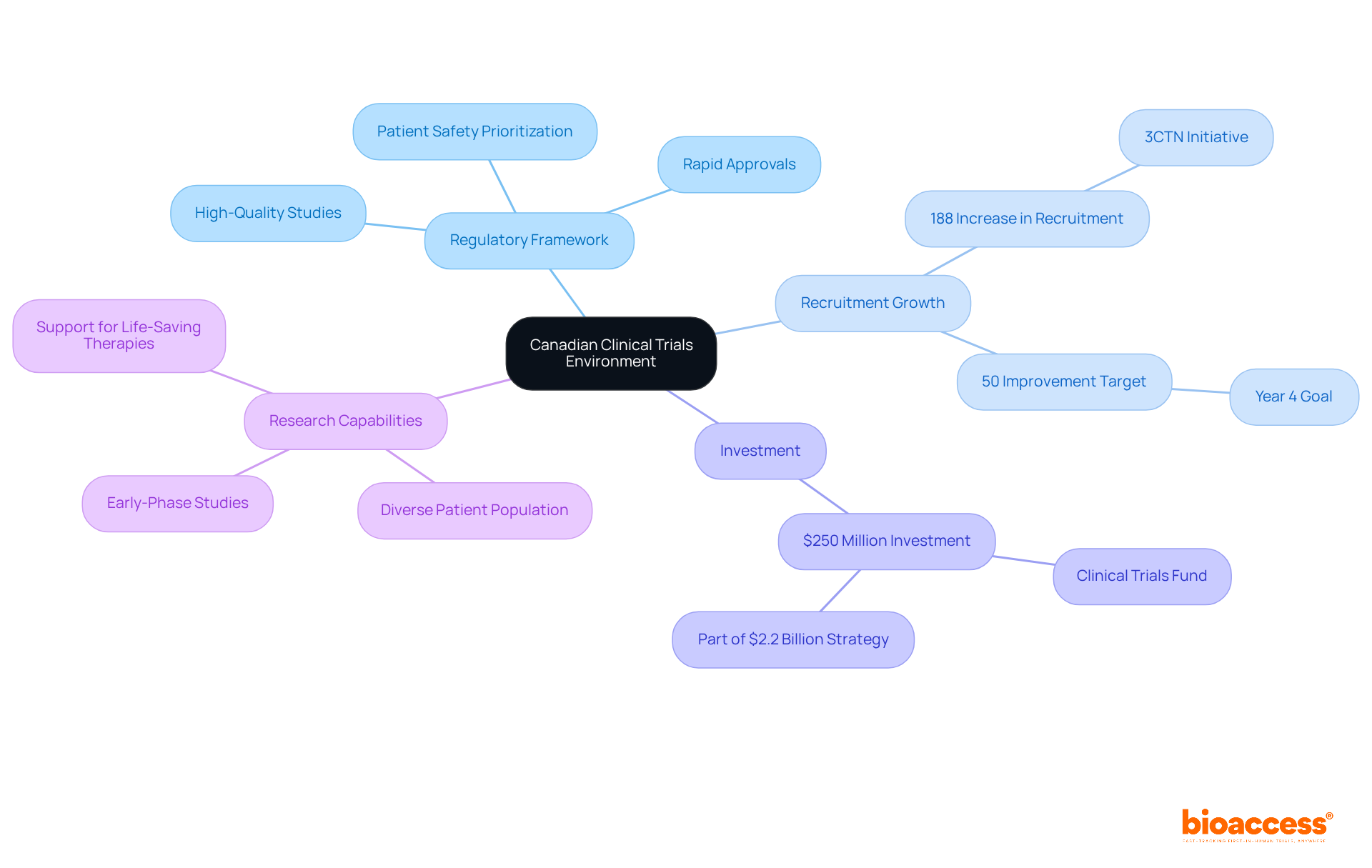
Canada's trial environment stands out due to a robust regulatory framework that prioritizes high-quality studies and patient safety. Notably, the country has experienced a remarkable increase in recruitment, with Ontario achieving a 188% rise above baseline during the 3CTN initiative. This commitment is further solidified by a $250 million investment over three years via the Clinical Trials Fund, aimed at enhancing the infrastructure for medical studies.
