


The article titled "10 Essential CDISC Standards Every Clinical Research Director Should Know" serves as a crucial resource for clinical research directors, emphasizing the importance of key CDISC standards. These standards, including SDTM, ADaM, and ODM, are vital for ensuring compliance and enhancing the quality of clinical trials. By facilitating regulatory submissions, improving data organization, and promoting transparency, these standards ultimately lead to faster approvals and better research outcomes. Understanding and implementing these standards is not merely beneficial; it is essential for directors aiming to navigate the complexities of clinical research effectively.
In the intricate realm of clinical research, adherence to standardized protocols transcends mere regulatory obligation; it is a pivotal element in the success of trials. The Clinical Data Interchange Standards Consortium (CDISC) provides a comprehensive framework that not only enhances data quality but also expedites regulatory approvals, ultimately leading to improved patient outcomes. Nevertheless, numerous clinical research directors encounter the daunting task of effectively navigating these standards while ensuring both compliance and operational efficiency. What essential CDISC standards must every research leader comprehend to optimize their clinical trials and adapt to the ever-evolving regulatory landscape?
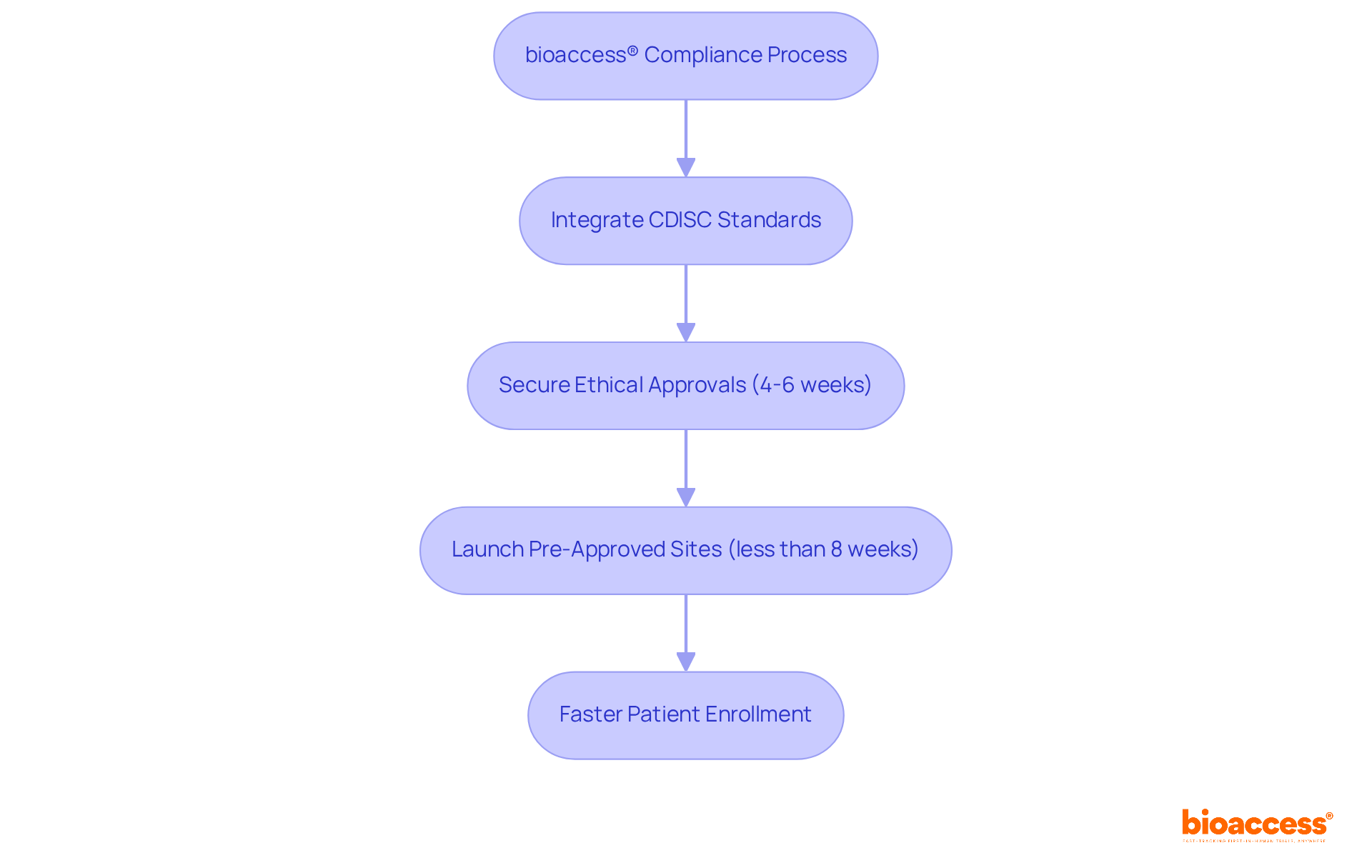
bioaccess® utilizes its extensive knowledge of CDISC standards to optimize trial processes, ensuring that all studies comply with regulatory requirements. By integrating CDISC standards, bioaccess® accelerates the approval process while enhancing the quality of information collected during experiments. This commitment to compliance is reflected in their capacity to secure ethical approvals in just 4-6 weeks—significantly quicker than conventional industry timelines.
