


The article outlines ten essential responsibilities of clinical trial coordinators, pivotal for the success of research studies. These responsibilities encompass:
Evidence underscores their significant impact on improving recruitment rates, ensuring ethical standards, and enhancing overall study integrity. This comprehensive overview not only highlights the crucial roles coordinators play but also emphasizes the necessity of their expertise in navigating the complexities of clinical research.
Clinical trial coordinators are the unsung heroes behind the scenes of medical research, ensuring that each study runs smoothly and ethically. Their responsibilities are multifaceted, encompassing:
Each is crucial for the success of clinical trials. As the demand for effective coordination grows, so does the necessity to comprehend the essential skills and strategies that define a successful clinical trial coordinator.
What challenges do they face in this evolving landscape? How can they effectively navigate these challenges to drive research outcomes?
This article delves into the ten critical responsibilities that every clinical trial coordinator must master to excel in their role and contribute meaningfully to the advancement of medical science.
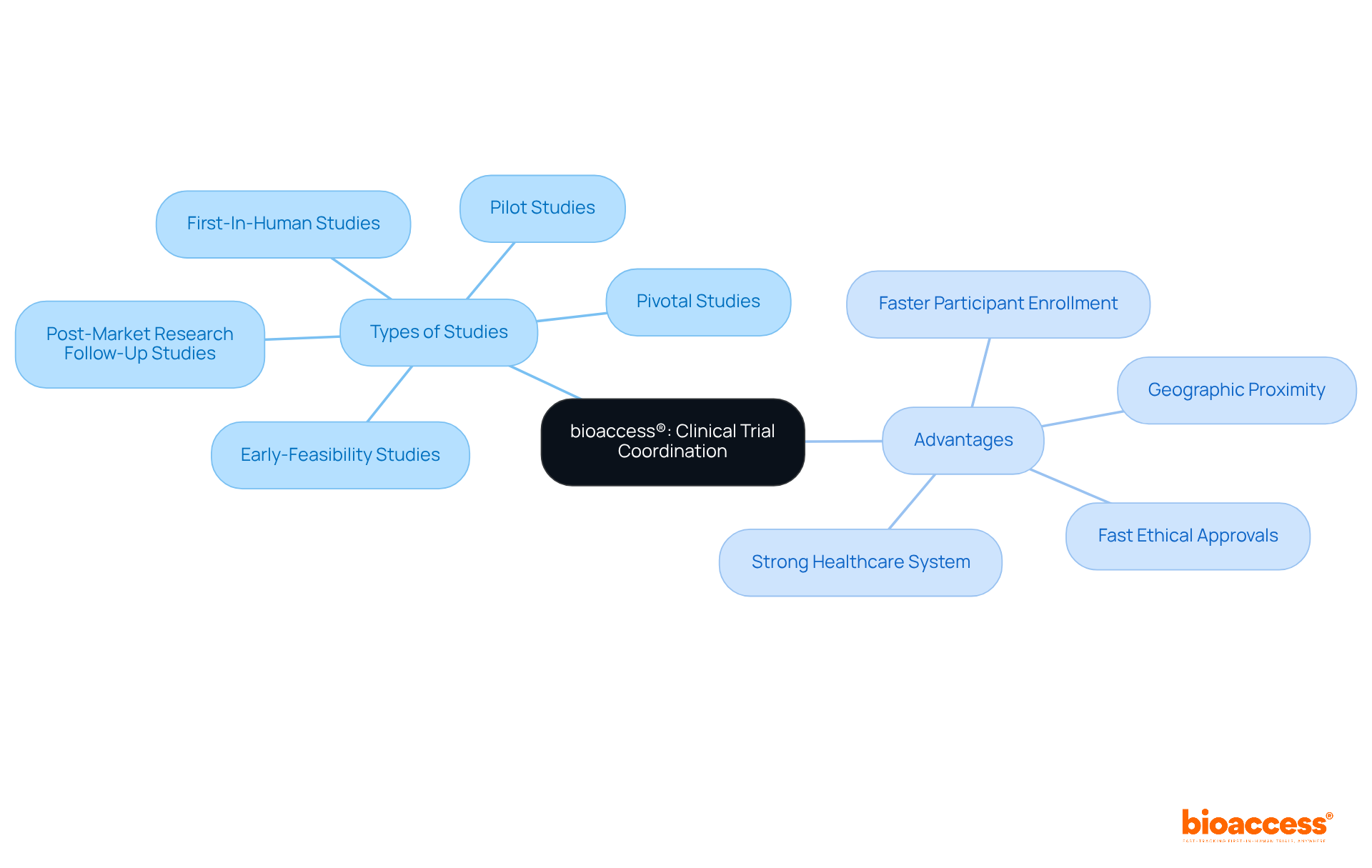
bioaccess® excels in coordinating studies within the research field by leveraging the regulatory efficiency of Latin America, particularly Colombia, which offers significant advantages for first-in-human research. With a strong emphasis on comprehensive research management services, bioaccess® specializes in:
This strategic focus allows bioaccess® to secure ethical approvals in an impressive timeframe of just 4-6 weeks, while achieving participant enrollment rates that are 50% faster than those in traditional markets. The geographic proximity and favorable time zone of Latin America further enhance its appeal for western biopharmaceutical firms, contributing to the region's growth in research studies.