With a diverse patient population, Canada is exceptionally positioned to conduct early-phase studies, attracting a significant share of global medical activity. The regulatory environment is designed to facilitate rapid approvals, ensuring that innovative therapies transition smoothly from concept to practical application.
Successful early-phase studies in Canada have showcased the effectiveness of this framework, highlighting the country's capability to support the development of life-saving vaccines and therapeutics. As Canada continues to bolster its research capabilities, it remains a pivotal location for Medtech, Biopharma, and Radiopharma innovators seeking to advance their studies.
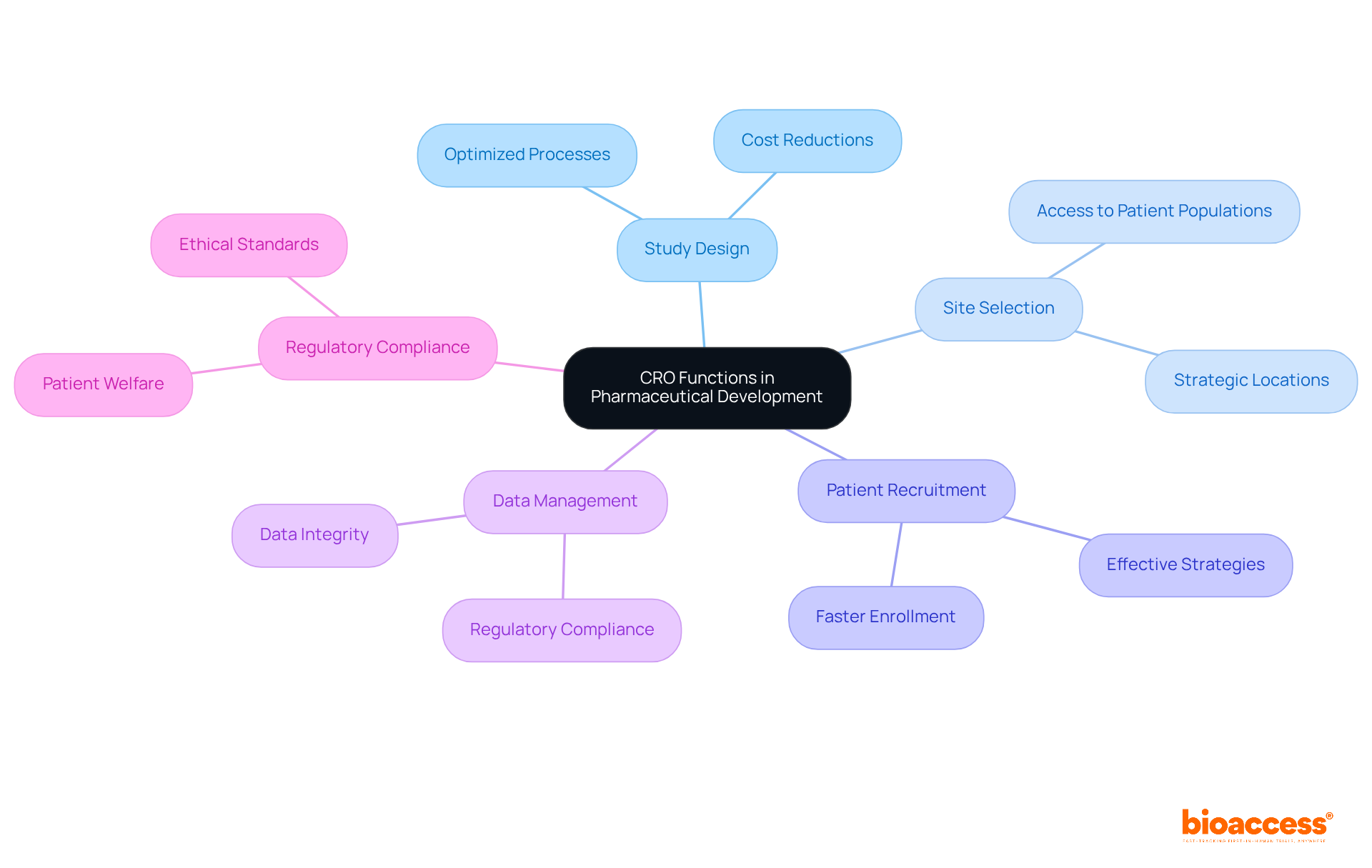
Contract research organizations play a pivotal role in pharmaceutical development by executing essential functions such as:
Their specialized knowledge allows sponsors to optimize the clinical study process, significantly lowering expenses and accelerating the time to market for new therapies. In fact, collaborating with contract research organizations can result in average cost reductions of up to 30% for sponsors, particularly when performing studies in nations like Colombia, where the savings can surpass this benchmark due to favorable economic conditions.
