


The article titled "10 Essential Clinical Trial Coordinator Duties for Research Success" outlines the critical responsibilities that clinical trial coordinators must fulfill to guarantee the success of research studies. It emphasizes that effective coordination is paramount, encompassing duties such as:
These elements are crucial for enhancing research efficiency and upholding ethical standards, ultimately leading to improved overall study outcomes.
The landscape of clinical trials is intricate and demanding, where the success of research hinges on meticulous coordination and adherence to ethical standards. This article explores the ten essential duties of a clinical trial coordinator, illuminating how these responsibilities not only facilitate smoother operations but also enhance the integrity and efficiency of research studies.
With increasing pressures to deliver results faster and more effectively, coordinators face the critical challenge of ensuring compliance with regulatory requirements while also addressing participant needs. How can they drive innovation in medical research amidst these demands?
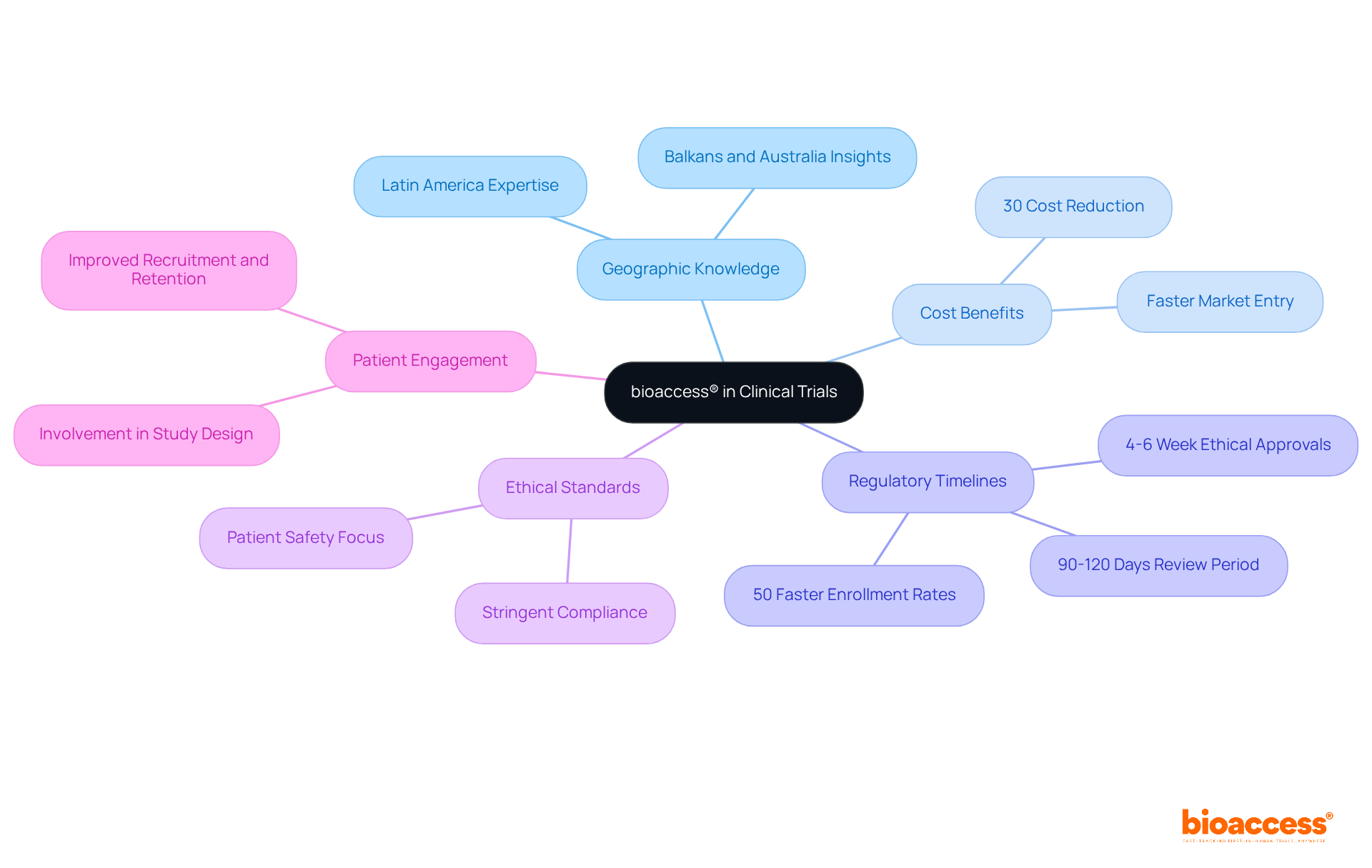
bioaccess® strategically harnesses its extensive knowledge across Latin America, the Balkans, and Australia to deliver exceptional coordination of research studies. By capitalizing on Colombia's competitive advantages—such as cost reductions exceeding 30% compared to North America and Western Europe, regulatory review periods of only 90-120 days, and a healthcare system recognized globally for its excellence—bioaccess® accelerates research studies while upholding stringent ethical standards. This approach facilitates ethical approvals within a mere 4-6 weeks and achieves enrollment rates that are 50% faster than those in traditional markets, positioning bioaccess® as an indispensable partner for innovators in the Medtech, Biopharma, and Radiopharma sectors.
The expert coordination provided by bioaccess® is vital for navigating the intricate landscape of clinical trial coordinator duties in medical studies. It guarantees that every aspect is managed with precision and efficiency, including the clinical trial coordinator duties, from regulatory compliance to patient engagement. Industry specialists emphasize that leveraging regional regulatory benefits significantly enhances research timelines and overall success rates, solidifying bioaccess®'s position as a leader in research facilitation. Furthermore, integrating patient participation in study design is essential, as it leads to improved recruitment and retention, ultimately contributing to the success of medical studies. Directors of Clinical Research are encouraged to reflect on how these insights can be applied to elevate their own research strategies.