Furthermore, with over 50 pre-approved sites launched in less than 8 weeks, bioaccess® facilitates faster patient enrollment and information gathering, establishing itself as a dependable ally for research directors. Their expertise spans diverse medical device research studies, including:
This ensures comprehensive support throughout the research process.
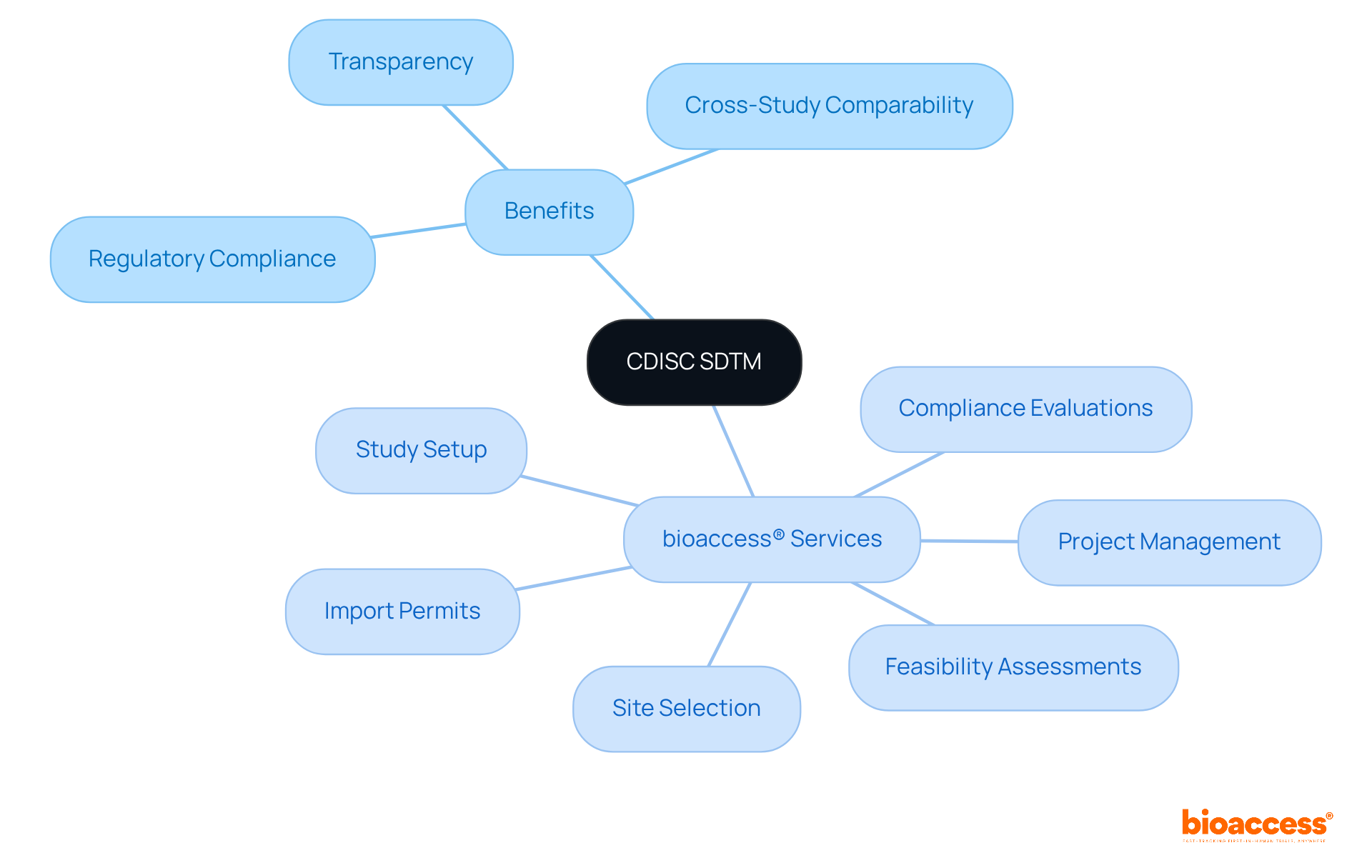
The Study Data Tabulation Model (SDTM) serves as a pivotal standard that adheres to CDISC standards, providing a structured approach for organizing and formatting research information. Adhering to CDISC standards allows study leaders to ensure their data is arranged in compliance with regulatory requirements, thereby facilitating smoother submissions to organizations like the FDA. This model enhances the transparency of information and promotes cross-study comparability, simplifying the evaluation and authorization of research findings by regulators.