Additionally, Colombia's healthcare system, recognized as one of the best in Latin America, facilitates efficient patient recruitment, supported by a population exceeding 50 million and 95% coverage under universal healthcare. However, challenges such as socio-economic barriers to informed consent remain critical considerations in the research landscape. Notably, annual funding in the research sector in Latin America has surged from $3-4 million to over $50 million, reflecting growing confidence in the region as a viable location for medical studies. Such remarkable agility is essential for Medtech, Biopharma, and Radiopharma innovators aiming to expedite product development and market entry, ensuring they maintain competitiveness in a rapidly evolving environment.
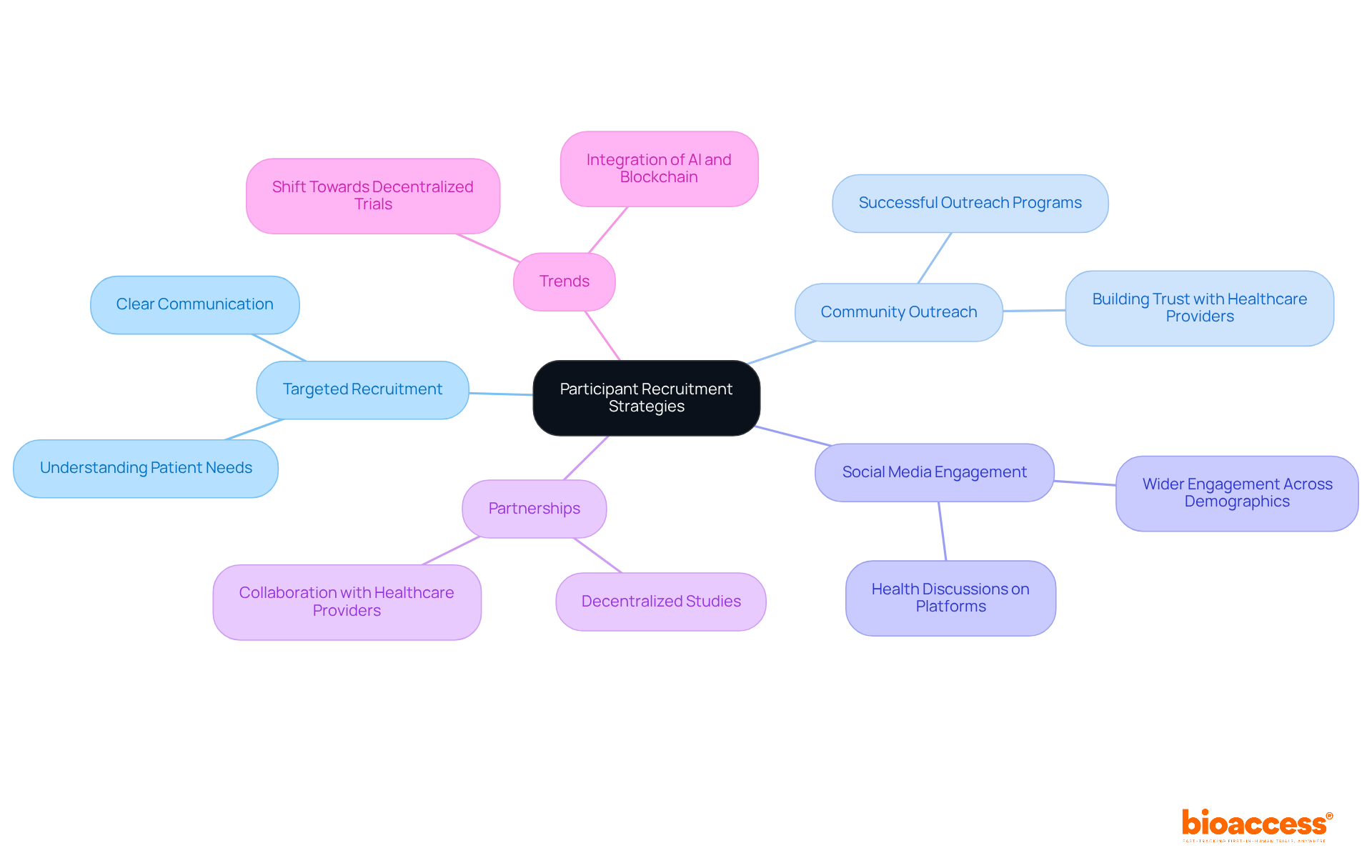
Finding appropriate individuals is crucial for fulfilling clinical trial coordinator responsibilities and ensuring the success of research studies. Coordinators must implement targeted recruitment strategies that resonate with potential candidates to fulfill clinical trial coordinator responsibilities. Successful community outreach programs can increase awareness and interest in clinical studies, while utilizing social media platforms enables wider engagement with various demographics. Working together with healthcare providers is essential for identifying qualified individuals and building trust.