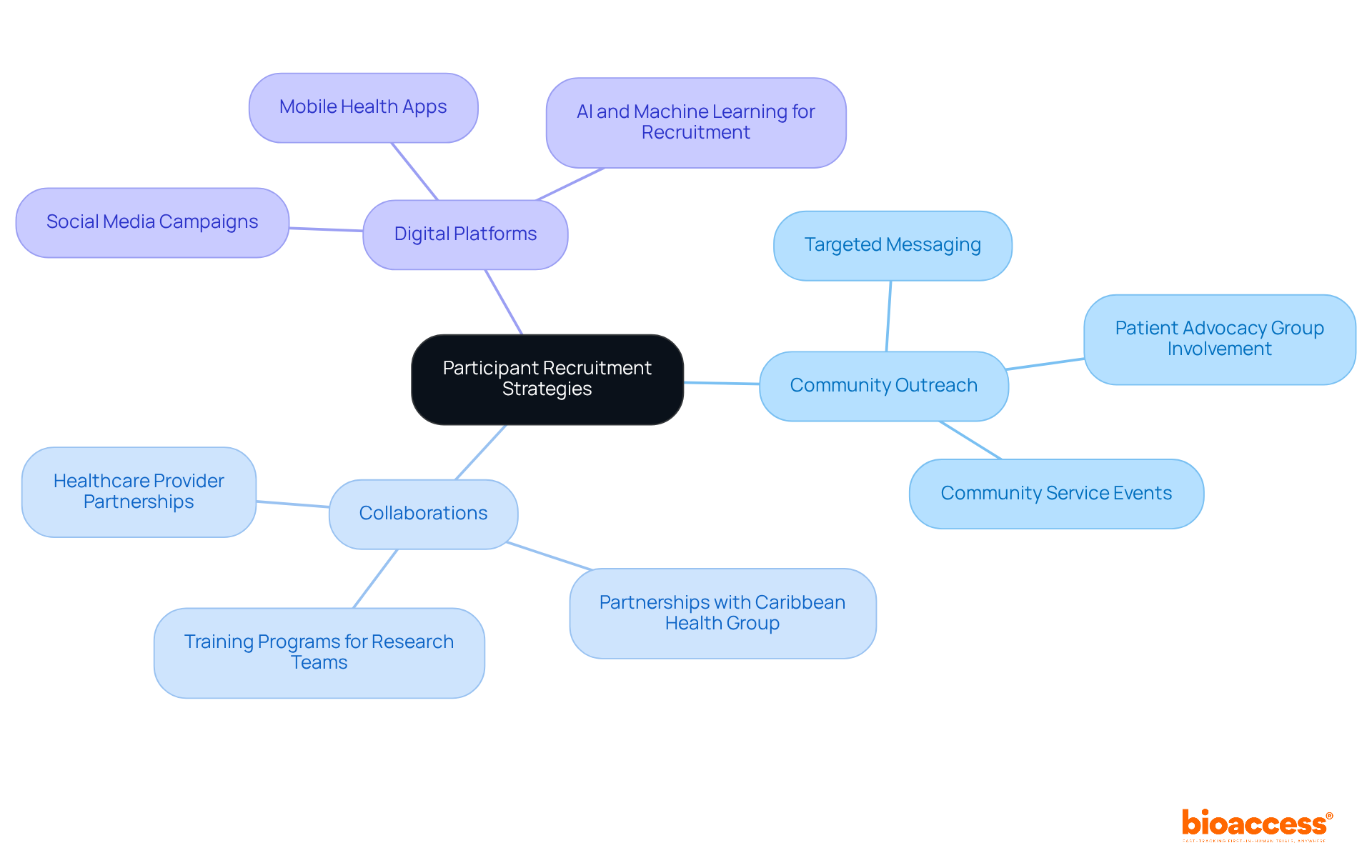
Enlisting the appropriate subjects is crucial for upholding the integrity of research studies. bioaccess™ implements targeted recruitment strategies that prioritize community outreach, collaborations with healthcare providers, and the use of digital platforms to engage diverse populations. This method is further enhanced by the partnership with Caribbean Health Group, aimed at establishing Barranquilla as a premier location for clinical research in Latin America, backed by Colombia's Minister of Health. By examining the demographics and health profiles of prospective individuals, bioaccess™ effectively aligns candidates with study criteria, greatly enhancing the likelihood of successful enrollment.
The significance of clear communication cannot be emphasized enough; bioaccess™ guarantees that prospective individuals fully grasp the study's objectives, processes, and possible advantages. This approach not only fosters trust but also enhances participant retention. Studies have shown that effective outreach can lead to a 25% increase in enrollment rates, particularly when tailored to the specific needs of diverse communities. By utilizing these insights, bioaccess™ is dedicated to closing awareness and access gaps, ultimately aiding in more equitable medicine in research studies. Moreover, it is essential to recognize that almost 70% of delays in the process stem from recruitment challenges, highlighting the necessity for strong strategies in this domain. The extensive research management services provided by bioaccess™, which encompass clinical trial coordinator duties including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, further improve the recruitment process and overall project success.