Furthermore, with bioaccess®'s comprehensive research study management services—including:
research directors can streamline their processes. By leveraging bioaccess®'s expertise, they can accelerate patient enrollment by 50% and achieve savings of $25K per patient with FDA-compliant information, ultimately overcoming regulatory hurdles and expediting approval processes for startups.
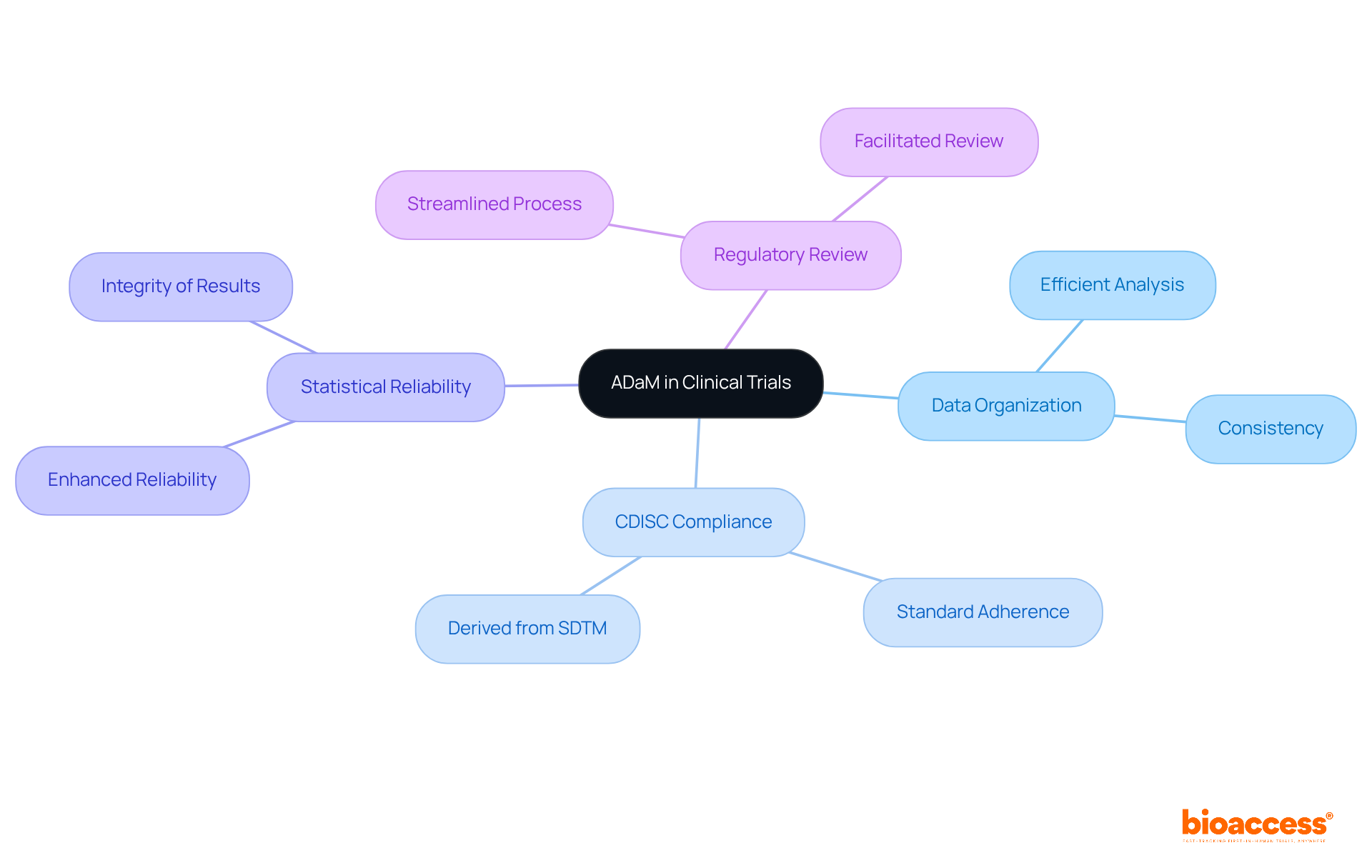
The Analysis Data Model (ADaM) is essential for organizing datasets that facilitate statistical evaluation in medical trials. By adhering to CDISC standards, study directors can produce analysis-ready datasets that facilitate efficient analysis and reporting. This standard ensures that datasets comply with CDISC standards, as they are derived from SDTM datasets, thereby maintaining consistency and integrity throughout the analysis process. Implementing ADaM not only enhances the reliability of statistical results but also streamlines the review process by regulatory agencies, underscoring its critical role in the clinical research landscape.
The Operational Data Model (ODM) serves as a vendor-neutral standard that significantly enhances the exchange and storage of trial information. By implementing ODM, study leaders can facilitate seamless information sharing across various systems, which is crucial for fostering collaboration and ensuring efficient information integration. This standard accommodates both raw and derived datasets, thereby promoting a continuous flow of information throughout the research lifecycle.
In 2025, the benefits of ODM in managing research information are particularly pronounced, as it not only simplifies information management procedures but also bolsters compliance with regulatory standards for information interchange. Organizations utilizing ODM standards have reported a remarkable 50% increase in information interchange efficiency, enabling quicker decision-making and improved study outcomes.
