


The article titled "10 Essential Clinical Research Associate Training Programs" serves to identify pivotal training programs that equip aspiring Clinical Research Associates (CRAs) with the essential skills required for success in the Medtech sector. It underscores that programs such as the bioaccess® Training Program and ACRP Certification impart crucial knowledge in regulatory compliance, study management, and practical skills. These competencies are vital for navigating the complexities of clinical research and significantly enhancing career prospects in an increasingly competitive job market.
In the rapidly evolving landscape of clinical research, the demand for skilled Clinical Research Associates (CRAs) is surging. This surge is driven by advancements in medical technologies and the pressing need for efficient trial management. This article explores ten essential training programs designed to equip aspiring CRAs with the critical skills and knowledge necessary to thrive in this competitive field. However, as the industry grows, a pivotal question arises: which training program will provide the most value and effectively prepare individuals for the challenges of tomorrow's clinical trials?
The bioaccess® Training Program is meticulously designed as one of the clinical research associate training programs to empower aspiring associates (CRAs) with the essential skills needed to excel in the Medtech sector. By addressing critical aspects such as regulatory adherence, study management, and patient safety, the clinical research associate training programs ensure that participants acquire a solid grasp of the industry's requirements. Through hands-on training and mentorship from seasoned professionals in clinical research associate training programs, participants enhance their practical skills, positioning themselves for accelerated career growth. This program holds particular relevance as the Medtech sector anticipates significant advancements in clinical research associate training programs for 2025, presenting an invaluable opportunity for individuals eager to make a substantial impact in healthcare.
Significantly, bioaccess® offers comprehensive management services for studies, including:
These elements are crucial for CRAs to understand fully. Organizations like GlobalCare Clinical Studies have showcased the effectiveness of collaborating with bioaccess®, achieving a remarkable reduction in recruitment time by over 50% and savings of $25K per patient with FDA-ready data. This evidence underscores the importance of strategic partnerships in enhancing operational efficiency within the clinical research landscape.
The ACRP Clinical Research Associate Certification Program serves as a vital pathway for CRAs to obtain industry-recognized qualifications, underscoring their commitment to excellence in health studies. This certification not only validates a professional's expertise but also signifies adherence to best practices and regulatory standards.
The program, which is one of the clinical research associate training programs, encompasses comprehensive training on crucial topics such as regulatory guidelines, ethical considerations, and study management, equipping CRAs with the essential knowledge for effective performance in their roles.
Furthermore, organizations like bioaccess provide extensive trial management services—including feasibility studies, compliance assessments, project oversight, and reporting—enabling CRAs to adeptly navigate the complexities of medical investigations.
As Jan S. Peterson, Vice President of Regulatory Affairs and Quality for Global Regulatory Partners, Inc., articulates, "Ultimately, certification showcases one’s dedication to the area of health studies."
Achieving ACRP certification can significantly enhance career advancement, establishing it as a strategic advantage for professionals aspiring to excel in the competitive realm of medical studies. Notably, the average pass rate for first-time test takers stands at 73 percent, reflecting both the rigorous standards and the preparedness instilled in candidates.
The SOCRA Certified Clinical Research Professional (CCRP) Online Course is an essential program for professionals aiming to validate their expertise in clinical trial studies through clinical research associate training programs. This comprehensive course, part of clinical research associate training programs, delves into critical elements such as:
All of which are fundamental for executing successful research initiatives. By participating in clinical research associate training programs, Clinical Research Associates (CRAs) not only enhance their grasp of industry standards but also significantly bolster their professional credibility. This elevated recognition positions them as formidable candidates in a competitive job market, particularly as the demand for skilled professionals in medical research continues to grow alongside the need for expedited regulatory approvals and effective patient enrollment strategies.
Furthermore, clinical research associate training programs equip CRAs with the essential knowledge to adeptly navigate study management processes, encompassing:
This ultimately contributes to the success of Medtech and Biopharma initiatives.