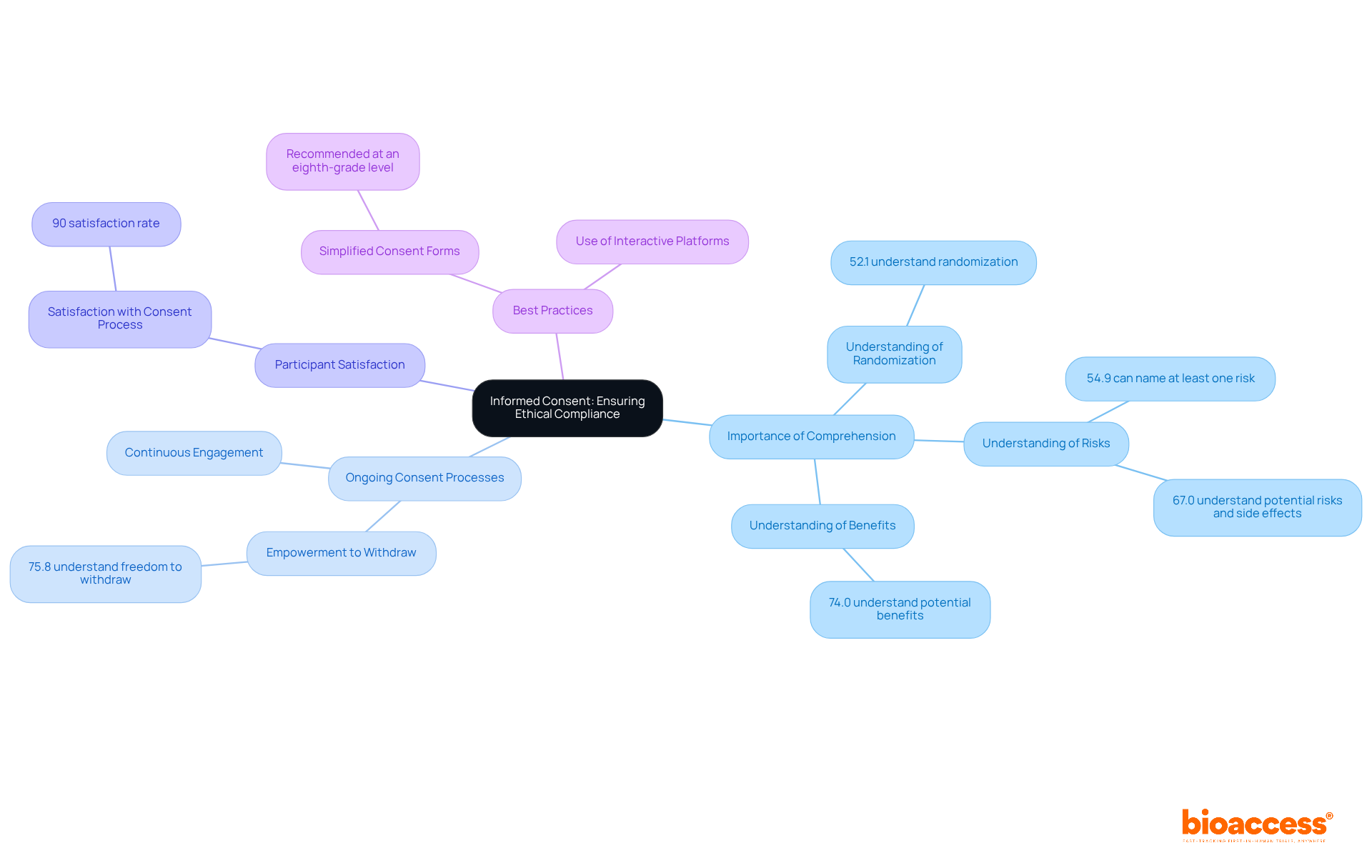
Informed consent stands as a fundamental pillar of clinical trials, ensuring that individuals fully grasp the nature of the research, including its associated risks and benefits. At bioaccess®, we emphasize this process by providing comprehensive information in straightforward, understandable language, enabling individuals to engage meaningfully with the material. This approach is crucial, as research indicates that comprehension of informed consent elements can vary significantly; only 52.1% of individuals understand the concept of randomization, while 54.9% can identify at least one risk linked to their participation. In fact, understanding of informed consent components ranges from 52.1% to 75.8%, underscoring the necessity for effective communication strategies.
To further enhance ethical compliance, bioaccess® implements ongoing consent processes, empowering individuals with the autonomy to withdraw from the study at any time. This practice not only fortifies ethical standards but also fosters trust and transparency among contributors and researchers. As emphasized by bioethicists, ensuring that individuals are well-informed and comfortable with their choices is vital for preserving the integrity of clinical research. In a survey of nearly 300 cancer trial participants, approximately 90% reported satisfaction with the consent process, underscoring the effectiveness of our practices. By adopting best practices in informed consent, we contribute to a more ethical and participant-centric research environment.