Clear communication regarding the study's purpose, procedures, and benefits is vital, as it significantly enhances the clinical trial coordinator responsibilities in recruitment efforts. Recent trends indicate a shift towards decentralized studies, which can further enhance accessibility and participant diversity, ultimately resulting in more successful outcomes. Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a prominent location for research studies in Latin America, backed by Colombia's Minister of Health. This initiative not only enhances recruitment strategies but also contributes to local economic growth through job creation and improved healthcare services.
The collaboration has achieved over a 50% reduction in recruitment time and a 95% retention rate, showcasing its effectiveness. Moreover, media attention from Clinical Leader underscores the increasing enthusiasm for research studies in Latin America, stressing the significance of these initiatives. However, it is important to recognize that roughly 80% of research studies encounter delays or shutdowns because of recruitment problems, and 37% of research sites under-enroll participants. Incorporating insights from industry leaders, such as Scott Gray's emphasis on understanding patient needs, can further strengthen recruitment strategies.
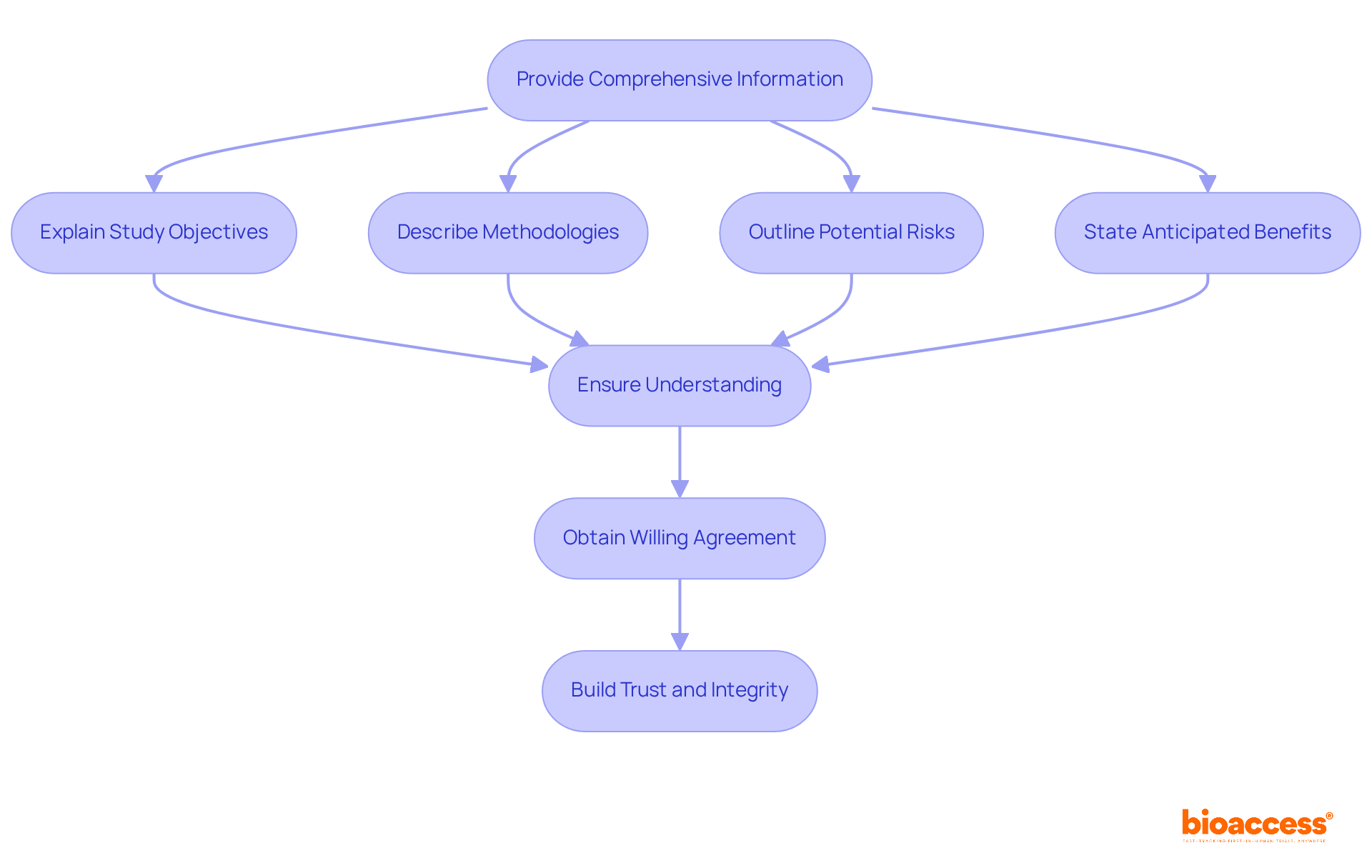
Obtaining informed consent is not merely a procedural formality; it is a fundamental responsibility of clinical trial coordinator responsibilities. This process requires providing prospective participants with comprehensive information about the study, including its objectives, methodologies, potential risks, and anticipated benefits. Coordinators must ensure that participants fully understand this information and willingly agree to participate. Such diligence not only fulfills ethical obligations but also fosters trust between participants and researchers, which is essential for the integrity of clinical research.
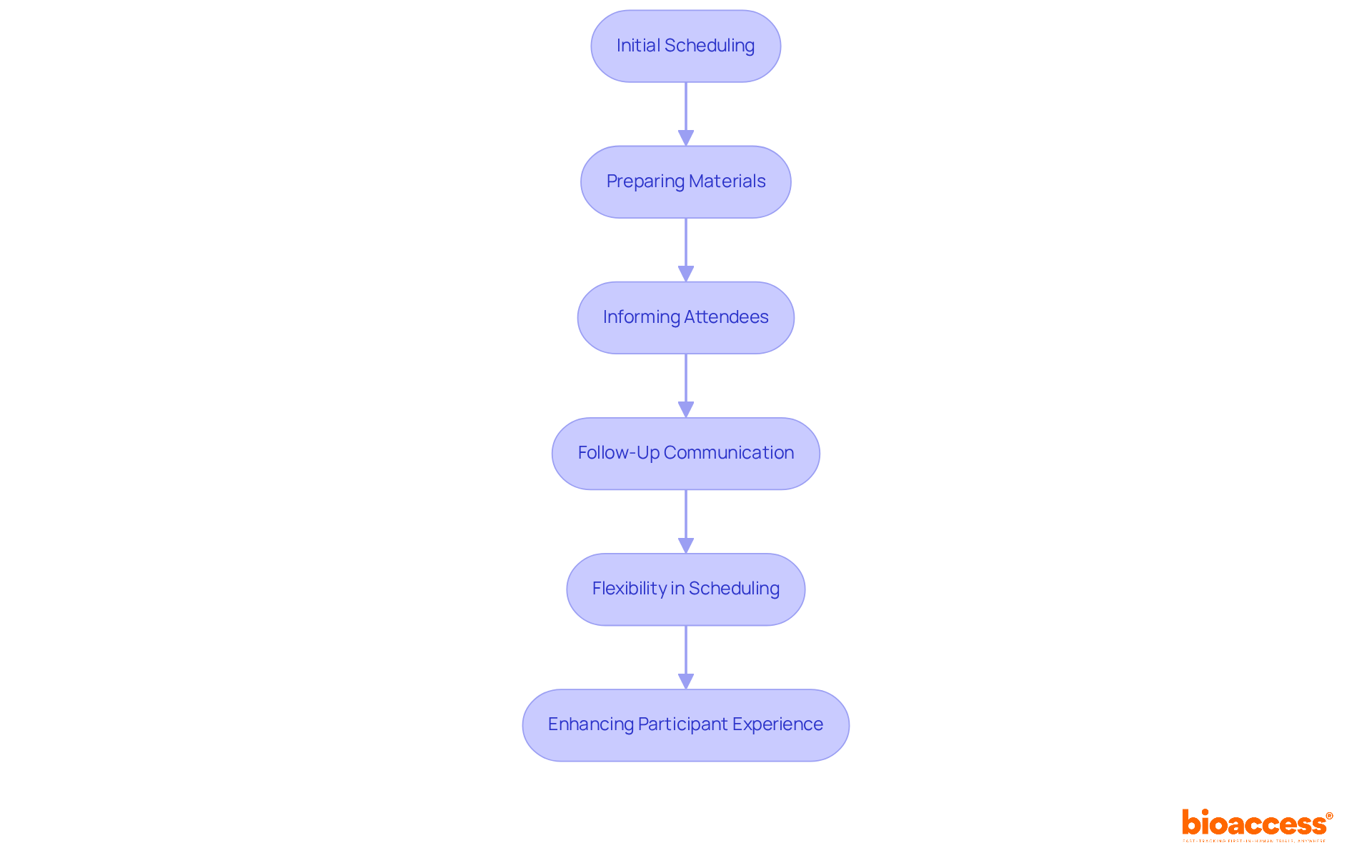
Organizing study visits necessitates careful planning and skilled handling of attendee interactions, a critical component in clinical research. Clinical trial coordinator responsibilities encompass playing a pivotal role in ensuring that visits are arranged effectively, accommodating attendees' availability while adhering to the research timeline. This preparation involves ensuring all essential materials are ready and that attendees are well-informed about what to expect. Effective communication is essential; studies indicate that timely follow-up can increase conversion rates by up to 60 times when initial contact occurs within 24 hours.
