


The article titled "10 CRDs Transforming MedTech Development and Compliance" emphasizes the revolutionary impact of Custom Resource Definitions (CRDs) on the medical technology sector. By enhancing development processes and ensuring regulatory compliance, CRDs are proving to be indispensable. The article outlines various applications of CRDs, including:
These applications not only demonstrate the critical role of CRDs in accelerating innovation but also highlight their importance in maintaining high standards within the MedTech industry.
In the rapidly evolving landscape of medical technology, efficiently navigating regulatory challenges is crucial for a company's success. As the demand for innovative solutions rises, MedTech companies are increasingly leveraging advanced tools and frameworks designed to streamline processes and enhance compliance.
The adoption of Custom Resource Definitions (CRDs) and platforms like Kubernetes is revolutionizing clinical research by accelerating ethical approvals and automating workflows. This article explores the pivotal role these technologies play in facilitating faster development cycles, ensuring regulatory adherence, and ultimately improving patient outcomes within the MedTech sector.
As the industry progresses, understanding these innovations is essential for organizations striving to maintain a competitive edge.
bioaccess® leverages its extensive experience and regional advantages to secure ethical approvals in an impressive 4-6 weeks. This expedited process is crucial for medical technology firms striving to accelerate trials and hasten the market launch of their innovations. By expertly navigating the complex regulatory landscape, bioaccess® empowers innovators to focus on their primary objective: developing life-saving technologies without unnecessary delays.
In 2025, the average duration for ethical approvals in medical technology trials remains a significant concern, with many companies grappling with prolonged timelines. However, bioaccess® distinguishes itself by consistently delivering faster approvals, which is vital in a landscape where the top five medical specialties account for 55% of devices cleared.
Recent collaborations, such as with Welwaze Medical Inc. for the Celbrea® medical device launch and Caribbean Health Group to position Barranquilla as a leading destination for clinical trials, underscore bioaccess®'s commitment to enhancing clinical trial efficiency. These partnerships have demonstrated the ability to achieve over a 50% reduction in recruitment time while maintaining 95% retention rates.
As Ben Wolf, a partner in Alston & Bird's Health Care Group, observed, 'The FDA should not have to parse anything; they should have all the material they need in a way that they can access it.' This perspective aligns with the industry's evolving emphasis on the significance of swift ethical approvals, positioning bioaccess® as a leader in facilitating timely access to innovative medical solutions.

Crossplane emerges as a powerful solution for medical technology companies aiming to streamline their crds. By providing a unified control plane, it simplifies the deployment and management of resources across various cloud environments—an essential capability in the highly regulated MedTech sector. The automation of resource provisioning through Crossplane not only accelerates deployment but also ensures that infrastructure evolves in response to the dynamic demands of clinical research. This agility is critical for maintaining regulatory standards, enabling organizations to swiftly align their resources with the latest requirements.
Moreover, the implementation of Crossplane has been shown to significantly enhance operational efficiency, with data indicating that organizations utilizing crds for management experience a notable reduction in deployment times. Notably, several medical technology firms have effectively harnessed Crossplane to improve their resource management, demonstrating its practical efficacy.
As the landscape of medical research continues to evolve, Crossplane stands out as an indispensable tool for healthcare technology innovators striving to navigate the complexities of regulatory compliance while expediting their development processes.

Kubernetes CRDs empower medical technology firms to customize their Kubernetes settings by defining specific resource types tailored to unique research needs. This adaptability enables organizations to effectively manage patient data and seamlessly integrate with a variety of medical devices. By leveraging CRDs, innovators in healthcare technology can develop more resilient and scalable solutions that significantly enhance research capabilities and improve patient outcomes.
The increasing adoption of CRDs within the medical technology sector underscores a growing recognition of their value in streamlining healthcare processes and ensuring compliance. Notably, these definitions facilitate compliance assessments, project oversight, and reporting—critical components of comprehensive trial management services. Such services not only amplify the impact of MedTech research on local economies but also contribute to job creation, economic growth, and advancements in healthcare.
Experts assert that enhancing Kubernetes capabilities through CRDs is vital for addressing the unique challenges faced in research, ultimately driving progress in medical technology.