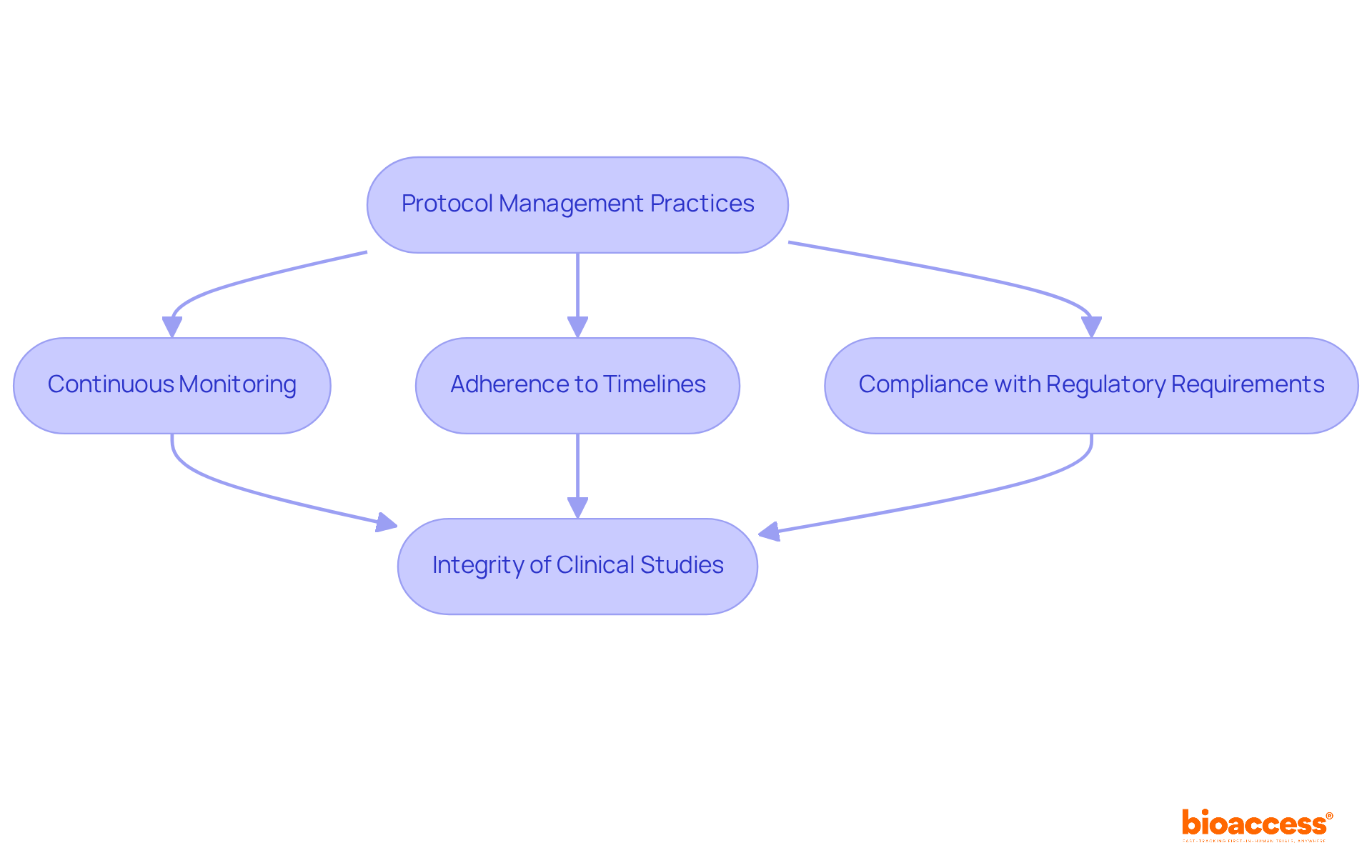
bioaccess® enforces rigorous protocol management practices to guarantee that all research activities are in strict conformity with the approved protocols. This involves continuous monitoring of study progress, adherence to established timelines, and compliance with regulatory requirements. Utilizing advanced project management tools, the organization effectively tracks milestones and identifies potential issues early, facilitating timely interventions.
The importance of protocol compliance cannot be exaggerated; it is vital for preserving the integrity of clinical studies and ensuring the reliability of gathered data. Research shows that strict compliance with protocols is essential for preventing data manipulation and bias, which can jeopardize study results. For instance, a study indicated that a median protocol adherence rate of 91.5% in superiority trials correlates with sound conclusions and informed decision-making in healthcare.
Successful examples of protocol adherence illustrate its influence on medical research. In one case study, effective communication with individuals significantly improved adherence rates, showcasing how comprehensive information fosters compliance and allows them to voice concerns. This proactive approach not only improves data integrity but also safeguards the rights and well-being of study participants, reinforcing the ethical foundation of medical research.
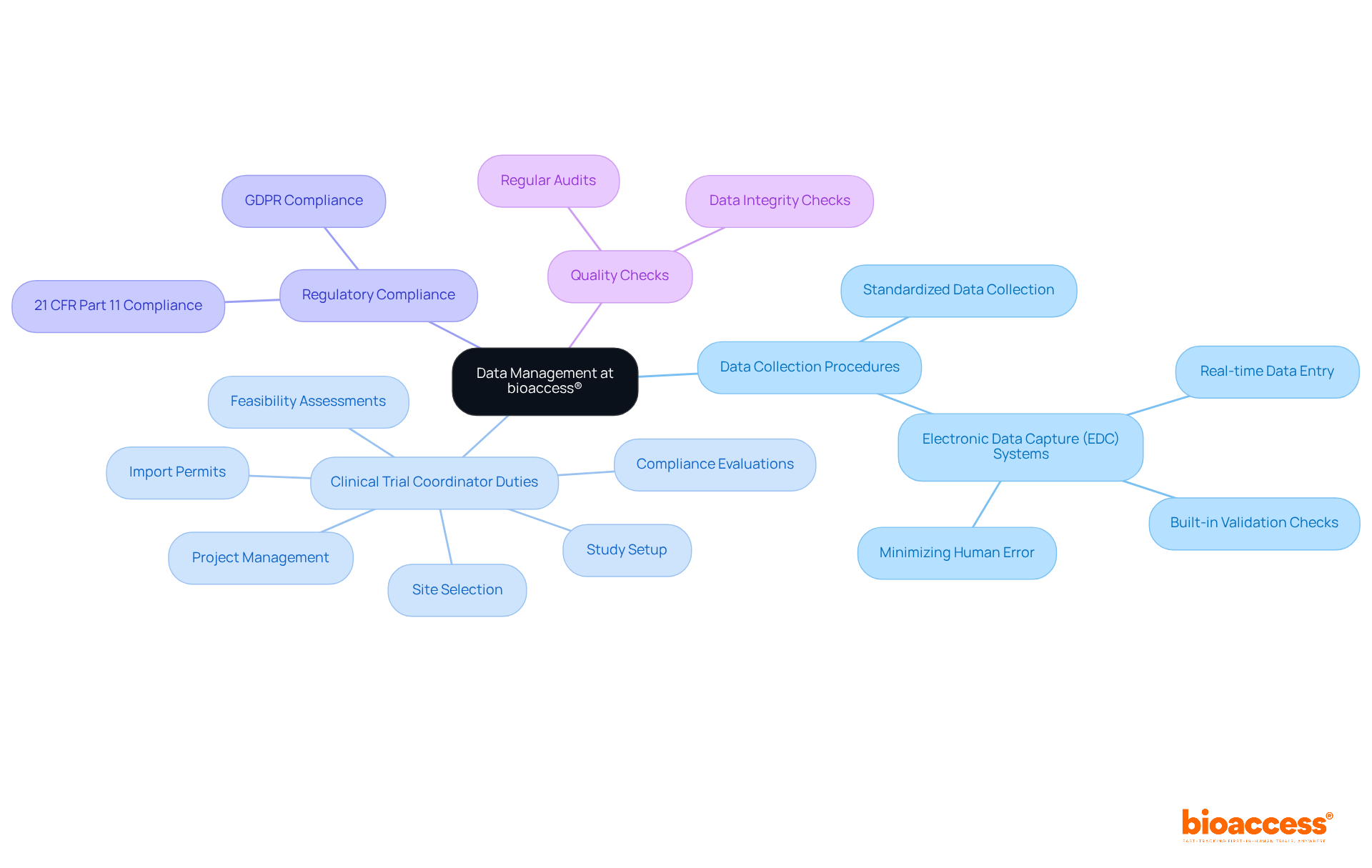
