


The article centers on "10 Essential CAPA Strategies for Clinical Research Compliance," aiming to underscore crucial strategies for ensuring compliance in clinical research through effective Corrective and Preventive Actions (CAPA). A robust CAPA program is not just beneficial; it is essential. Supported by thorough documentation, regular audits, and innovative technologies, such a program is vital for enhancing adherence and quality in clinical trials. This ultimately leads to improved patient safety and regulatory compliance.
In the ever-evolving Medtech landscape, the role of bioaccess in addressing key challenges cannot be overstated. By implementing these strategies, organizations can navigate the complexities of clinical research more effectively. Consider this: how prepared is your organization to tackle compliance challenges? The integration of CAPA strategies can significantly bolster your efforts.
In conclusion, collaboration is paramount. By embracing these CAPA strategies, clinical research entities can not only meet regulatory demands but also enhance the overall quality of their trials. The next steps involve assessing your current CAPA practices and identifying areas for improvement. Take action now to ensure your compliance and safeguard patient welfare.
In the realm of clinical research, compliance stands as a fundamental pillar—not just a regulatory checkbox—ensuring patient safety and the integrity of study outcomes. As organizations navigate the complexities of corrective and preventive action (CAPA) strategies, they face numerous challenges that can hinder progress. This article explores ten essential CAPA strategies that not only enhance compliance but also drive quality improvement in clinical research.
How can organizations effectively implement these strategies to overcome obstacles and foster a culture of continuous improvement? The insights provided will illuminate the path forward, empowering researchers to elevate their practices and achieve their goals.
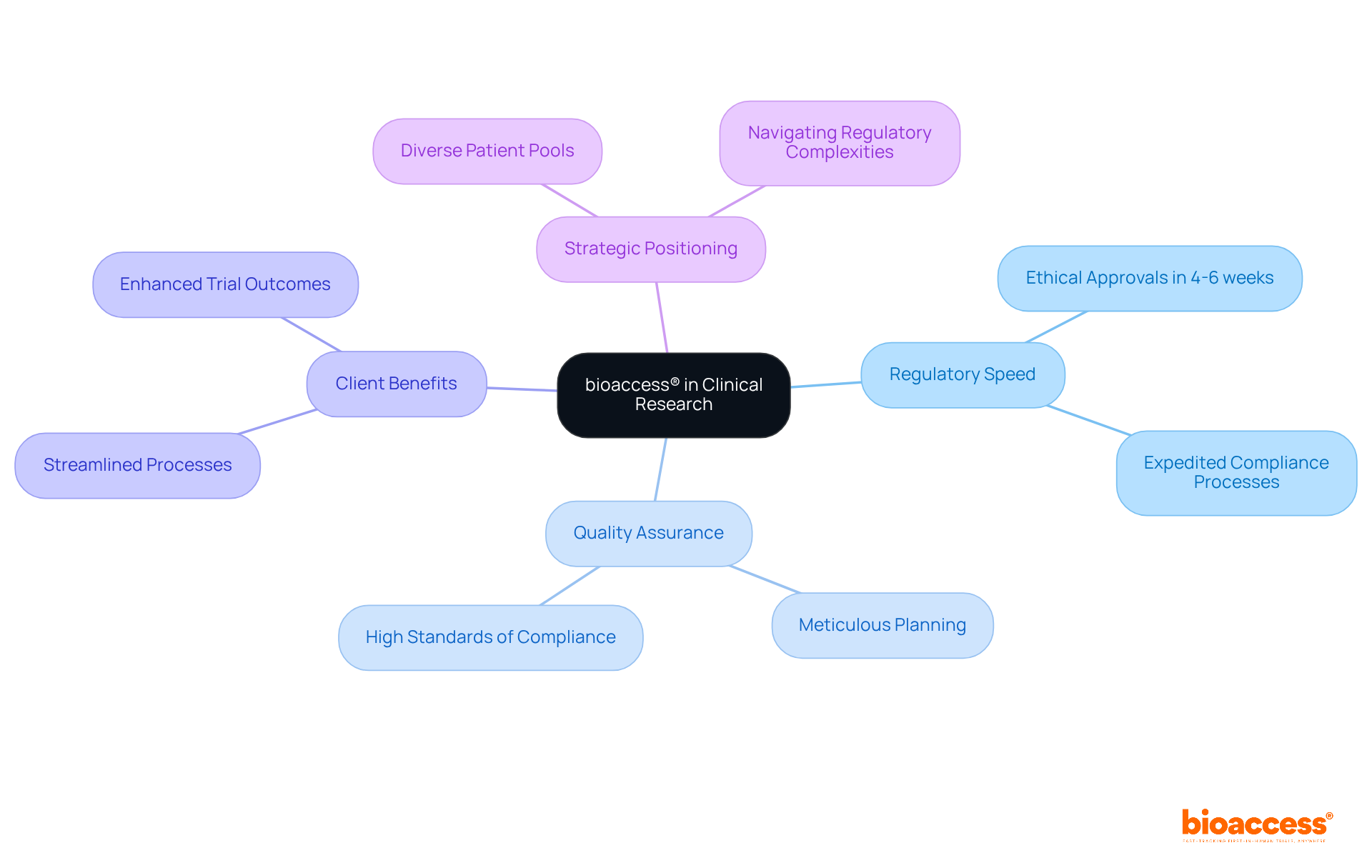
bioaccess® stands at the forefront of capa clinical research, leveraging its extensive knowledge of regulatory requirements and experimental processes to expedite compliance and quality assurance. By harnessing the regulatory speed of Latin America and its diverse patient pools, bioaccess® guarantees that trials are not only compliant but also efficient. This strategic positioning allows for ethical approvals in just 4-6 weeks, significantly enhancing the overall quality of trial outcomes. Their unwavering dedication to quality assurance is evident in the meticulous planning and execution of research studies, ensuring that every aspect meets the highest standards of compliance.