The GitHub Actions Runner Controller is essential in automating workflows within healthcare research. By enabling the creation of self-hosted runners, it empowers organizations to optimize their CI/CD processes, ensuring efficient testing and deployment of code changes. This level of automation accelerates project timelines and minimizes human error, allowing research teams to concentrate on critical tasks such as data analysis and patient engagement.
Studies indicate that automation in medical research workflows can yield significant efficiency improvements, with some organizations reporting up to a 50% reduction in time spent on manual processes. Furthermore, bioaccess's comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—enhance these efficiencies.
The collaboration between bioaccess and the Caribbean Health Group aims to position Barranquilla as a leading site for research trials in Latin America, supported by Colombia's Minister of Health. This initiative not only encourages international collaboration but also drives local economic growth through job creation and healthcare enhancement.
Additionally, insights from the case study titled 'Interventions and Their Effectiveness' underscore the significance of targeted interventions in fostering shared decision-making and improving outcomes such as quality of life and patient activation.

Custom Resource Definitions, or CRDs, are revolutionizing the MedTech sector with their versatile applications. A notable application is the development of CRDs tailored for managing trial data. This strategy significantly enhances the tracking of patient outcomes and ensures compliance with regulatory standards, ultimately streamlining the trial process. Moreover, CRDs can be crafted for device management, facilitating the seamless integration of new medical devices into existing healthcare systems. The impact of CRDs on trial data management is profound. By enabling effective data collection and analysis, these resources bolster patient safety and the overall success of medical trials. Insights from experts indicate that efficient data management not only streamlines processes but also improves the capacity to swiftly identify adverse events, which is vital for safeguarding patient safety. As medical technology evolves, the application of clinical research devices will be essential in addressing the specific challenges faced by innovators in the sector, thereby fostering advancements in healthcare technology and enhancing patient outcomes.
Bioaccess offers comprehensive clinical trial management services, including:
These services are crucial for the successful execution of clinical trials and contribute to local economic growth through job creation and healthcare improvement.

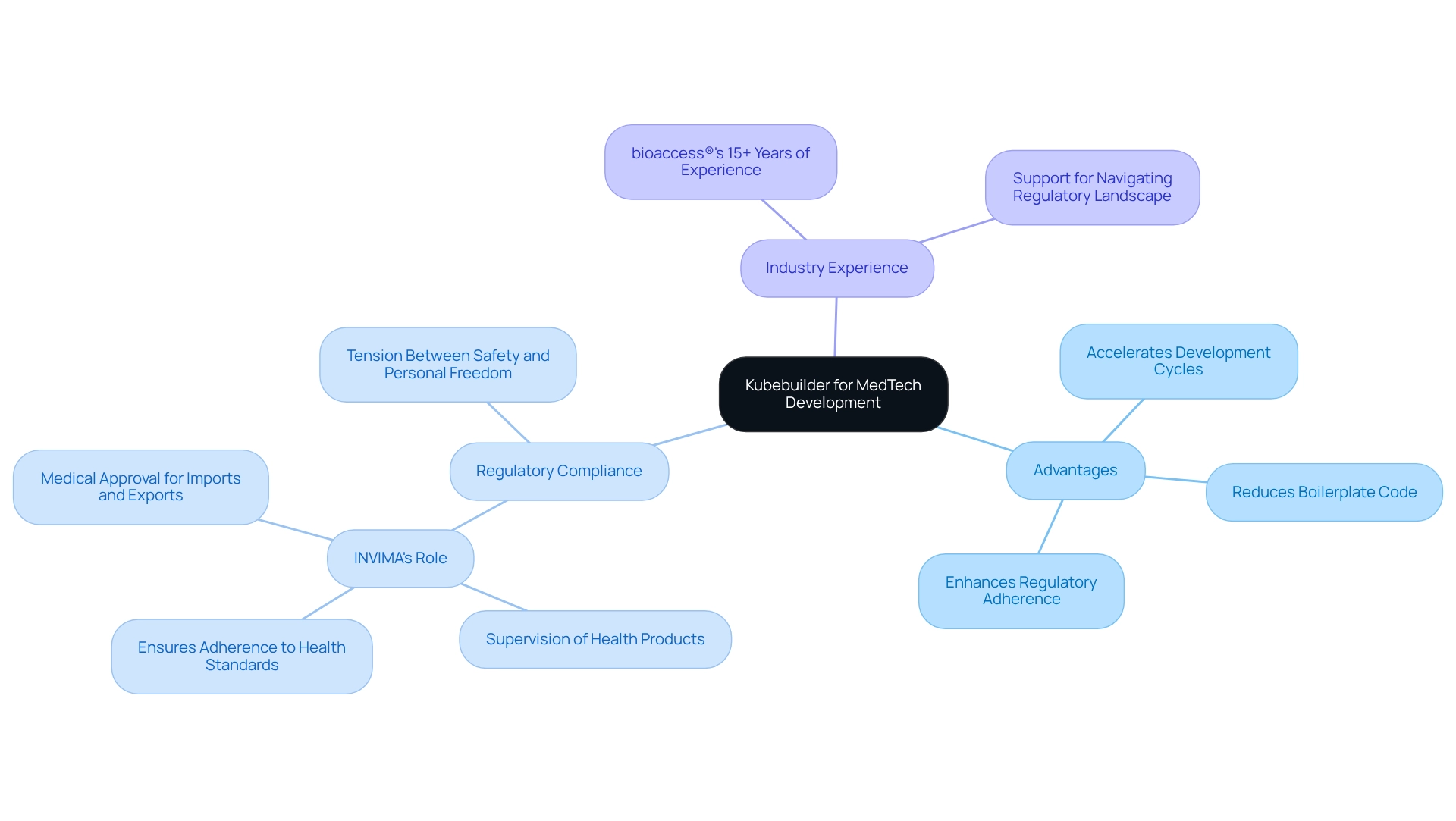
Kubebuilder serves as a powerful framework that streamlines the creation of Kubernetes APIs through CRDs, providing significant advantages for MedTech companies. By leveraging Kubebuilder, developers can accelerate their development cycles, enabling them to craft customized solutions that align with specific regulatory requirements, such as those set forth by INVIMA, the Colombia National Food and Drug Surveillance Institute.
INVIMA plays a crucial role in supervising the marketing and production of health products, ensuring adherence to health standards and granting medical approval for imports and exports. This focus on efficiency allows teams to prioritize writing business logic over repetitive boilerplate code, resulting in a notable reduction in time to market for innovative medical technologies.
Organizations employing Kubebuilder have reported development speed enhancements of up to 50%, highlighting its effectiveness in improving regulatory adherence and accelerating the launch of new products to the market. As James Q. Wilson aptly noted, addressing safety in regulations is often more straightforward than tackling health hazards, making frameworks like Kubebuilder essential for navigating the complexities of medical technology compliance.
Moreover, the persistent conflict between safety and individual liberty in regulatory frameworks emphasizes the significance of employing tools that can assist technology firms in balancing these opposing interests effectively. With over 15 years of experience in the industry, bioaccess® understands these challenges and supports innovators in navigating the regulatory landscape, ensuring adherence to INVIMA's standards and fostering innovation in Latin America.
Ongoing research in CRDs is paving the way for innovative applications in MedTech, underscoring their relevance in clinical research. Recent studies focus on enhancing the interoperability of CRDs with existing healthcare systems, which improves data management and ensures compliance with evolving regulatory standards. In this context, extensive trial management services—including:
play a crucial role. By staying informed about these advancements, medical technology firms can leverage new discoveries to enhance their product offerings and optimize clinical research processes. This not only aids in job creation and economic development but also contributes significantly to healthcare enhancement.