Flexibility in scheduling is equally vital, as logistical challenges can significantly impact participant retention—44% of individuals have reported finding travel to study clinics cumbersome, underscoring the necessity for convenient study designs. By applying optimal methods in engagement coordination, such as utilizing warm transfers to simplify scheduling, clinical trial coordinator responsibilities can significantly enhance the overall experience for participants and improve retention rates, which typically average around 30% dropout in research studies.
Ultimately, a considerate approach to handling participant interactions not only builds trust but also contributes to the success of research studies.
Clinical trial coordinator responsibilities include data oversight, which is a fundamental duty encompassing the precise collection, documentation, and organization of study information throughout the trial. Effective data handling systems are essential for ensuring data integrity and adherence to regulatory standards. Discrepancies in medical research databases can reach as high as 27%, underscoring the urgent need for meticulous oversight. Routine audits and checks are vital to confirm data precision and thoroughness. Research indicates that only 50% of research sites have a data oversight plan established, highlighting a significant gap in best practices.
Implementing robust data handling practices not only enhances the reliability of study outcomes but also aligns with industry standards, such as utilizing electronic data capture systems to minimize errors associated with manual data entry. Furthermore, bioaccess provides extensive research study oversight services, including:
These services are essential for upholding high standards in data handling and ensuring the integrity of research findings.
Training for personnel involved in data oversight is also crucial to ensure precise data interpretation and entry. By prioritizing data integrity and leveraging the comprehensive services offered by bioaccess, clinical trial coordinator responsibilities can help significantly enhance the quality and credibility of their findings. This commitment to excellence not only fosters trust in clinical research but also drives advancements in the Medtech landscape.
Regulatory adherence is paramount in research project oversight, requiring coordinators to be thoroughly informed about the ethical guidelines and regulations governing medical investigations. This includes obtaining necessary approvals from regulatory bodies and ensuring that all study activities align with established protocols.
Bioaccess offers comprehensive clinical study management services, encompassing:
Notably, compliance rates have markedly improved since 2017, with reporting rates climbing from 8.3% to 23.2% within just 12 months of study completion. Despite these advancements, challenges persist, as many assessments continue to encounter issues with timely reporting due to factors such as ambiguous guidance and technical difficulties.
To navigate these complexities effectively, coordinators must remain vigilant about regulatory changes and foster open communication with regulatory agencies. This proactive approach not only facilitates smoother testing operations but also bolsters the ethical integrity of the research process.
The efficient preparation of research groups is critical for the success of medical studies. Coordinators must establish comprehensive training programs that encompass all facets of study oversight, which includes the clinical trial coordinator responsibilities such as:
This is particularly significant given the extensive clinical trial coordinator responsibilities linked to research management, such as:
Regular training sessions and workshops not only enhance team skills and knowledge but also cultivate a collaborative environment that propels high-quality research outcomes.
Current trends indicate a shift towards adaptable, online learning platforms, allowing teams to engage in training at their convenience, thereby minimizing disruptions to ongoing projects. Furthermore, investing in specialized training initiatives, such as Good Clinical Practice (GCP) and ethics in research studies, substantially boosts staff proficiency—an essential component for maintaining compliance and ensuring participant safety. A well-prepared group can proactively manage workflows, including the complexities of setup and import permits, thereby reducing the risk of errors and enhancing overall efficiency.
Ultimately, the importance of staff training cannot be overstated, as it directly correlates with the integrity of medical research and the successful advancement of innovative healthcare solutions.