In the ever-evolving Medtech landscape, bioaccess® plays a crucial role in addressing key challenges faced by clinical researchers. With a commitment to excellence, they navigate the complexities of regulatory frameworks, ensuring that their clients can focus on what truly matters: advancing healthcare solutions. As you consider your own challenges in clinical research, think about how partnering with a knowledgeable ally like bioaccess® could streamline your processes and enhance your trial outcomes.
In summary, collaboration with bioaccess® not only facilitates compliance but also drives innovation in capa clinical research. Their expertise and strategic approach position them as a vital partner in navigating the complexities of the Medtech environment. Take the next step towards achieving your research goals by engaging with bioaccess® today.
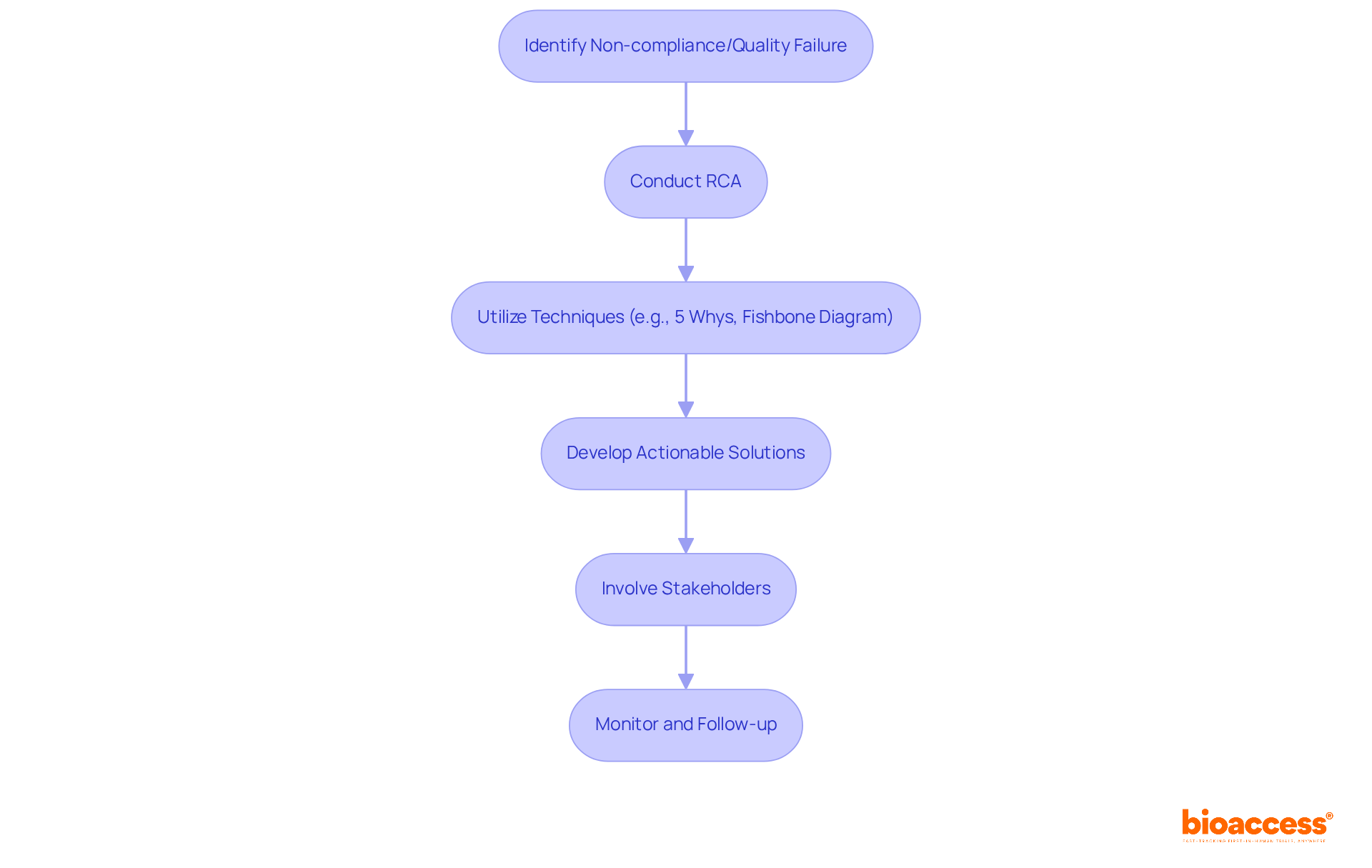
Root cause analysis (RCA) is a crucial element of any CAPA clinical research strategy, serving as a powerful tool to identify the fundamental reasons behind non-compliance and quality failures in medical studies. By systematically examining incidents, medical investigation teams can uncover root causes and implement corrective measures that effectively address these challenges. This proactive approach not only resolves current issues but also minimizes the risk of future incidents, thereby enhancing the overall quality of CAPA clinical research in the medical field.
Current trends in RCA techniques underscore the necessity of a structured approach. Methods such as the '5 Whys' technique, which involves repeatedly asking 'why' to drill down to the core issue, and fishbone diagrams, which visually map out potential causes of a problem, are widely adopted. These techniques foster a comprehensive understanding of the issues at hand, enabling teams to devise targeted solutions.
For instance, a recent systematic review highlighted that while RCA effectively identifies causes of safety incidents, its implementation often lacks follow-up, diminishing its potential to prevent recurrence. This underscores the importance for organizations to not only conduct RCA but also ensure that recommendations are systematically addressed and monitored. Notably, only two studies in CAPA clinical research demonstrated an improvement in patient safety due to RCAs, revealing the limitations of this approach.