Moreover, contract research organizations are crucial in ensuring that studies comply with ethical standards, thus protecting patient welfare and data integrity. Effective patient recruitment strategies utilized by contract research organizations have demonstrated the capability to enroll participants 50% quicker than conventional methods, especially in regions with robust healthcare systems such as Colombia, which boasts a population exceeding 50 million and provides universal healthcare coverage for 95% of its residents.
As industry leaders affirm, 'Contract Research Organizations are essential in guaranteeing that studies are carried out effectively and ethically.' As the landscape of medical research evolves, the contributions of contract research organizations remain vital in fostering innovation and enhancing patient outcomes.
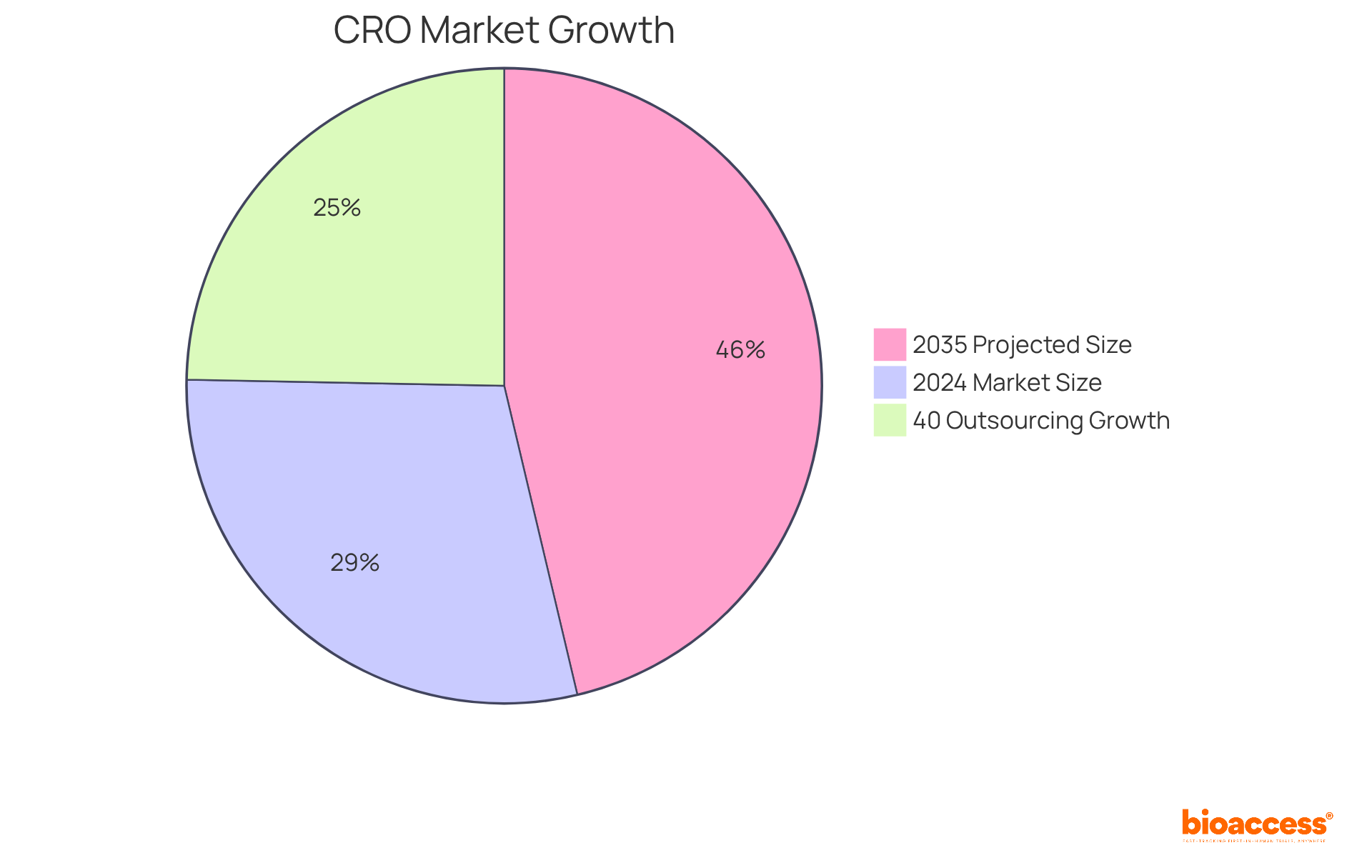
The CRO industry is experiencing significant growth, primarily driven by the surging demand for outsourced clinical trial services. Pharmaceutical and biotechnology companies are increasingly turning to contract research organizations to enhance operational efficiency and reduce costs. Notably, outsourcing activities have risen by 40% over the past five years.