Key markers in Clinical Research Development (CRD) for medical technology regulations encompass strict adherence to regulatory standards, meticulous documentation, and comprehensive testing protocols, which are essential for CRDs. For MedTech firms, adherence to these markers is essential to reduce expensive delays and legal issues. By applying best practices in CRDs development, organizations not only maintain high quality and safety standards but also improve patient outcomes and build trust among stakeholders. The influence of regulatory standards on CRDs development timelines cannot be underestimated; organizations that emphasize adherence to CRDs often experience smoother approval processes. For instance, bioaccess® leverages over 15 years of clinical research knowledge to effectively manage these challenges, obtaining ethical approvals in just 4–6 weeks.
Additionally, the collaboration between Satio and Nanowear showcases innovative strategies for regulation in home-based diagnostics. By combining AI-driven biomarker diagnostics with home blood collection technologies, they are establishing new standards for regulatory adherence while enhancing patient care.
As the landscape of medical technology evolves, remaining aware of regulatory matters is essential. Statistics indicate that non-compliance can lead to significant setbacks, underscoring the need for robust documentation and testing practices for CRDs. By embracing these optimal methods, medical technology firms can ensure adherence to regulations and promote progress in healthcare.

Creating and removing CRDs is essential for effective resource management in MedTech innovation. This process begins with defining the schema for the CRDs, followed by implementing controllers that oversee the lifecycle of the CRDs. Ensuring compliance with regulatory standards throughout these changes is crucial, particularly with authorities such as INVIMA (Colombia National Food and Drug Surveillance Institute), which oversees the marketing and manufacturing of health products. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its competence in guaranteeing the safety, efficacy, and quality of medical devices.
Moreover, organizations must create a clear strategy for decommissioning CRDs that are no longer necessary, which aids in preserving a streamlined and efficient Kubernetes environment. This proactive approach not only enhances operational efficiency but also aligns with best practices in resource management. Furthermore, the effect of medical technology clinical studies on local economies, including job creation and healthcare enhancement, underscores the significance of efficient resource management strategies.
By utilizing efficient lifecycle management of CRDs, technology firms in the medical sector can enhance their operations, ultimately resulting in quicker development and adherence in a competitive environment.