Post-trial responsibilities encompass essential tasks that are critical to the integrity of clinical research, including the closing out of studies and the transparent reporting of findings. The clinical trial coordinator responsibilities include playing a pivotal role in this process, ensuring that all data is meticulously documented and that final reports adhere to stringent regulatory requirements. This comprehensive process involves:
Proper closure not only fulfills ethical obligations but also enriches the collective body of medical knowledge.
Optimal methods for concluding research studies involve:
For instance, the prompt sharing of research results through platforms like ClinicalTrials.gov illustrates the importance of clear reporting, boosting accountability and fostering trust within the research community. By adhering to these best practices, coordinators effectively meet their clinical trial coordinator responsibilities and significantly contribute to the advancement of medical science.
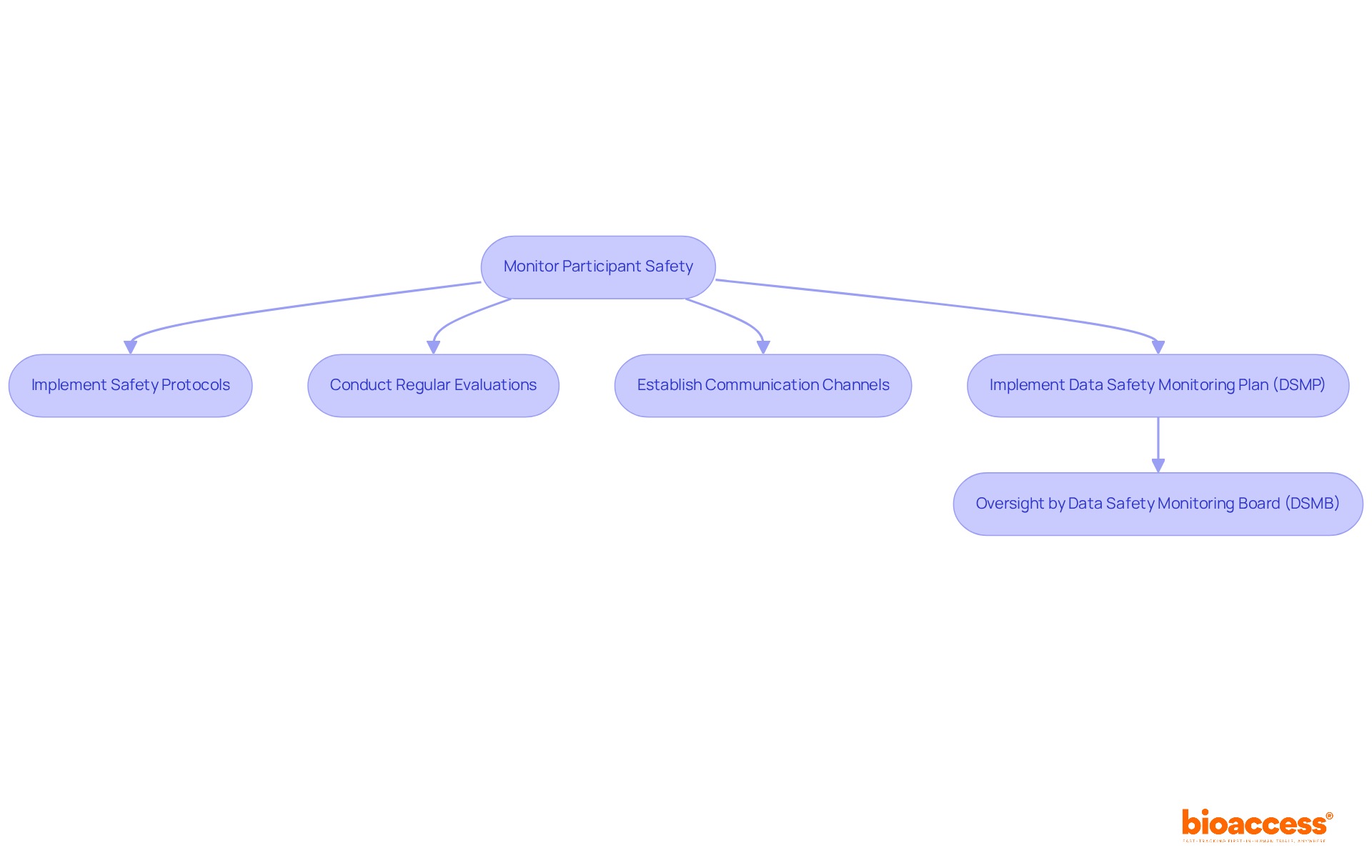
Monitoring participant safety is a key aspect of clinical trial coordinator responsibilities, as they play a crucial role in implementing and overseeing safety protocols. These protocols are meticulously designed to safeguard individuals by systematically identifying and addressing any adverse events or safety issues that may arise during the trial. Regular evaluations of individual well-being are vital, as they ensure that any potential risks are swiftly addressed.