Fastrack offers a fast-tracked training program that is one of the clinical research associate training programs designed for individuals eager to swiftly enter the workforce in clinical research. This intensive curriculum encompasses essential skills required for entry-level positions, such as:
Participants engage in practical project experiences and receive tailored guidance, ensuring they are thoroughly prepared for immediate job opportunities across various healthcare settings. Graduates frequently secure positions shortly after completing the course, with many reporting job offers within weeks. The program's effectiveness is underscored by endorsements from industry leaders, who emphasize the critical importance of practical skills and adaptability in the evolving healthcare landscape.
With the medical study market projected to surpass $80 billion by 2025, the demand for skilled professionals continues to rise, making clinical research associate training programs a valuable investment for those aspiring to become Clinical Study Associates.
The University of Washington Clinical Trials Certificate Program establishes a robust foundation in clinical research methodologies and practices, equipping participants with essential knowledge of the entire clinical trial process, from study design to data analysis. This program is particularly advantageous for individuals aiming to enhance their qualifications through clinical research associate training programs for Clinical Research Associate (CRA) positions.
Success stories from former participants underscore the initiative's effectiveness in improving job placement rates, with many graduates securing roles in esteemed organizations shortly after completion. Furthermore, updates for 2025 include enhanced education in experimental methodologies, ensuring that participants are well-prepared for the evolving landscape of medical studies.
The learning outcomes of clinical research associate training programs are meticulously designed to cultivate critical thinking and practical skills, rendering them a valuable asset for aspiring CRAs and significantly contributing to their professional development in the field.
The bioaccess clinical research associate training programs provide aspiring research associates with a unique opportunity to gain practical experience in research management. Participants engage in exercises that replicate real-world scenarios, enabling them to apply theoretical knowledge within a controlled setting. This hands-on approach not only equips graduates with the essential skills needed to excel as CRAs but also emphasizes the role of clinical research associate training programs in enhancing research studies.
With bioaccess® generating $25K in patient savings and accelerating PMA data submissions by 11 months, the training initiative prepares CRAs to tackle challenges in patient recruitment, thereby supporting the success of medtech and biopharma startups.
Furthermore, the comprehensive trial management services provided by bioaccess—including:
highlight the crucial role CRAs play in advancing healthcare outcomes and fostering economic growth through international collaboration.
The DIA Clinical Investigation Training Online course offers a comprehensive examination of trial processes, encompassing vital aspects such as study design, regulatory requirements, and data management. This program, specifically the clinical research associate training programs, is tailored for both newcomers and seasoned professionals, equipping participants with essential knowledge that can be directly applied to their current roles or leveraged for future career advancements.
Notably, thorough education in medical studies has been shown to significantly enhance career progression, with recent surveys indicating that clinical research associate training programs related to medical trials have surged to 37.84%. Furthermore, industry leaders underscore the importance of understanding regulatory frameworks and effective study design, which are crucial for successful trials.
As the landscape of medical investigation continues to evolve, this initiative stands as an invaluable resource for professionals aiming to advance their careers through clinical research associate training programs.
The Barnett International clinical research associate training programs are meticulously crafted to elevate the skills of trial associates and coordinators. This program explores advanced topics such as project management, regulatory compliance, and innovative patient recruitment strategies. By engaging in this training, professionals not only refine their skills but also significantly enhance their effectiveness in healthcare roles.
Industry leaders assert that effective project management is vital in medical research, with insights underscoring that 'operations keep the lights on, strategy provides a light at the end of the tunnel, but project management is the train engine that moves the organization forward,' as articulated by Joy Gumz.
Success stories from participants illustrate that advanced training leads to improved project outcomes and greater adaptability in dynamic healthcare environments. For instance, one participant reported a 30% increase in project efficiency after completing the course.
As we approach 2025, the significance of clinical research associate training programs for study associates cannot be overstated, as they provide the essential tools to navigate the complexities of contemporary medical experiments.
With bioaccess®'s capabilities, including the ability to enroll treatment-naive cardiology or neurology cohorts 50% faster and achieve $25K savings per patient through FDA-ready data, professionals can effectively leverage these insights to tackle patient recruitment challenges.
A goal without a timeline is merely a dream, reinforcing the necessity for structured training that prepares professionals for the challenges that lie ahead.