Moreover, involving frontline professionals and stakeholders in the RCA process can significantly enhance the effectiveness of the analysis. Their insights provide unique perspectives on care delivery, which are essential for developing actionable solutions. As Jimmy Martin-Delgado noted, many recommendations from RCA often fall short in preventing the recurrence of incidents, highlighting the need for a robust implementation strategy. By fostering a culture of transparent communication and education, organizations can greatly improve their RCA outcomes, ultimately contributing to enhanced patient safety and adherence in CAPA clinical research.
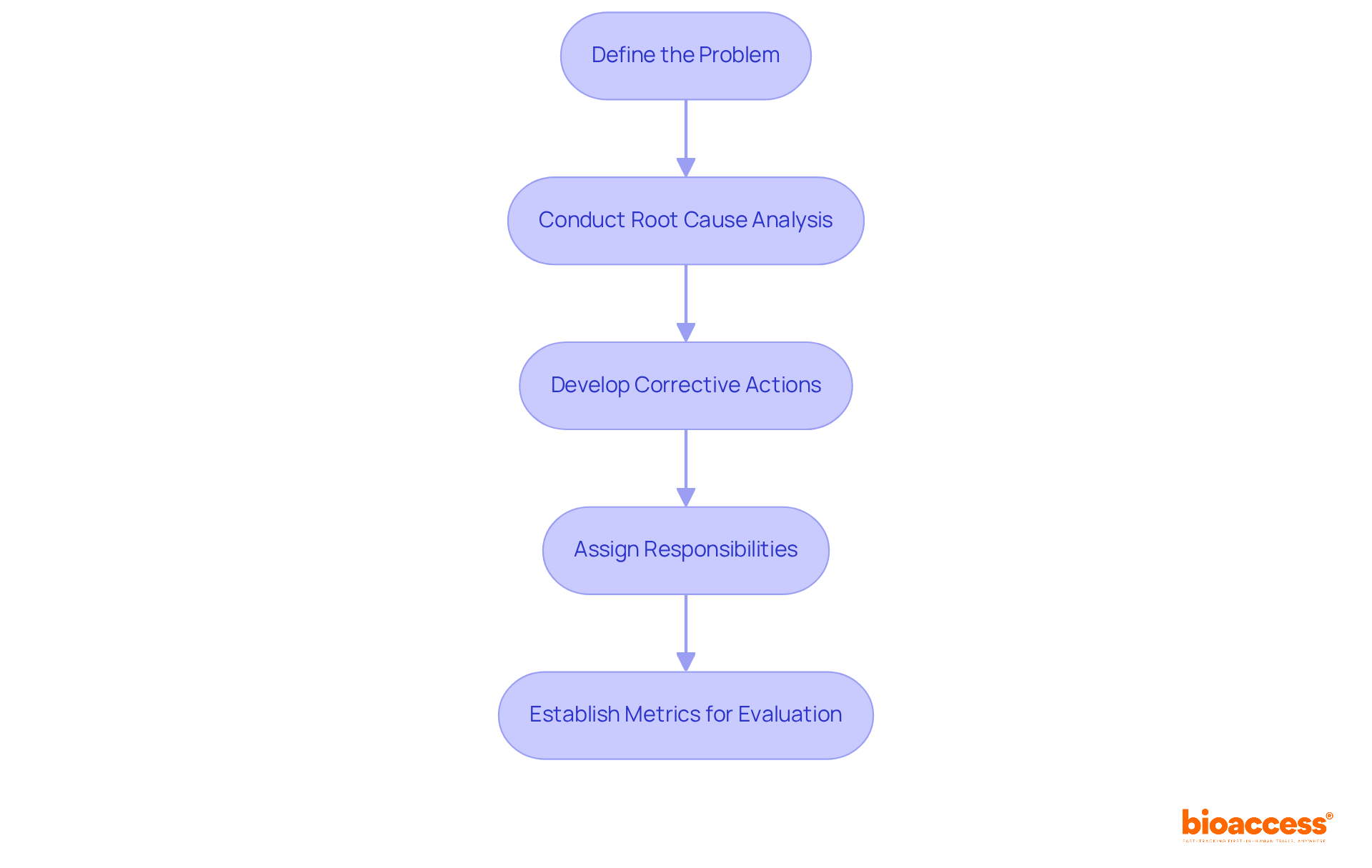
Establishing a robust Corrective and Preventative Action strategy is crucial for ensuring compliance in clinical trials. This process starts with a precise definition of the problem and its implications for adherence. Following this, a comprehensive root cause analysis is conducted, employing techniques like Fishbone diagrams and the '5 Whys' method to reveal underlying issues. Prompt intervention in the corrective and preventive action process is vital to mitigate potential impacts. Once root causes are identified, specific corrective actions must be developed to address them, along with preventive measures aimed at preventing future occurrences.
Assigning clear responsibilities and timelines for each action fosters accountability within the team. This structured approach not only facilitates swift action but also increases the likelihood of successful implementation. As Meghan Hosely articulates, "As a methodology, the corrective and preventive action process goes beyond issue resolution; it aims to drive continuous product and process improvements while enhancing the organization’s understanding of both its products and processes." Ultimately, establishing metrics to evaluate the effectiveness of the corrective action plan is essential for ongoing improvement. Consistent oversight and adjustments to the plan ensure it remains effective in addressing regulatory challenges and supports the overall integrity of capa clinical research. Recognizing challenges in executing corrective and preventive actions, such as adapting to evolving regulatory requirements, is also necessary to provide a comprehensive view of the process.