Market forecasts suggest that the global CRO market will reach approximately USD 46.99 billion by 2024 and is projected to grow to USD 75.0 billion by 2035, underscoring the vital role these organizations play in the drug development process. This trend is further bolstered by the growing complexity of drug development, which requires the specialized expertise and resources that contract research organizations provide.
As the sector evolves, the integration of cutting-edge technologies, such as artificial intelligence and machine learning, is transforming how clinical research organizations conduct studies, enhancing patient recruitment and data management capabilities.
The demand for outsourced research services is expected to continue its upward trajectory, driven by the need for innovative therapies and the rising prevalence of chronic illnesses, which emphasizes the significance of the list of clinical research organizations as indispensable partners in the healthcare sector.
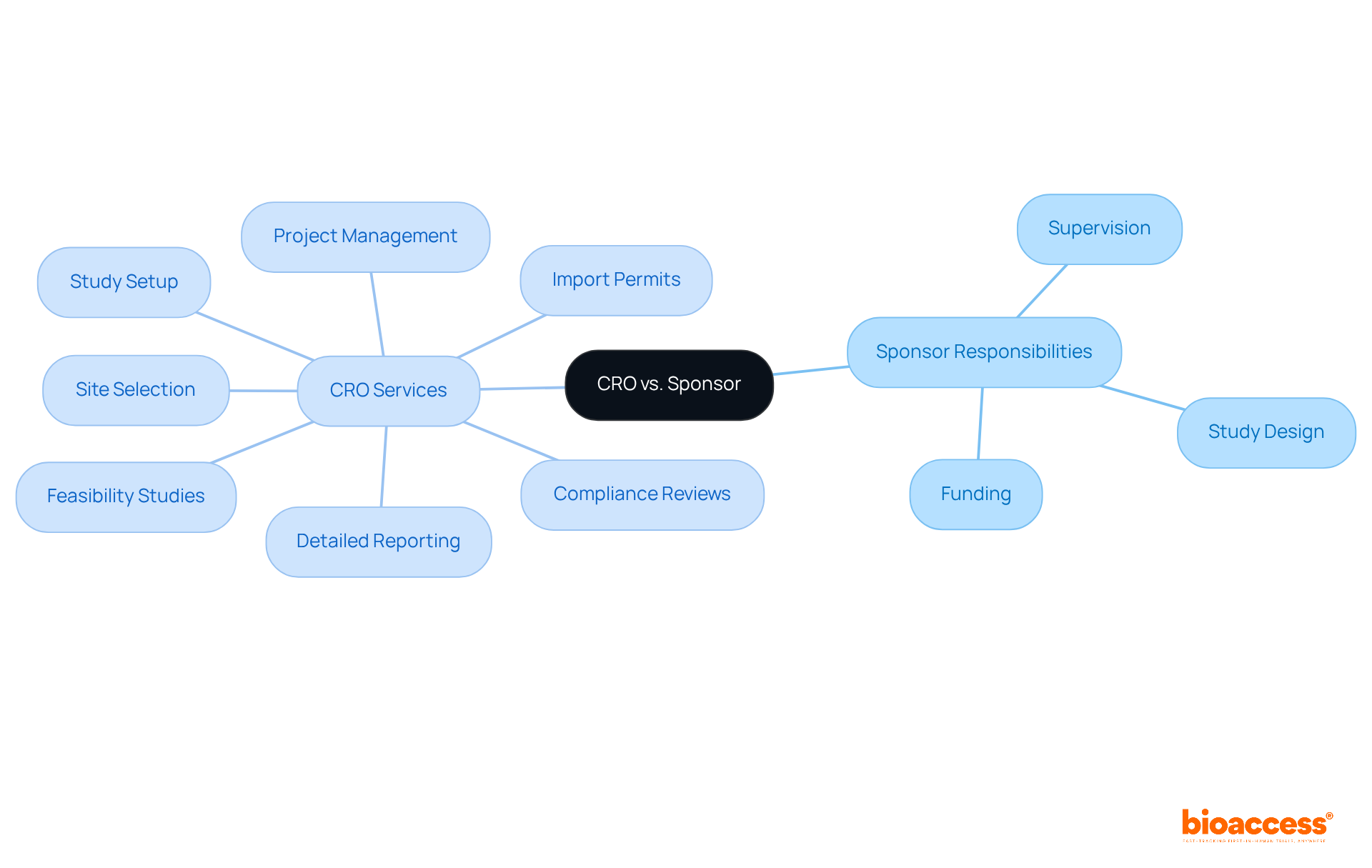
In research studies, the sponsor assumes primary responsibility for the overall study design, funding, and supervision, while the Contract Research Organization (CRO) delivers specialized services that facilitate study execution. This clear delineation of roles is essential for fostering effective collaboration. Sponsors depend on a list of clinical research organizations for their expertise in managing various study elements, enabling them to concentrate on strategic decision-making and resource allocation.
Notably, research studies conducted by contract research organizations, such as bioaccess®, are completed, on average, 30 days more swiftly than those overseen by sponsors. This statistic underscores the effectiveness of a robust partnership with a contract research organization. Bioaccess® excels in comprehensive clinical study management services, encompassing:
As the biotech industry grapples with talent shortages, 41% of companies have heightened their reliance on a list of clinical research organizations through Functional Service Provider (FSP) partnerships, signaling the growing acknowledgment of these organizations as vital collaborators in navigating the intricate R&D landscape. This collaborative approach not only enhances efficiency in studies but also ensures that sponsors can maintain oversight and accountability for data integrity, ultimately fostering innovation in the Medtech sector.
Ethical guidelines are paramount in clinical studies, shaping the execution of experiments to prioritize participant safety and uphold data integrity. Central to these principles are informed consent, risk-benefit analysis, and the role of independent review boards. A robust informed consent process empowers participants by ensuring they are fully aware of the study's purpose, methods, risks, and benefits, enabling them to make autonomous decisions about their involvement. This transparency is vital for fostering trust, as participants must feel assured that their rights and well-being are respected throughout the research process.