Expert insights indicate that ODM enhances collaboration among stakeholders by providing a shared framework for information exchange, ultimately leading to more robust and trustworthy research studies. As the demand for effective data management continues to rise, the adoption of ODM will be essential for directors seeking to optimize their data strategies.

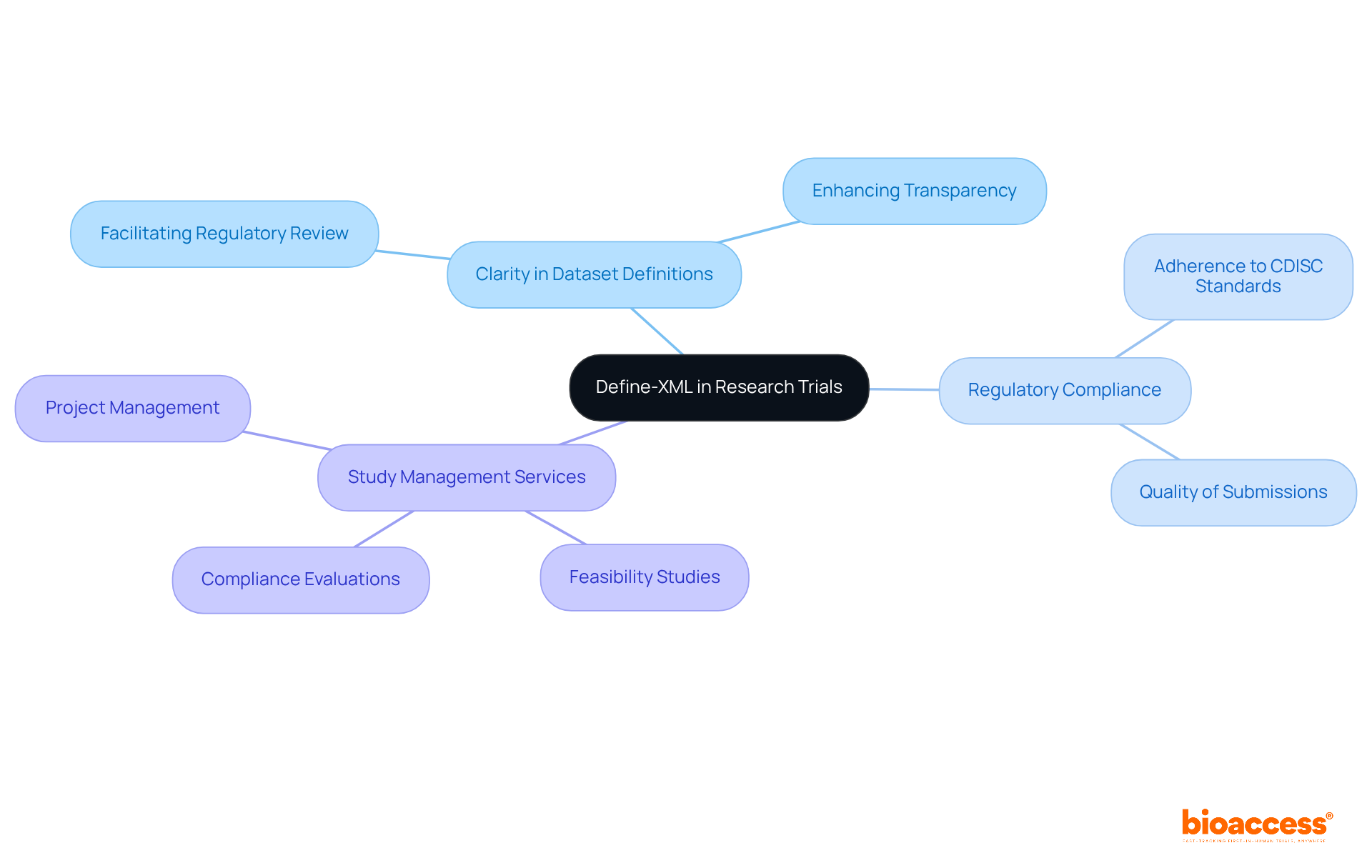
Define-XML stands as a pivotal standard that articulates the metadata defining the structure of datasets employed in research trials. By leveraging Define-XML and adhering to CDISC standards, study directors ensure that their dataset definitions are both clear and consistent, thereby facilitating regulatory review and approval. This standard significantly enhances the transparency of data submissions, enabling regulatory agencies to readily comprehend the organization and content of the datasets. The implementation of Define-XML not only elevates the quality of submissions but also ensures compliance with regulatory requirements in accordance with CDISC standards.
Comprehensive study management services, such as those offered by bioaccess—including feasibility studies, compliance evaluations, and project management—are crucial in guaranteeing that these standards are effectively integrated throughout the medical investigation process.
Controlled terminology represents a collection of standardized terms utilized in medical studies, ensuring consistency across datasets. By adopting controlled terminology, clinical research directors can significantly enhance the quality and comparability of information collected during trials. This standardization not only reduces the risk of mistakes and discrepancies but also facilitates easier analysis and interpretation of information across various studies.