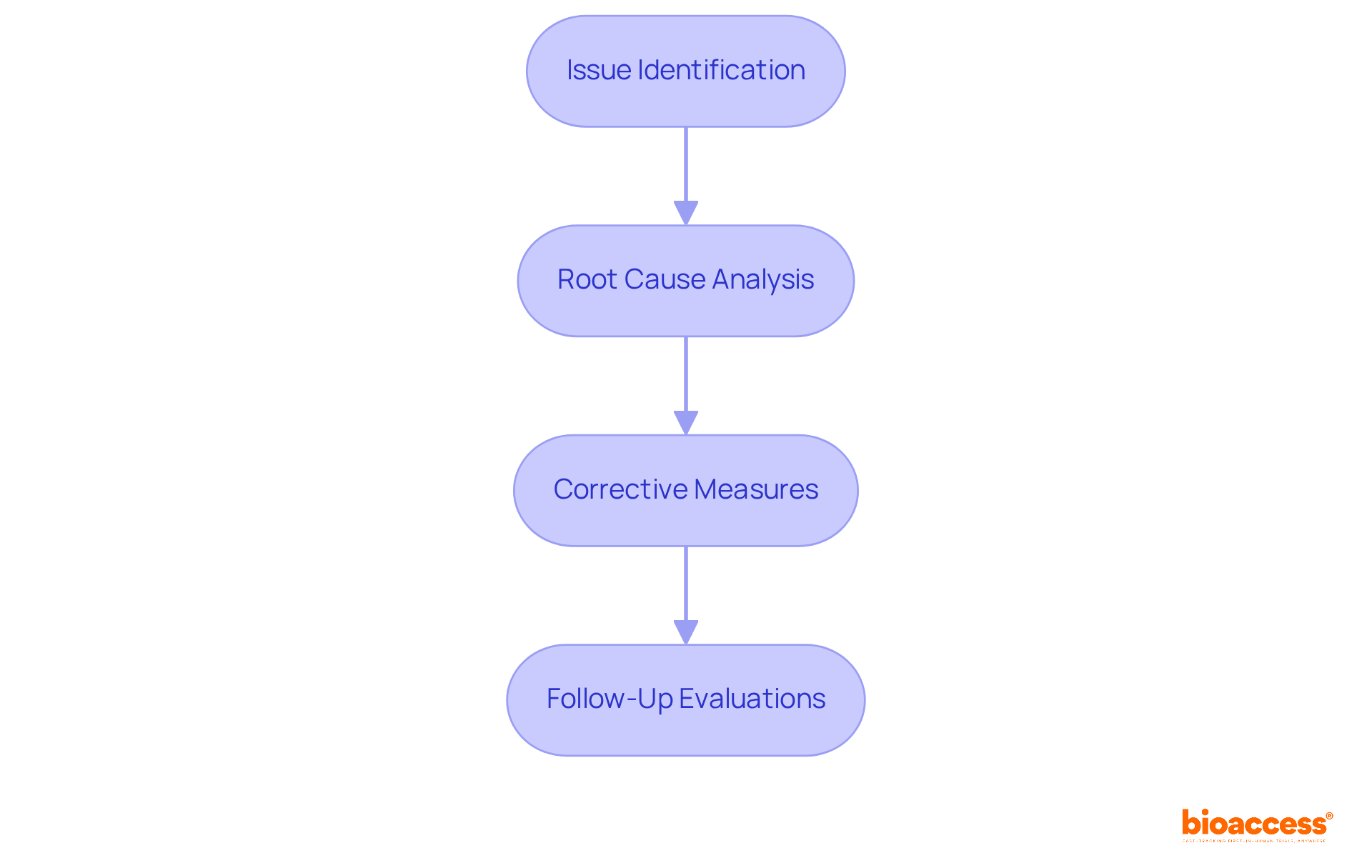
Integrating technology into corrective and preventive action management is crucial for enhancing adherence initiatives in CAPA clinical research. Tools such as electronic document management systems (EDMS) and corrective action software significantly improve the monitoring of regulatory issues and remedial actions. These technologies provide real-time data access, enabling teams to track progress effectively and ensure the timely implementation of corrective measures.
Furthermore, automated reporting functions simplify documentation, making it easier to maintain accurate records and demonstrate compliance with regulatory standards. By leveraging these advancements in CAPA clinical research, organizations can streamline their processes and enhance their overall operational efficiency.

Successful execution of corrective and preventive actions hinges on comprehensive training and education for all team members involved in capa clinical research. Routine training sessions must cover the fundamentals of corrective and preventive actions, the importance of regulatory adherence, and the specific steps to take when issues arise within the context of capa clinical research. By fostering a culture of continuous learning, organizations empower their staff to identify potential regulatory challenges early and respond appropriately.
Moreover, refresher courses and workshops play a crucial role in keeping the team updated on the latest regulatory changes and best practices in corrective and preventive action management. Organizations that implement corrective and preventive action management systems report compliance rates soaring to between 99% and 100%, compared to 85% to 97% for those lacking such systems. This stark contrast underscores the vital role of training in achieving high compliance rates.
Additionally, a correlation of 0.748 between total complaints and total deviations illustrates the effectiveness of robust corrective and preventive action strategies. Investing in employee education yields a remarkable return of $2.29 for every dollar spent, with 95% of participants finding training beneficial for their roles. To enhance the efficiency of corrective and preventive action processes, organizations should prioritize timely closure, establishing specific targets for resolution.
As a practical tip, consider developing personalized learning paths for team members. This approach can lead to a 20% increase in engagement, ensuring that training is not only informative but also motivating.

Efficient documentation methods are essential for upholding regulations in corrective and preventive action (CAPA) processes. Each stage of the CAPA process must be meticulously documented, covering:
This comprehensive documentation serves not only as a compliance record but also offers valuable insights for future CAPA initiatives. As emphasized in the industry, 'Good Documentation Practices (GDP) are essential for maintaining data quality in research trials.'
Utilizing standardized templates and checklists can streamline the documentation process, ensuring consistency and completeness across all CAPA activities. Notably, statistics reveal that "6 out of 10 warning letters issued by the US-FDA to research investigators in 2010 cited failure to maintain adequate case histories," underscoring the critical need for robust documentation. By prioritizing careful documentation, organizations can enhance data integrity, support regulatory adherence, and ultimately improve the quality of health-related outcomes.