At bioaccess®, data management is meticulously crafted to uphold the accuracy, reliability, and security of research data. The organization employs standardized data collection procedures in conjunction with advanced electronic data capture (EDC) systems, which significantly minimize errors and streamline documentation processes. These systems not only facilitate real-time data entry but also incorporate built-in validation checks, thereby enhancing data accuracy and integrity.
In addition to robust data management, bioaccess® excels in comprehensive research study management services that encompass clinical trial coordinator duties. The clinical trial coordinator duties include:
Regular audits and quality checks are vital for maintaining data integrity throughout the process, ensuring compliance with regulatory standards such as 21 CFR Part 11 and GDPR. This rigorous approach safeguards sensitive patient information and enhances the overall quality of research outcomes.
By prioritizing effective data management practices and implementing a robust data management plan, bioaccess® positions itself as a leader in delivering high-quality research. This commitment ultimately contributes to the successful advancement of innovative medical solutions.
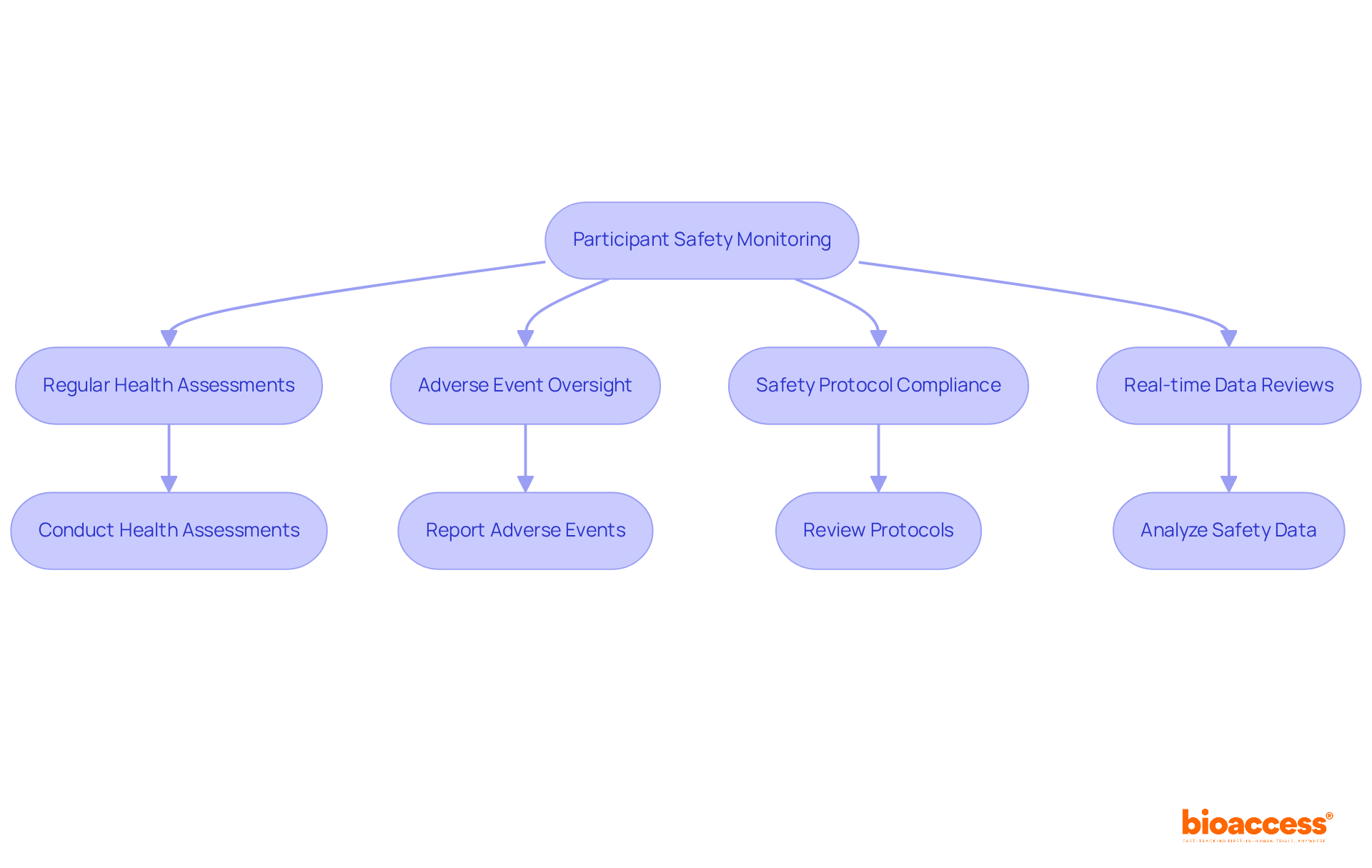
bioaccess® underscores the critical importance of safety monitoring for subjects as a cornerstone of the research process. This commitment includes regular health assessments, vigilant oversight for adverse events—both serious and non-serious—and strict adherence to established safety protocols. A dedicated safety monitoring team conducts real-time data reviews, enabling swift action in response to any emerging safety concerns.