Frequently asked questions about crds in MedTech often center on their implementation, management, and compliance. Key inquiries include:
Addressing these inquiries is essential for creators seeking to optimize the advantages of Customer Reference Documents in their development processes.
Optimal approaches for producing Customer Reference Documents include ensuring clarity and precision in definitions, which can significantly enhance their applicability across various projects. Integration with current systems should prioritize compatibility and accessibility, enabling teams to utilize custom resource definitions without disrupting established workflows. Compliance requirements are especially crucial, as they vary by area and can influence the pace of product development.
Statistical insights reveal that organizations adopting best practices for compliance requirements experience a 30% increase in operational efficiency, underscoring the importance of a systematic approach. Furthermore, a case study on surrogate endpoints illustrates how clear guidelines and robust training programs can lead to improved compliance and reduced errors in clinical trials. As Denis H. Y. Leung notes, 'In this paper, the technical issues are discussed in detail, and an alternative structure for evaluating the validity of surrogate endpoints is proposed.' By providing clear answers to these FAQs, organizations can empower their teams to utilize crds effectively and confidently, ultimately driving innovation in the MedTech landscape.

The integration of advanced technologies such as Custom Resource Definitions (CRDs) and platforms like Kubernetes is transforming the MedTech sector by streamlining clinical research and accelerating ethical approvals. Insights indicate that tools like bioaccess® and Crossplane are essential for navigating the complex regulatory landscape, enabling faster development cycles and improving compliance. By leveraging these innovations, MedTech companies can focus on their core mission: delivering life-saving technologies more efficiently.
Moreover, the benefits of automation through GitHub Actions and the flexibility offered by Kubernetes CRDs are crucial for enhancing operational efficiency and patient outcomes. As organizations adapt to the evolving demands of clinical trials, implementing best practices in CRD management and compliance will be pivotal for success. Ongoing research and development in this field promise to further advance the capabilities of MedTech innovators, ultimately contributing to better healthcare solutions and economic growth.
As the MedTech industry continues to evolve, embracing these technologies and methodologies will not only foster innovation but also ensure that patient safety and regulatory compliance remain at the forefront. The path ahead is clear: organizations that prioritize the adoption of these advanced tools and frameworks will be well-positioned to thrive in a competitive landscape, making significant strides in improving healthcare outcomes for patients worldwide.
What is bioaccess® and how does it benefit clinical research?
bioaccess® is a service that accelerates ethical approvals for clinical trials, achieving them in an impressive 4-6 weeks. This expedited process helps medical technology firms hasten trials and market launches by navigating the complex regulatory landscape efficiently.
Why is the duration of ethical approvals significant in 2025?
The average duration for ethical approvals in medical technology trials remains a concern, as many companies face prolonged timelines that can delay innovation. Faster approvals are essential in a competitive landscape where a few specialties dominate device clearances.
What recent collaborations has bioaccess® undertaken to improve clinical trials?
bioaccess® has collaborated with Welwaze Medical Inc. for the Celbrea® device launch and with Caribbean Health Group to position Barranquilla as a clinical trial destination. These partnerships have achieved over a 50% reduction in recruitment time while maintaining a 95% retention rate.
How does Crossplane simplify CRD management for MedTech applications?
Crossplane provides a unified control plane for medical technology companies to streamline the deployment and management of resources across cloud environments. It automates resource provisioning, enhancing operational efficiency and ensuring compliance with regulatory standards.
What impact does Crossplane have on deployment times for medical technology firms?
Organizations using Crossplane for resource management have experienced a notable reduction in deployment times, significantly enhancing operational efficiency in the highly regulated MedTech sector.
How do Kubernetes CRDs extend functionality for MedTech solutions?
Kubernetes CRDs allow medical technology firms to customize their Kubernetes settings by defining specific resource types suited to their research needs, thus improving patient data management and integration with medical devices.
What are the broader implications of using CRDs in medical technology research?
The adoption of CRDs facilitates compliance assessments, project oversight, and reporting, which are crucial for trial management. This not only enhances MedTech research's impact on local economies but also contributes to job creation and advancements in healthcare.
Why are CRDs considered vital for addressing challenges in medical technology research?
Enhancing Kubernetes capabilities through CRDs addresses unique research challenges, driving progress in medical technology by enabling more resilient and scalable solutions that improve research capabilities and patient outcomes.