For instance, a case study on 'Accurate Documentation Leading to Patient Safety' illustrates how efficient documentation practices can effectively monitor patient progress and ensure compliance with regulatory standards, thereby enhancing overall trial integrity. This highlights the importance of adopting rigorous documentation practices in CAPA clinical research to mitigate risks and improve outcomes.
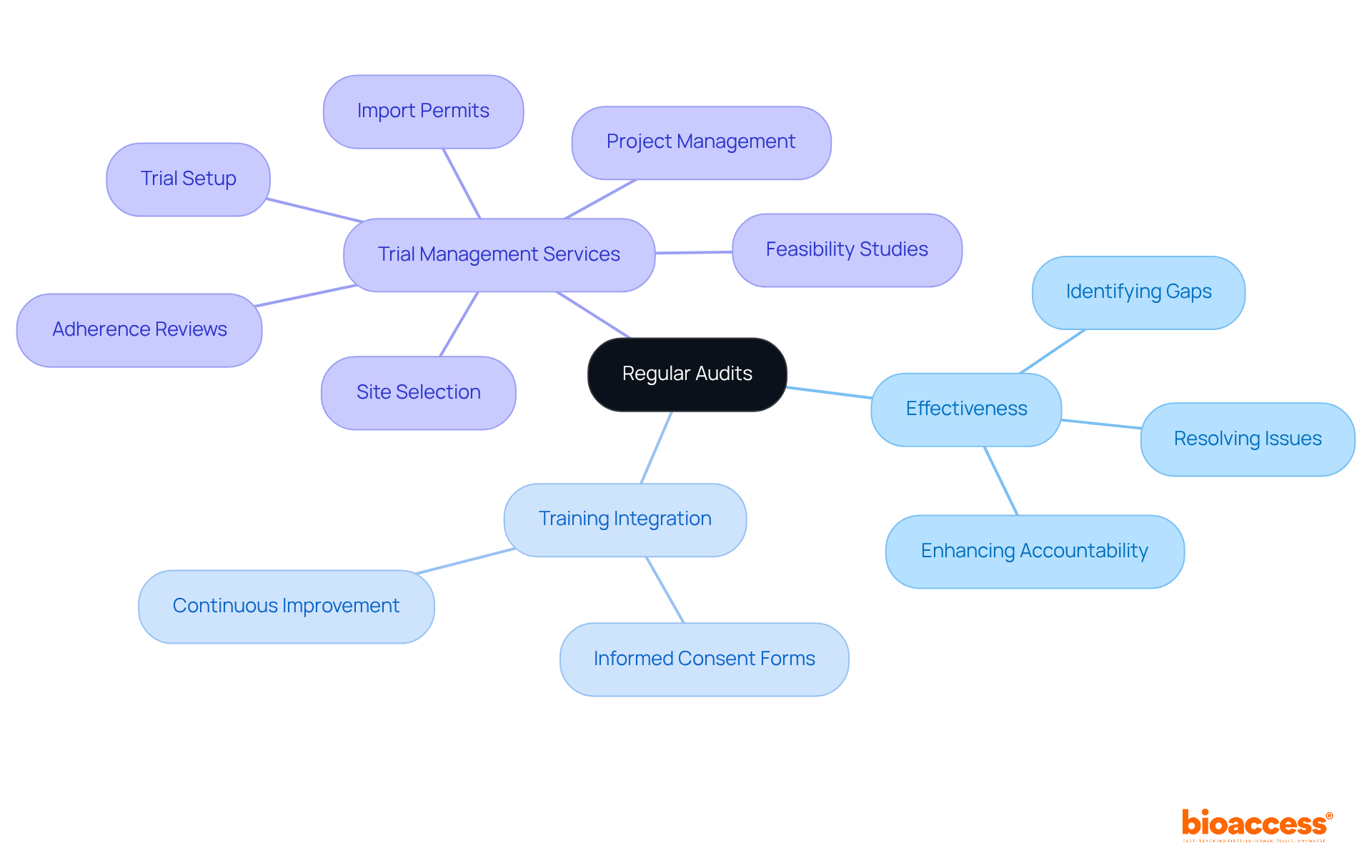
Regular audits are crucial for ensuring ongoing adherence and quality assurance in CAPA clinical research. These evaluations focus on the effectiveness of the CAPA clinical research processes, confirming that corrective measures are not only implemented but also effectively resolve identified issues. By systematically reviewing adherence records, documentation, and training practices, organizations can pinpoint gaps and areas needing improvement. This proactive strategy not only enhances adherence but also fosters a culture of accountability and quality within CAPA clinical research teams.
For instance, audits can reveal discrepancies in data entry, with an average error rate of one per 300 keystrokes, prompting immediate corrective actions. Furthermore, integrating audit findings into regular training sessions can significantly improve the percentage of properly executed informed consent forms, a critical metric in site management. At Bioaccess, our extensive trial management services encompass:
All aimed at promoting regulatory excellence.
Ultimately, regular audits not only strengthen adherence but also empower healthcare professionals to champion essential changes. This collaborative atmosphere emphasizes quality in research trials, ensuring that all stakeholders are aligned in their commitment to excellence.