Furthermore, controlled terminology plays a vital role in enhancing information integrity, ensuring that the details collected are precise and trustworthy. It also aids in adhering to regulatory requirements for information reporting, which is crucial in today’s stringent regulatory environment.
For instance, when research studies employ standardized terminology, they achieve a greater level of consistency in information, thereby facilitating the aggregation and comparison of outcomes across various trials. This approach simplifies the analysis procedure and enhances the overall trustworthiness of the findings.
Therapeutic Area User Guides (TAUGs) extend CDISC standards to meet the unique requirements of specific disease areas. By utilizing TAUGs, study directors can ensure that their investigations align with the best practices and standards relevant to their therapeutic area. These guides provide valuable illustrations and advice on applying CDISC standards, thereby improving the quality and relevance of research data. The adoption of TAUGs enhances the efficiency of study design while also supporting compliance with regulatory expectations for disease-specific research.
Furthermore, bioaccess® offers extensive management services for research studies, which include:
This comprehensive approach ensures that research endeavors are executed smoothly and effectively.

The cdisc standards for submission are essential for ensuring that trial information meets the criteria established by regulatory agencies. By adhering to these standards, research directors can streamline the submission process, significantly reducing the likelihood of delays and rejections. These standards offer a definitive framework for organizing and presenting data, facilitating regulatory reviewers' assessment of submission quality and integrity. The application of CDISC standards not only enhances compliance but also accelerates the overall approval timeline for research trials.