In the Medtech and Biopharma industries, efficient safety oversight methods are paramount, as the reliability of study results hinges on the well-being of participants. By implementing robust safety protocols alongside comprehensive management services—including feasibility assessments, site selection, compliance reviews, setup, and project management—bioaccess® not only enhances the reliability of results but also fosters trust and confidence among participants, which is essential for successful outcomes.
The importance of comprehensive safety monitoring is further emphasized by industry experts who assert that prompt reporting of adverse events is vital for safeguarding individuals' well-being and maintaining the credibility of clinical research. Optimal methods for evaluating individual well-being during experiments involve thorough health assessments and ongoing dialogue with subjects, which are crucial for early identification of potential risks and upholding ethical standards throughout the research. Additionally, oversight committees play a pivotal role in ensuring subject safety by reviewing trial protocols and monitoring compliance with safety standards. The incorporation of frameworks like the Aggregate Safety Assessment Plan (ASAP) can significantly enhance the systematic approach to safety data integration and analysis.
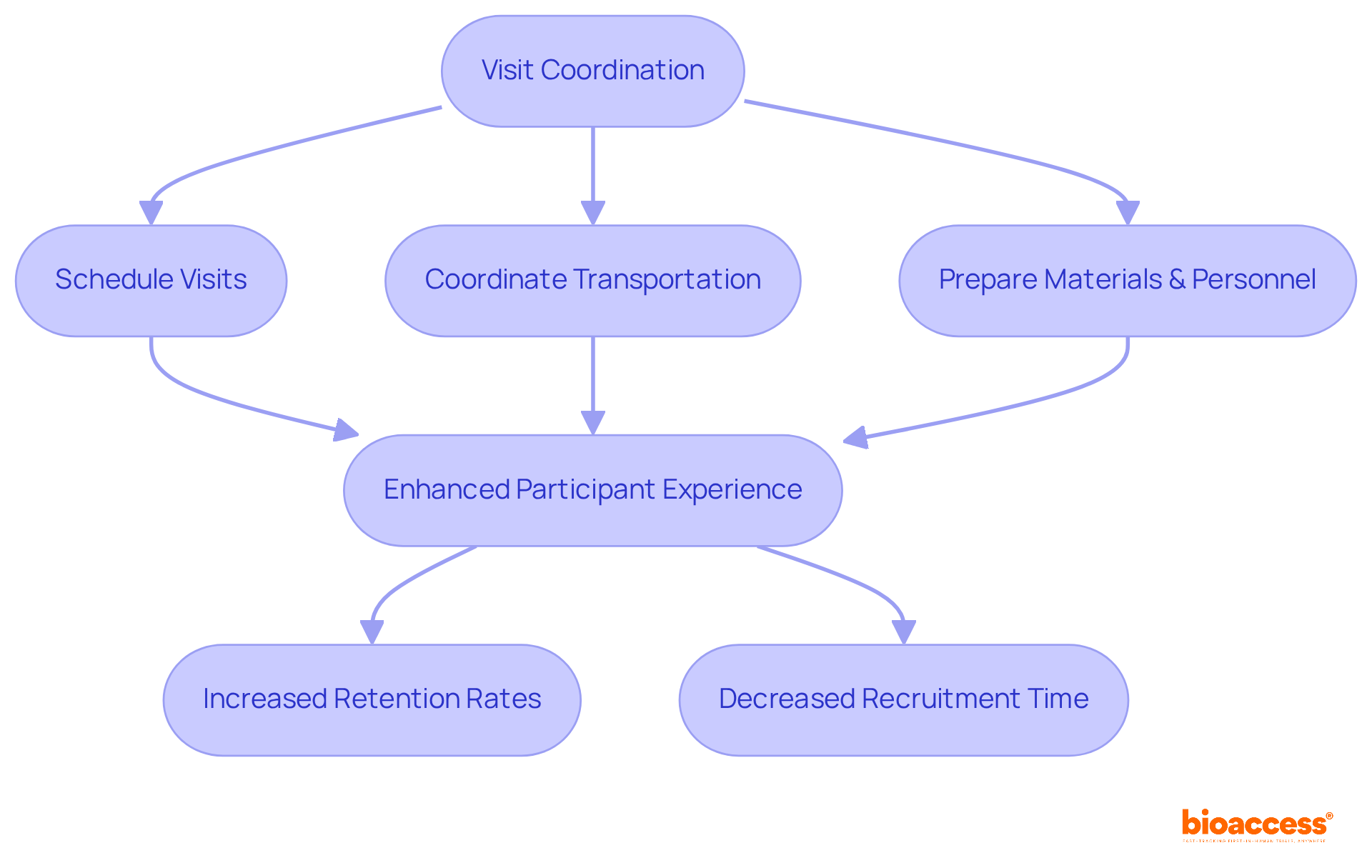
bioaccess® excels in visit coordination, meticulously planning and executing all logistical aspects to ensure a seamless experience for attendees. This includes:
Efficient logistics administration is essential; research indicates that logistical obstacles significantly impact attendee retention. For instance:
By optimizing the visit process, bioaccess® not only improves participant experience but also encourages greater retention rates, ultimately aiding the success of research studies.