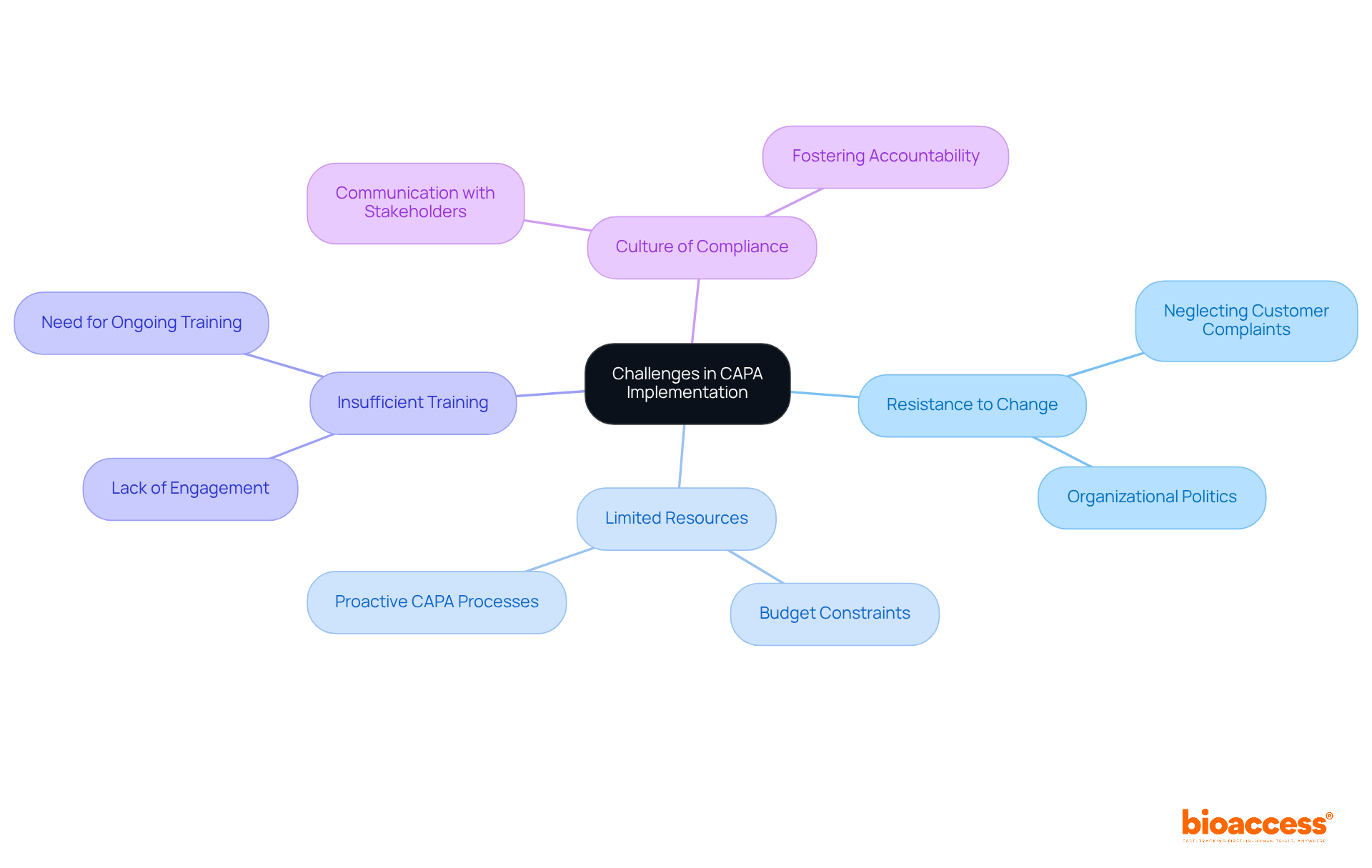
Implementing a CAPA clinical research program presents significant challenges, including resistance to change, limited resources, and insufficient training. Organizations often struggle to cultivate a culture of compliance, especially when team members are not fully engaged in the CAPA process. For instance, neglecting to investigate customer complaints can lead to delays in addressing critical issues, as evidenced by cases where non-compliance resulted in serious repercussions, including FDA Warning Letters.
Resource distribution is crucial for the success of CAPA programs. Limited budgets and staffing can severely impede the effective implementation of corrective and preventive action strategies. However, organizations that prioritize communication about the importance of CAPA to all stakeholders can create a more compliant environment. Providing adequate resources and ongoing training is essential to ensure that team members understand their roles in the CAPA process.
Even in resource-constrained environments, successful CAPA programs have emerged. For example, a proactive CAPA process aimed at addressing document control failures prior to an audit demonstrated that thorough documentation and awareness can significantly reduce nonconformities. This approach not only lessened the severity of the issues but also extended the time before the next audit, allowing for a comprehensive resolution of the problems.
Statements from industry experts underscore the importance of overcoming challenges in CAPA clinical research. As Aaron S. Kesselheim noted, "Setting up training and protocols for medical studies can alleviate regulatory pressures for investigators." This highlights the necessity of investing in training and resources to effectively manage the complexities of CAPA implementation.