CDISC implementation guides provide essential directions for utilizing the CDISC standards in medical studies. These guides serve as invaluable tools for study directors, offering practical strategies for integrating standards into study design, information gathering, and analysis. By adhering to these implementation guides and CDISC standards, investigation teams can ensure that their studies meet regulatory requirements and align with industry best practices. The use of CDISC implementation guides not only enhances the quality of research information but also facilitates effective study management.
CDISC plays a pivotal role in enhancing transparency in medical studies by establishing standards that facilitate the open sharing of study information. By adhering to CDISC standards, study leaders can significantly improve the availability and practicality of their data, which fosters teamwork and innovation within the scientific community.
At Bioaccess, our comprehensive study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—are designed to support these efforts by ensuring meticulous management and strict adherence to regulatory requirements.
Transparency in information not only cultivates public confidence in medical research but also aids in meeting regulatory obligations for information sharing. Implementing CDISC standards for data transparency guarantees that trial results are readily accessible for scrutiny and analysis, ultimately advancing medical knowledge and enhancing patient outcomes.
Furthermore, these clinical studies contribute to local economies through job creation and economic growth, thereby improving healthcare and fostering international collaboration through our dedicated project management and compliance services.
The integration of CDISC standards is paramount for clinical research directors who seek to enhance the efficiency and compliance of their studies. By comprehensively understanding and implementing these essential standards—such as SDTM, ADaM, and ODM—research leaders can effectively streamline their processes, elevate data quality, and facilitate smoother regulatory submissions. This commitment to these standards not only accelerates patient enrollment and reduces costs but also guarantees that the research conducted is both ethical and transparent.
Key insights from the article underscore the significance of each CDISC standard in promoting data integrity and regulatory compliance. For example:
Moreover, the emphasis on data transparency fosters trust within the scientific community, enhancing collaboration and cooperation.
Adopting CDISC standards transcends mere compliance; it represents a strategic approach that can yield more robust and trustworthy clinical research outcomes. As the clinical trial landscape continues to evolve, embracing these standards becomes essential for research directors who aspire to optimize their studies, meet regulatory requirements, and ultimately contribute to advancements in healthcare. Engaging with organizations like bioaccess® can provide invaluable support in navigating this complex terrain, ensuring that clinical research is conducted efficiently and effectively.
What is bioaccess® and how does it relate to CDISC standards?
bioaccess® is a service that utilizes extensive knowledge of CDISC (Clinical Data Interchange Standards Consortium) standards to optimize clinical trial processes, ensuring compliance with regulatory requirements and accelerating the approval process.
How does bioaccess® improve the approval process for clinical trials?
By integrating CDISC standards, bioaccess® enhances the quality of information collected during experiments and secures ethical approvals in just 4-6 weeks, which is significantly quicker than conventional industry timelines.
What types of studies does bioaccess® support?
bioaccess® supports a variety of medical device research studies, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies.
What is the Study Data Tabulation Model (SDTM)?
The SDTM is a pivotal standard that provides a structured approach for organizing and formatting clinical trial data, ensuring compliance with regulatory requirements for smoother submissions to organizations like the FDA.
How does adhering to CDISC standards benefit clinical trials?
Adhering to CDISC standards enhances the transparency of information, promotes cross-study comparability, and simplifies the evaluation and authorization of research findings by regulators.
What services does bioaccess® offer to streamline research processes?
bioaccess® offers comprehensive research study management services, including feasibility assessments, site selection, study setup, import permits, project management, and compliance evaluations.
What are the benefits of using bioaccess® for patient enrollment?
By leveraging bioaccess®'s expertise, research directors can accelerate patient enrollment by 50% and achieve savings of $25K per patient with FDA-compliant information.
What is the Analysis Data Model (ADaM) and its importance in clinical trials?
ADaM is essential for organizing datasets that facilitate statistical evaluation in medical trials. It ensures that datasets are analysis-ready and comply with CDISC standards, maintaining consistency and integrity throughout the analysis process.
How does implementing ADaM impact the review process by regulatory agencies?
Implementing ADaM enhances the reliability of statistical results and streamlines the review process by regulatory agencies, highlighting its critical role in the clinical research landscape.