Case studies reveal that addressing logistical challenges, such as providing transportation options, can significantly enhance participation rates, particularly among demographics facing mobility issues. This commitment to logistics is further strengthened by bioaccess®'s collaboration with Caribbean Health Group, aimed at establishing Barranquilla as a premier location for medical research in Latin America. Additionally, the partnership with GlobalCare Clinical Trials has resulted in over a 50% decrease in recruitment time and 95% retention rates. This initiative is also supported by Colombia's Minister of Health, ensuring that participants can focus on their health and the study itself, rather than the complexities of travel and scheduling.
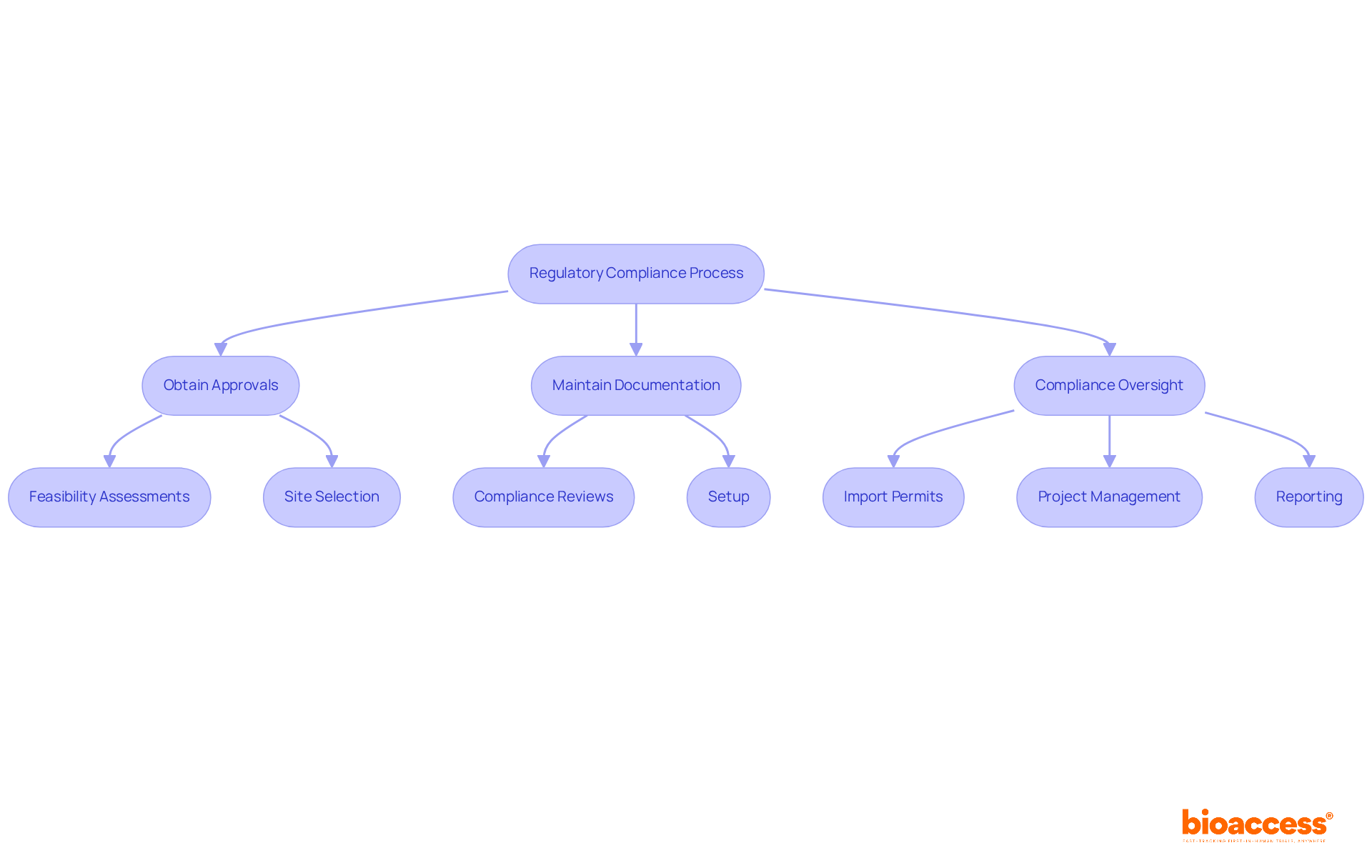
bioaccess® is dedicated to expertly navigating the intricate landscape of regulatory compliance, a crucial factor in the success of medical studies. The organization meticulously monitors both local and international regulations, ensuring that all medical studies adhere to the necessary legal standards. This encompasses obtaining approvals from ethics committees and regulatory bodies, alongside maintaining comprehensive documentation to support compliance initiatives.
Beyond regulatory oversight, bioaccess® offers a wide range of management services for studies, which include clinical trial coordinator duties such as:
These services are designed to streamline the research process, empowering Medtech, Biopharma, and Radiopharma startups to accelerate their studies and achieve regulatory approval with greater efficiency.