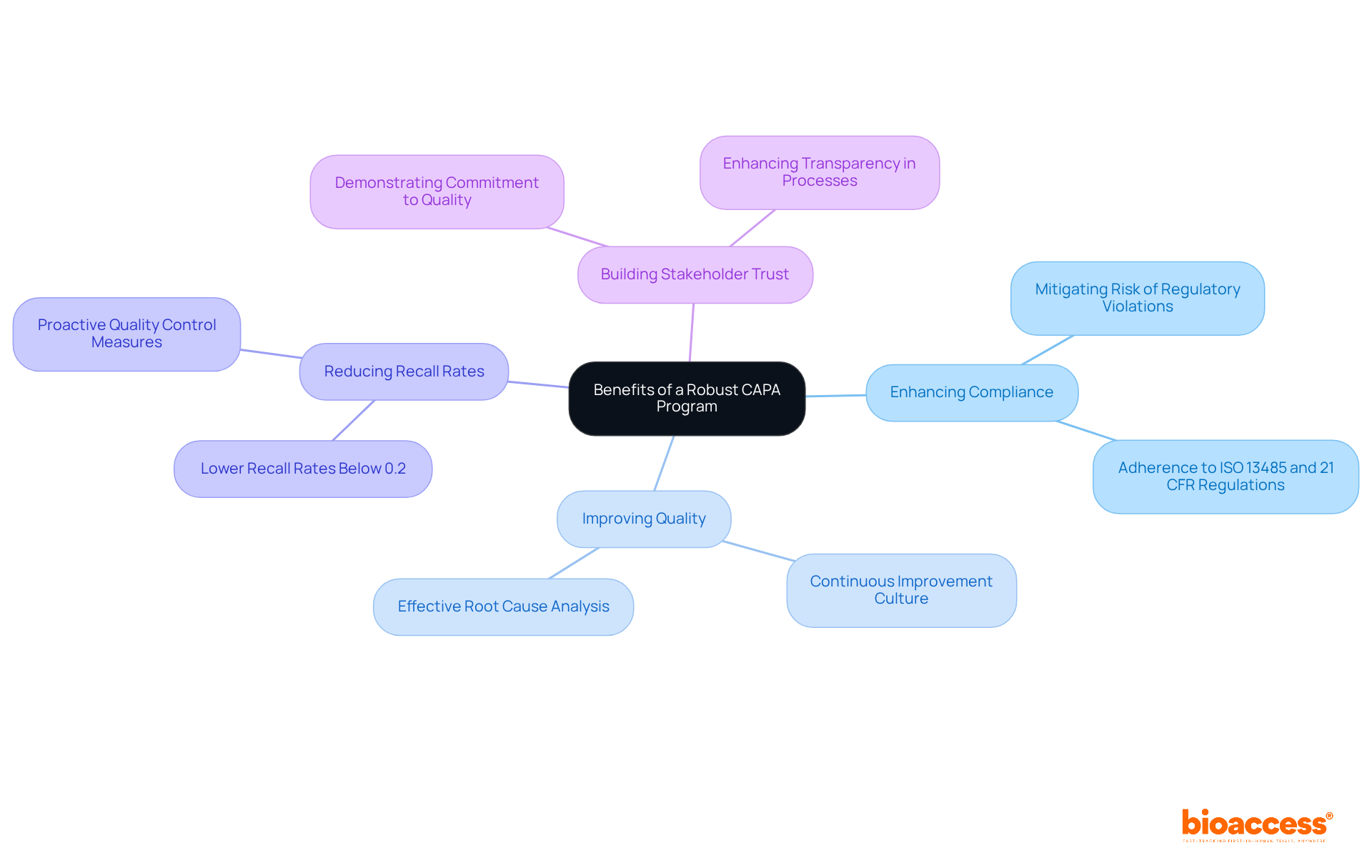
A strong Corrective and Preventive Action program significantly enhances adherence and quality in capa clinical research. By systematically addressing adherence issues, organizations can mitigate the risk of regulatory violations—an essential consideration, given that 96% of organizations have reported facing product recalls in the last five years. Effective corrective and preventive action strategies foster a culture of continuous improvement, allowing teams to learn from past mistakes and implement precautionary measures. For instance, at MediFeck, the implementation of a structured corrective and preventive action process led to swift corrective actions, such as halting production and recalling affected devices, ultimately improving product safety and compliance.
Moreover, a robust corrective action program enhances stakeholder trust by demonstrating a commitment to maintaining high standards throughout the investigation process. Organizations with effective corrective and preventive action systems typically experience lower recall rates, often below 0.2% of dispatched units, highlighting the importance of thorough audits and proactive quality control. By prioritizing corrective and preventive actions, capa clinical research organizations can achieve better study outcomes while ensuring that patient safety and regulatory compliance remain at the forefront of their operations. Additionally, comprehensive trial management services provided by bioaccess—including feasibility studies, site selection, adherence reviews, trial setup, import permits, project management, and reporting—support these corrective and preventive action initiatives. This holistic approach not only boosts adherence but also contributes to local economies through job creation, economic development, healthcare improvement, and global collaboration.
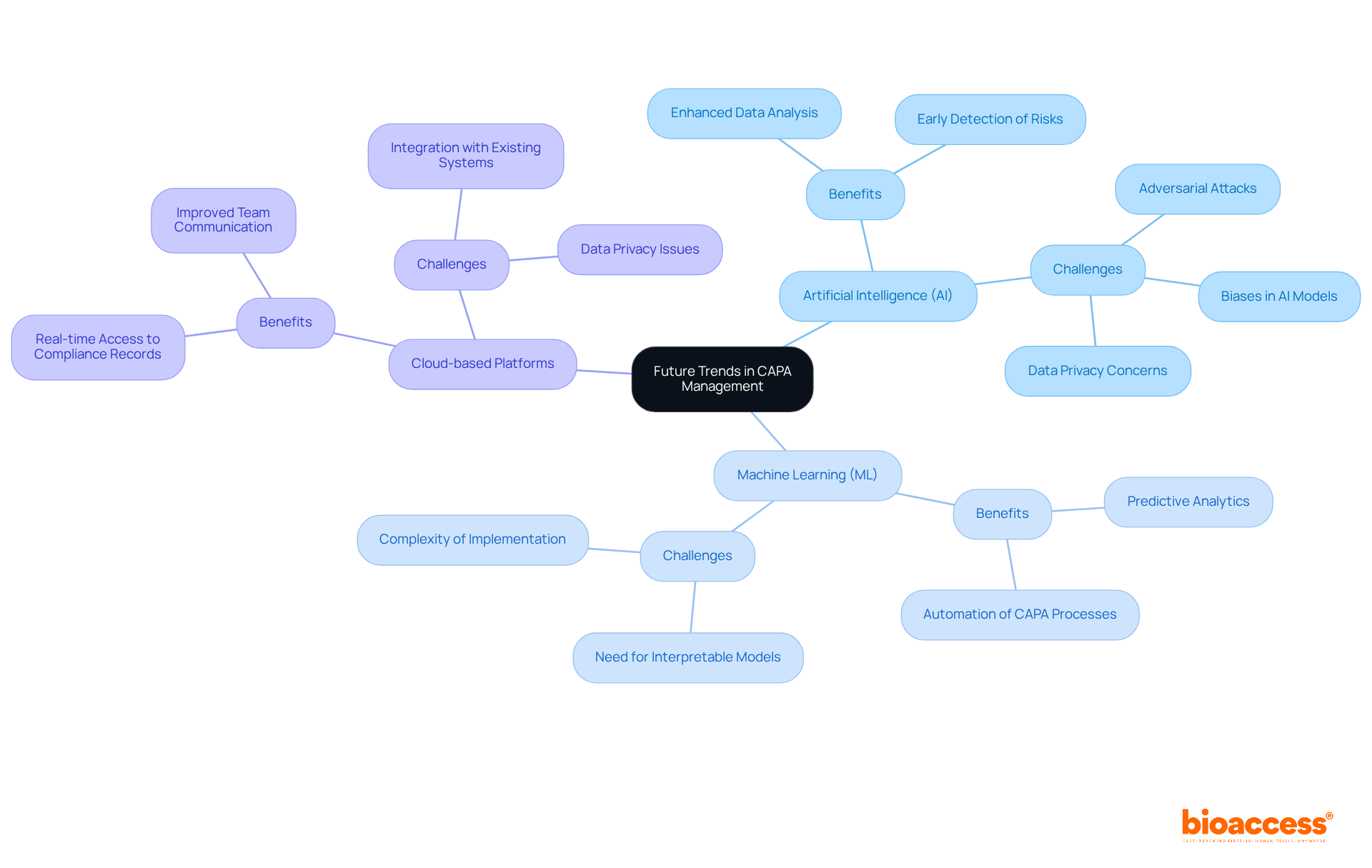
As the clinical research environment evolves, significant trends in corrective and preventive action (CAPA) management are emerging, all aimed at enhancing adherence. Innovations such as artificial intelligence (AI) and machine learning (ML) are now integral to CAPA processes, enabling organizations to analyze data more effectively and anticipate potential regulatory issues before they arise. A recent survey of 330 studies highlighted the effectiveness of AI in early detection of regulatory risks, underscoring its critical role in this landscape.
Moreover, the adoption of cloud-based platforms for documentation and collaboration is gaining traction, providing real-time access to compliance records and fostering improved communication among team members. However, organizations must navigate challenges like data privacy and the necessity for interpretable models to fully harness these technologies. By embracing these trends, organizations can refine their CAPA clinical research strategies and secure a competitive edge in the sector.
As John Smith Mabere aptly noted, "The integration of Artificial Intelligence (AI) and Machine Learning (ML) in cybersecurity has revolutionized threat detection, risk mitigation, and security automation," reflecting the transformative potential of these technologies in CAPA management. The path forward is clear: organizations must act decisively to integrate these innovations and address the accompanying challenges.
The significance of implementing effective Corrective and Preventive Action (CAPA) strategies in clinical research is paramount. These strategies not only ensure compliance with regulatory standards but also enhance the overall quality of research outcomes. By embracing a structured approach to CAPA, organizations can proactively address issues, mitigate risks, and foster a culture of continuous improvement.