The ProRelix Education CRA Online Certification Program serves as a robust entry-level training pathway within clinical research associate training programs for aspiring Clinical Research Associates. This comprehensive curriculum encompasses essential topics such as:
By equipping participants with crucial knowledge and skills, clinical research associate training programs significantly enhance their readiness for entry-level positions in healthcare studies, ultimately contributing to improved job placement outcomes. Insights from industry leaders underscore the critical importance of foundational knowledge in trial processes, further validating the program's role in cultivating the next generation of CRAs.
The Global CCRA® Exam Committee Certification stands as a pivotal credential for aspiring trial associates, affirming their expertise and commitment to professional excellence. Attaining this certification not only reflects adherence to industry standards but also significantly boosts career prospects in a competitive job market.
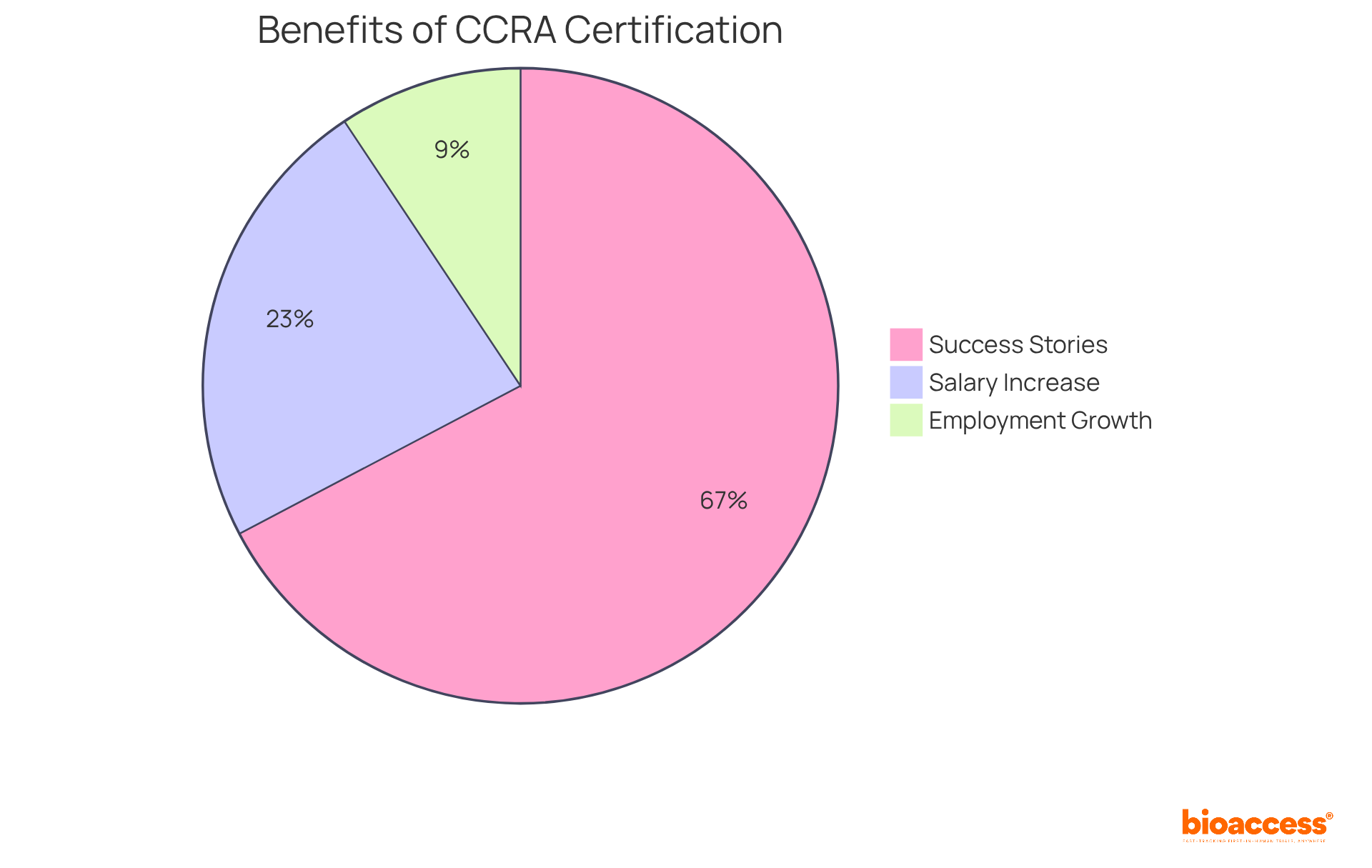
Preparation for the exam demands a thorough understanding of medical study principles, regulatory frameworks, and ethical practices, typically involving a study period of three to six months. Numerous success stories highlight this journey, with many Clinical Research Associates (CRAs) reporting substantial career advancements following certification.