Furthermore, the evaluation of risks and benefits is crucial in determining the ethical viability of a study. Researchers are urged to design studies that minimize unnecessary risks while maximizing potential benefits, in alignment with established ethical standards. For instance, studies should incorporate methods that guarantee participant safety, such as conducting procedures in healthcare settings where prompt responses to adverse reactions can be efficiently managed.
The impact of these ethical considerations extends beyond individual studies; they enhance the overall credibility of medical investigations. By adhering to ethical standards, organizations can bolster public confidence in studies, ultimately leading to increased participation and more significant findings. Additionally, a list of clinical research organizations that provide comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential in upholding these ethical standards. These services ensure thorough oversight and adherence to regulatory requirements, aligning with the functions of INVIMA, the Colombian regulatory authority, which plays a critical role in overseeing medical devices and safeguarding participant welfare. As the landscape of clinical research evolves in 2025, the commitment to ethical principles remains a cornerstone for advancing medical innovation and ensuring participant welfare.

The landscape of clinical research organizations (CROs) is pivotal in driving innovation within the Medtech sector. By providing essential services that streamline the drug development process, CROs like bioaccess and PPD exemplify how specialized expertise can enhance research efficiency and accelerate the delivery of life-saving treatments. Their roles are not merely supportive; they are integral to ensuring that clinical trials are conducted ethically and effectively.
Throughout this exploration, key insights have emerged regarding the transformative impact of CROs. From bioaccess's rapid regulatory approvals in Latin America to PPD's comprehensive management services, these organizations are reshaping the clinical research environment. The emphasis on education and certification by ACRP further underscores the importance of skilled professionals in maintaining high standards within the industry. Additionally, the increasing reliance on advanced technologies signifies a shift towards more efficient and data-driven research methodologies.
As the clinical research field continues to evolve, it is essential for stakeholders to recognize the significance of CROs in fostering innovation and improving patient outcomes. Embracing partnerships with these organizations can lead to enhanced operational efficiencies and ultimately a faster route to market for groundbreaking therapies. The future of Medtech innovation hinges on a collaborative approach that values the expertise of CROs, ensuring that advancements in healthcare can be realized more swiftly and ethically.
What is bioaccess and what distinguishes it in clinical research?
bioaccess® is a premier contract research organization (CRO) in Latin America, recognized for its ability to secure ethical approvals in just 4-6 weeks. It leverages Colombia's advantages, such as significant cost reductions, a quick regulatory review process, and a high-quality healthcare system.
What are the advantages of conducting clinical research in Colombia?
Colombia offers cost reductions exceeding 30% compared to North America and Western Europe, a regulatory review process of 90-120 days, a diverse patient population with over 95% covered by universal healthcare, and significant R&D tax incentives, including a 100% tax deduction for investments in science and technology.
What types of studies does bioaccess focus on?
bioaccess focuses on early-phase studies and provides customized solutions for Medtech, Biopharma, and Radiopharma innovators.
Can you provide an example of a successful partnership involving bioaccess?
A successful partnership example is the collaboration with Welwaze Medical Inc. for the launch of Celbrea®, which highlights bioaccess's proficiency in facilitating market access and ensuring regulatory compliance in Colombia.
What services does PPD offer in the clinical trial management sector?
PPD offers a comprehensive array of study management services tailored for pharmaceutical and biotechnology firms, covering all phases of drug development and ensuring efficient and compliant trial execution.
How does PPD ensure a faster time-to-market for new therapies?
PPD has a proven track record of significantly reducing time-to-market for new therapies through its operational excellence and strict adherence to regulatory standards.
What is the role of ACRP in advancing clinical research?
The Association of Clinical Study Professionals (ACRP) promotes medical investigations through education and certification, enhancing the skills and knowledge of research professionals to ensure high standards of practice.
What types of services are provided by organizations like bioaccess in relation to clinical research?
Organizations like bioaccess provide services such as feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.