To enhance safety oversight, coordinators must establish clear communication channels that facilitate easy reporting of issues or concerns by participants. This proactive approach not only strengthens the monitoring process but also fosters trust between contributors and the research team. Furthermore, the implementation of a Data Safety Monitoring Plan (DSMP) is essential for maintaining subject safety and data integrity, while the oversight provided by a Data Safety Monitoring Board (DSMB) guarantees that safety data is regularly reviewed, prioritizing participant protection.
In addition to these safety measures, the clinical trial coordinator responsibilities encompass:
By emphasizing the safety of individuals through efficient protocols and comprehensive research oversight services, the clinical trial coordinator responsibilities significantly enhance the integrity of the study and the well-being of those involved.
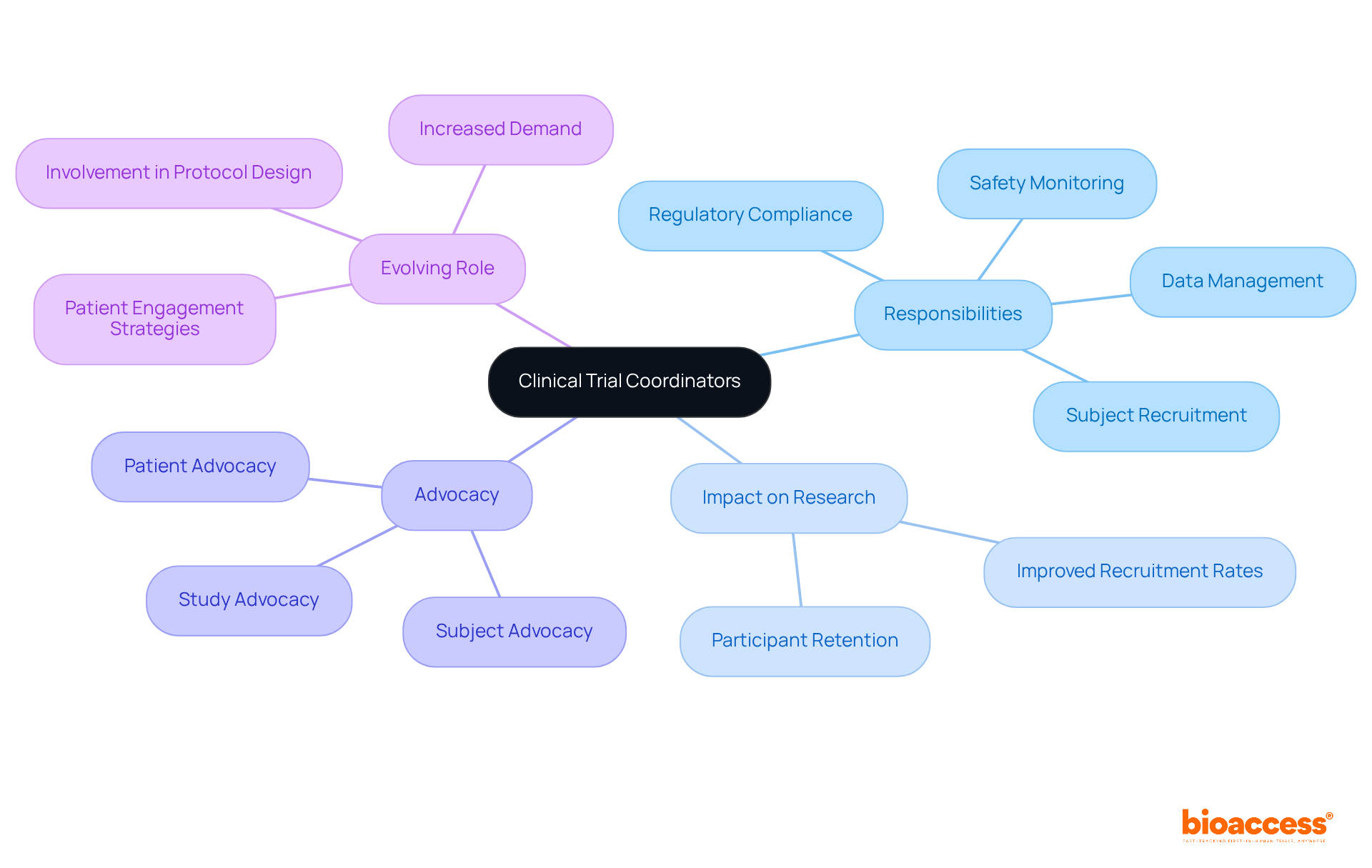
Research coordinators are pivotal to the success of medical studies, as their clinical trial coordinator responsibilities encompass a diverse array of tasks including subject recruitment, data management, regulatory compliance, and safety monitoring. Their role transcends mere administration; they act as advocates for patients, ensuring individuals are well-informed about their rights and the study's objectives. This advocacy is crucial, as it fosters trust and enhances participant retention, which is vital for the integrity of research studies.