The emphasis on regulatory adherence not only mitigates risks but also significantly enhances the credibility of research studies. Research indicates that compliance with regulations can lead to increased success rates in medical studies, with findings showing that the involvement of non-industrial collaborators can improve success rates by over 11%. Furthermore, effective navigation of legal requirements is essential, as approximately 80% of medical studies fail to meet initial enrollment targets, often due to regulatory hurdles. By fostering a culture of adherence, bioaccess® positions itself as a frontrunner in facilitating successful research outcomes in healthcare. To further bolster compliance, organizations should consider implementing regular compliance training for their teams.
With the expertise of specialists like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, an authority in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, bioaccess® is well-equipped to support research coordinators in fulfilling their clinical trial coordinator duties, ensuring that studies are conducted efficiently and in full compliance with all regulatory standards.
bioaccess® emphasizes the continuous training and development of its trial personnel through comprehensive onboarding programs and ongoing education. This commitment includes regular updates on regulatory changes and specialized training in critical areas such as data management and participant safety. By fostering a culture of learning, bioaccess® ensures that its team is well-prepared to navigate the complexities of clinical research, particularly in relation to the clinical trial coordinator duties associated with overseeing diverse projects such as Early-Feasibility Trials, First-In-Human Trials, and Post-Market Clinical Follow-Up Trials.
This dedication to employee training significantly enhances the execution of studies, which includes the clinical trial coordinator duties, such as setup, compliance reviews, and project management. It nurtures a positive work atmosphere, essential for sustaining team morale and productivity. Research indicates that organizations investing in continuous education experience improved outcomes; for instance, a study found that 95.7% of healthcare professionals believed that training programs enhanced the quality of care they provided. Furthermore, placing seasoned experts into permanent positions at research locations has been shown to stabilize operations and enhance team unity, ultimately resulting in improved study outcomes.
Insights from research leaders underscore that forming skilled teams through efficient onboarding and ongoing education is paramount for the success of trials. This strategic approach not only addresses immediate operational requirements but also equips personnel for forthcoming challenges in the evolving landscape of clinical research, thereby enhancing the overall success of clinical trials and their positive impact on local economies.
bioaccess® recognizes that effective reporting of research is crucial for advancing medical knowledge and improving patient outcomes. The organization plays a pivotal role in assisting researchers with the preparation of comprehensive study reports and manuscripts for publication in peer-reviewed journals. This support encompasses data analysis, result interpretation, and strict compliance with publication standards, which are essential for ensuring the integrity and credibility of medical research.
By facilitating the distribution of research results, bioaccess® not only aids the wider scientific community but also significantly enhances the prominence and influence of its studies. Successful examples of research publications underscore the importance of timely and transparent reporting; studies indicate that only 29% of completed clinical trials conducted by faculty at major academic centers are published within two years. This statistic highlights the pressing need for organizations like bioaccess® to champion the timely sharing of results, thereby fostering a culture of accountability and progress in medical research.
The role of a clinical trial coordinator is pivotal in ensuring the success of research studies. This article has explored the essential duties that these professionals undertake, emphasizing their impact on the integrity and efficiency of clinical trials. By effectively managing participant recruitment, ensuring informed consent, overseeing protocol adherence, and maintaining rigorous data management practices, clinical trial coordinators play a crucial role in advancing medical research.
Key insights highlighted include:
Moreover, the article underscores how organizations like bioaccess® leverage regional advantages to streamline regulatory compliance and improve study logistics, ultimately enhancing the overall research experience.
In light of these discussions, it is clear that investing in the training and development of clinical trial coordinators is essential for fostering a competent workforce capable of navigating the complexities of clinical research. As the landscape of medical trials continues to evolve, embracing best practices in coordination and management will not only facilitate more efficient studies but also contribute to the ethical advancement of medical knowledge. Engaging with these insights presents an opportunity for stakeholders to enhance their research strategies and ensure that clinical trials remain a cornerstone of innovation in healthcare.
What is bioaccess® and what does it offer for clinical trials?
bioaccess® specializes in coordinating research studies across Latin America, the Balkans, and Australia, leveraging Colombia's advantages such as cost reductions, quick regulatory review periods, and a high-quality healthcare system to accelerate clinical trials while maintaining ethical standards.
How does bioaccess® enhance the speed of clinical trials?
bioaccess® accelerates clinical trials by achieving ethical approvals in 4-6 weeks and enrollment rates that are 50% faster than traditional markets, making it a valuable partner for Medtech, Biopharma, and Radiopharma sectors.
What strategies does bioaccess® use for participant recruitment?
bioaccess® employs targeted recruitment strategies that include community outreach, partnerships with healthcare providers, and digital platforms to engage diverse populations, thereby improving enrollment success.
How does bioaccess® ensure effective communication with potential participants?
bioaccess® guarantees that prospective participants understand the study's objectives, processes, and potential benefits, which fosters trust and enhances participant retention.
What role does informed consent play in bioaccess®'s clinical trials?
Informed consent is fundamental at bioaccess®, ensuring individuals understand the research's nature, risks, and benefits. The process includes clear communication and ongoing consent options, allowing individuals to withdraw at any time.
How does bioaccess® address the challenges of informed consent comprehension?
bioaccess® focuses on providing information in simple language and implements ongoing consent processes to enhance understanding and ensure participants are comfortable with their choices.
What are the benefits of integrating patient participation in study design according to bioaccess®?
Integrating patient participation in study design leads to improved recruitment and retention rates, contributing to the overall success of medical studies.
What are the key services provided by bioaccess® to support clinical trial management?
bioaccess® offers extensive research management services, including feasibility assessments, site selection, compliance evaluations, project oversight, and reporting, which enhance the recruitment process and project success.