Key strategies have been highlighted throughout this discussion, including:
Each element plays a critical role in creating a robust CAPA framework that supports compliance and enhances the integrity of clinical research processes. Additionally, regular audits and meticulous documentation practices are essential for maintaining high standards and addressing any discrepancies that may arise.
In conclusion, the evolving landscape of clinical research underscores the need for organizations to adopt innovative CAPA management practices. By leveraging advancements such as artificial intelligence and cloud-based solutions, companies can refine their strategies and stay ahead of regulatory challenges. Taking decisive action to implement these essential CAPA strategies will not only facilitate compliance but also drive quality improvements that ultimately benefit patient safety and healthcare outcomes. Engaging with experienced partners like bioaccess® can further amplify these efforts, paving the way for successful clinical research initiatives.
What is bioaccess® and its role in clinical research?
bioaccess® is a leader in capa clinical research, utilizing its knowledge of regulatory requirements and experimental processes to expedite compliance and quality assurance, ensuring efficient and compliant clinical trials.
How quickly can bioaccess® facilitate ethical approvals for clinical trials?
bioaccess® can facilitate ethical approvals in just 4-6 weeks, significantly enhancing the overall quality of trial outcomes.
What is the importance of root cause analysis (RCA) in CAPA clinical research?
RCA is essential for identifying the fundamental reasons behind non-compliance and quality failures, allowing teams to implement corrective measures that resolve issues and minimize future risks.
What techniques are commonly used in root cause analysis?
Common techniques include the '5 Whys' method, which involves repeatedly asking 'why' to uncover core issues, and fishbone diagrams, which visually map potential causes of a problem.
What are some limitations of root cause analysis in clinical research?
A significant limitation is the lack of follow-up on RCA findings, which can diminish its effectiveness in preventing recurrence of incidents.
How can involving frontline professionals enhance the effectiveness of RCA?
Involving frontline professionals provides unique insights into care delivery, which are crucial for developing actionable solutions to identified issues.
What are the essential steps in developing a CAPA plan for clinical trials?
Essential steps include defining the problem, conducting a root cause analysis, developing specific corrective and preventive actions, assigning responsibilities, and establishing metrics for evaluating effectiveness.
Why is prompt intervention important in the CAPA process?
Prompt intervention is vital to mitigate potential impacts of identified issues and to ensure swift action towards corrective and preventive measures.
How does establishing metrics contribute to CAPA plan effectiveness?
Establishing metrics allows for ongoing evaluation of the corrective action plan's effectiveness, ensuring it addresses regulatory challenges and supports the integrity of clinical research.
What challenges might organizations face in executing corrective and preventive actions?
Organizations may face challenges such as adapting to evolving regulatory requirements, which can complicate the execution of corrective and preventive actions.