Often referred to as the 'policemen of the protocol,' coordinators ensure adherence to regulatory standards while balancing the needs of participants. Their adeptness at navigating complex challenges—such as unexpected patient withdrawals or regulatory shifts—showcases their problem-solving abilities and adaptability. Research indicates that centers with specialized coordinators experience significantly improved recruitment rates and overall study quality, with over 80% of participating locations acknowledging the positive impact of coordinators on research outcomes.
Moreover, the evolving role of research coordinators includes expanding clinical trial coordinator responsibilities, with increasing expectations for their involvement in protocol design and patient engagement strategies. Industry experts emphasize that the clinical trial coordinator responsibilities include being process-driven and possessing strong communication skills to effectively implement study protocols and safeguard participant safety. This multifaceted role not only contributes to advancing medical knowledge but also enhances patients' quality of life, as coordinators often observe the beneficial outcomes of their efforts.
In the context of bioaccess's comprehensive healthcare study management services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—the responsibilities of a clinical trial coordinator are vital in ensuring these processes function seamlessly. Their contributions are essential in bridging the gap between innovative research and real-world applications, ultimately leading to breakthroughs in medical science. Furthermore, with an anticipated 10% increase in demand for clinical trial coordinator responsibilities from 2016 to 2026, their significance in the evolving landscape of medical studies is more apparent than ever. This is particularly relevant as partnerships, such as that between bioaccess and Caribbean Health Group, aim to position Barranquilla as a premier location for research studies in Latin America, supported by initiatives from Colombia's Minister of Health. Additionally, the economic impact of clinical studies on local economies—encompassing job creation and healthcare advancements—highlights the crucial role clinical trial coordinators play in promoting international collaboration and growth.
The essential responsibilities of clinical trial coordinators are pivotal for the success of medical research, encompassing a variety of critical tasks that ensure studies are conducted ethically and effectively. By managing participant recruitment, overseeing data integrity, ensuring regulatory compliance, and prioritizing participant safety, coordinators play a crucial role in bridging the gap between innovative research and real-world applications.
Key insights highlighted throughout the article include:
Moreover, the impact of bioaccess® in facilitating efficient clinical trial coordination in Latin America demonstrates how strategic partnerships can enhance research outcomes and accelerate the development of new medical solutions.
Ultimately, the role of clinical trial coordinators transcends administrative oversight; it embodies the fostering of trust and the promotion of ethical practices within the research community. As the demand for skilled coordinators continues to rise, there exists a significant opportunity for professionals in this field to make a lasting impact on healthcare advancements. Embracing best practices and innovative strategies will be essential for driving success in clinical research, ensuring that the focus remains on participant well-being and the integrity of study findings.
What is bioaccess® and what services does it provide?
bioaccess® specializes in coordinating clinical studies, particularly in Latin America, with a focus on comprehensive research management services. Its key offerings include Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Research Follow-Up Studies.
Why is Latin America, especially Colombia, advantageous for clinical trials?
Latin America, particularly Colombia, offers regulatory efficiency, allowing bioaccess® to secure ethical approvals in 4-6 weeks and achieve participant enrollment rates that are 50% faster than traditional markets. Additionally, Colombia's healthcare system supports efficient patient recruitment due to its large population and high coverage under universal healthcare.
What are the challenges faced in participant recruitment for clinical trials?
Challenges in participant recruitment include socio-economic barriers to informed consent, delays or shutdowns of research studies due to recruitment problems, and under-enrollment at research sites.
How does bioaccess® enhance participant recruitment strategies?
bioaccess® enhances recruitment by implementing targeted strategies, engaging in community outreach, utilizing social media for wider engagement, and collaborating with healthcare providers to identify qualified individuals. Their partnership with Caribbean Health Group has significantly reduced recruitment time and improved retention rates.
What role does informed consent play in clinical trials?
Informed consent is a fundamental responsibility of clinical trial coordinators, requiring them to provide prospective participants with comprehensive information about the study, including objectives, methodologies, risks, and benefits. This process ensures ethical compliance and fosters trust between participants and researchers.
What trends are currently influencing clinical trials in Latin America?
There is a shift towards decentralized studies, which enhances accessibility and participant diversity. Additionally, there has been a significant increase in annual funding for research in Latin America, reflecting growing confidence in the region as a viable location for medical studies.