Notably, approximately 72% of respondents revealed that they secured their first CRA position at larger organizations that offered comprehensive clinical research associate training programs, underscoring the increasing recognition of certification's value within the industry. As Brian Achille aptly states, 'Certification not only boosts your credibility but also opens doors to better job opportunities, higher salaries, and career advancement.'
Furthermore, certified professionals earn 20-30% more than their uncertified counterparts, further accentuating the advantages of certification. With the employment rate for Clinical Research Associates anticipated to grow by 10% annually, pursuing clinical research associate training programs to obtain the Global CCRA certification in 2025 represents more than a mere credential; it is a strategic decision for those committed to excelling in clinical research.
Additionally, maintaining certification necessitates ongoing education, ensuring professionals remain informed about industry developments and continue to refine their skills.
The landscape of clinical research is rapidly evolving, and the significance of specialized training programs for Clinical Research Associates (CRAs) is paramount. These programs not only impart essential skills and knowledge but also substantially enhance career prospects in an increasingly competitive field. Aspiring CRAs who engage in comprehensive training are strategically positioned to meet the industry's demands and contribute meaningfully to advancements in healthcare.
This article has highlighted various training programs, each offering unique benefits tailored to different stages of a CRA's career. From the hands-on experience provided by the bioaccess® program to the industry-recognized credentials of the ACRP certification, these educational opportunities equip participants with the necessary tools for success. The emphasis on practical skills, regulatory knowledge, and ethical considerations is evident across all programs, underscoring their relevance in the current healthcare landscape.
As the demand for skilled professionals in clinical research continues to grow, investing in these training programs emerges as a strategic decision for those aiming to excel. The insights and certifications gained from these courses not only enhance individual careers but also contribute to the overall efficiency and effectiveness of clinical trials. For anyone considering a career as a Clinical Research Associate, now is the time to explore these essential training programs and take the first step toward a rewarding future in medical research.
What is the bioaccess® Clinical Research Training Program?
The bioaccess® Clinical Research Training Program is designed to empower aspiring clinical research associates (CRAs) with essential skills for the Medtech sector, focusing on regulatory adherence, study management, and patient safety.
What services does bioaccess® provide for clinical studies?
bioaccess® offers comprehensive management services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
How has bioaccess® impacted recruitment efficiency in clinical studies?
Organizations like GlobalCare Clinical Studies have reported a reduction in recruitment time by over 50% and savings of $25K per patient by collaborating with bioaccess®, which highlights the effectiveness of strategic partnerships in clinical research.
What is the ACRP Clinical Research Associate Certification Program?
The ACRP Clinical Research Associate Certification Program is a pathway for CRAs to gain industry-recognized qualifications, validating their expertise and commitment to best practices and regulatory standards in health studies.
What topics are covered in the ACRP certification program?
The program covers regulatory guidelines, ethical considerations, and study management, equipping CRAs with the necessary knowledge for effective performance in their roles.
What is the average pass rate for first-time test takers of the ACRP certification?
The average pass rate for first-time test takers of the ACRP certification is 73 percent.
What does the SOCRA Certified Clinical Research Professional (CCRP) Online Course focus on?
The SOCRA CCRP Online Course focuses on Good Clinical Practice (GCP), regulatory compliance, and ethical considerations, which are fundamental for successful clinical trial studies.
How do clinical research associate training programs benefit CRAs?
These programs enhance CRAs' understanding of industry standards, bolster their professional credibility, and prepare them to navigate study management processes effectively, making them competitive candidates in the job market.