


This article identifies ten essential clinical trials courses designed to equip research leaders with the critical skills and knowledge necessary for effective trial management. It highlights programs from reputable institutions, such as bioaccess® and York University, which prioritize hands-on training, ethical considerations, regulatory compliance, and practical applications. These elements are crucial in preparing participants to successfully navigate the complexities of clinical research. By understanding these courses, research leaders can enhance their capabilities and contribute more effectively to the field.
As the landscape of clinical research evolves, the demand for skilled leaders capable of navigating the complexities of clinical trials has reached unprecedented levels. This article explores ten essential courses tailored specifically for research leaders, providing insights into the latest methodologies, regulatory frameworks, and ethical considerations vital for success in this field. However, with a plethora of options available, how can aspiring leaders discern which programs will genuinely equip them to confront the challenges posed by modern clinical trials?
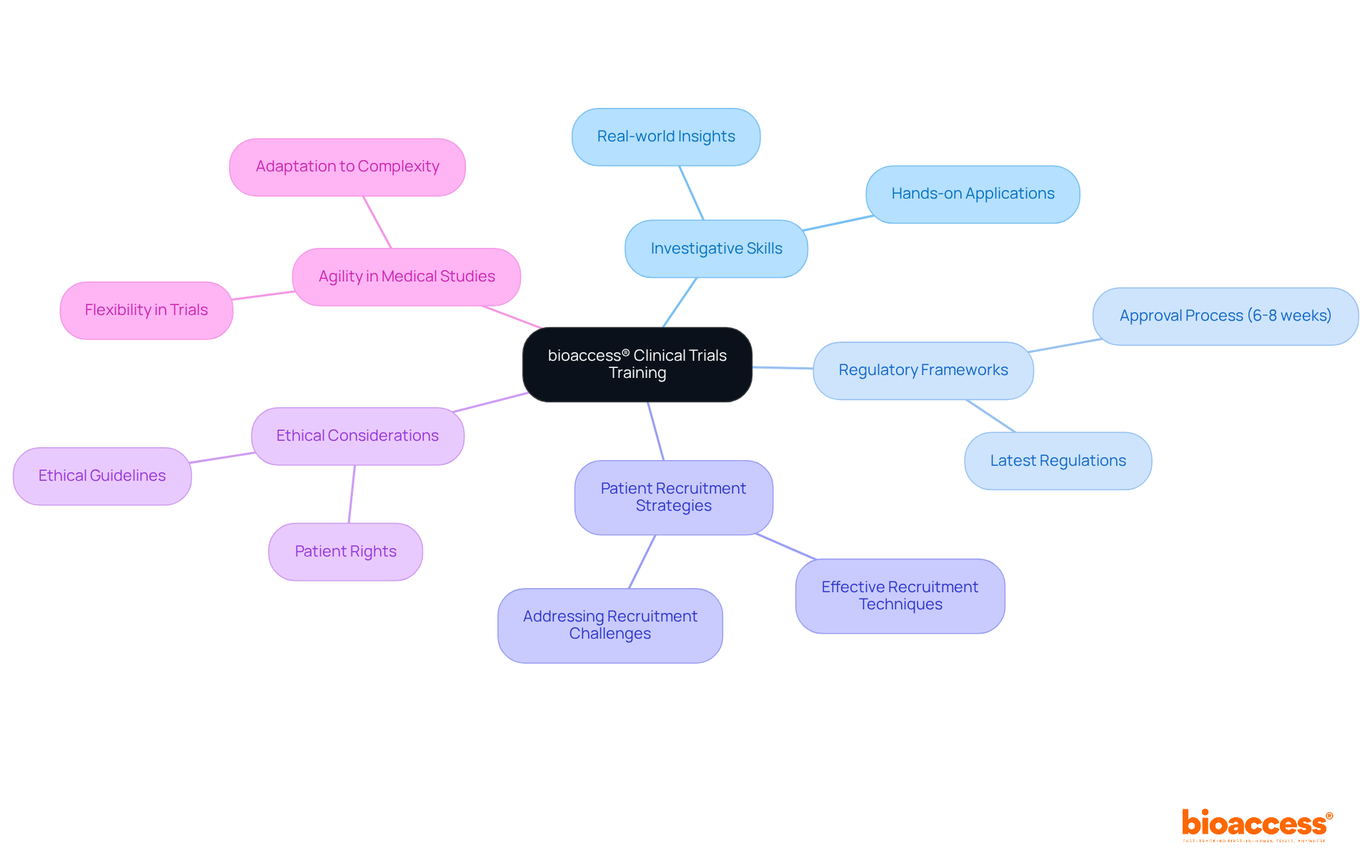
The bioaccess® clinical trials courses program is meticulously designed to enhance investigative skills through hands-on, real-world applications.
Through clinical trials courses, participants will gain crucial insights into the latest regulatory frameworks, effective patient recruitment strategies, and essential ethical considerations, preparing them to lead research trials with confidence.
This training underscores the critical requirement for agility in medical studies, demonstrating bioaccess®'s unwavering commitment to accelerating advancements in medical technology.
With a sped-up regulatory approval process of merely 6-8 weeks, in stark contrast to the usual 6-12 months in the US/EU, and the capacity to enroll treatment-naive cardiology or neurology groups 50% quicker than Western locations, bioaccess® empowers leaders in trials to navigate these challenges efficiently.
As research studies become increasingly intricate, this program equips leaders to confront recruitment challenges head-on.
By fostering a culture of ongoing education and flexibility, bioaccess® ensures that its participants are not only well-informed but also adept at applying innovative solutions in their projects, supported by bioaccess®'s expertise and tailored approach in overseeing studies.
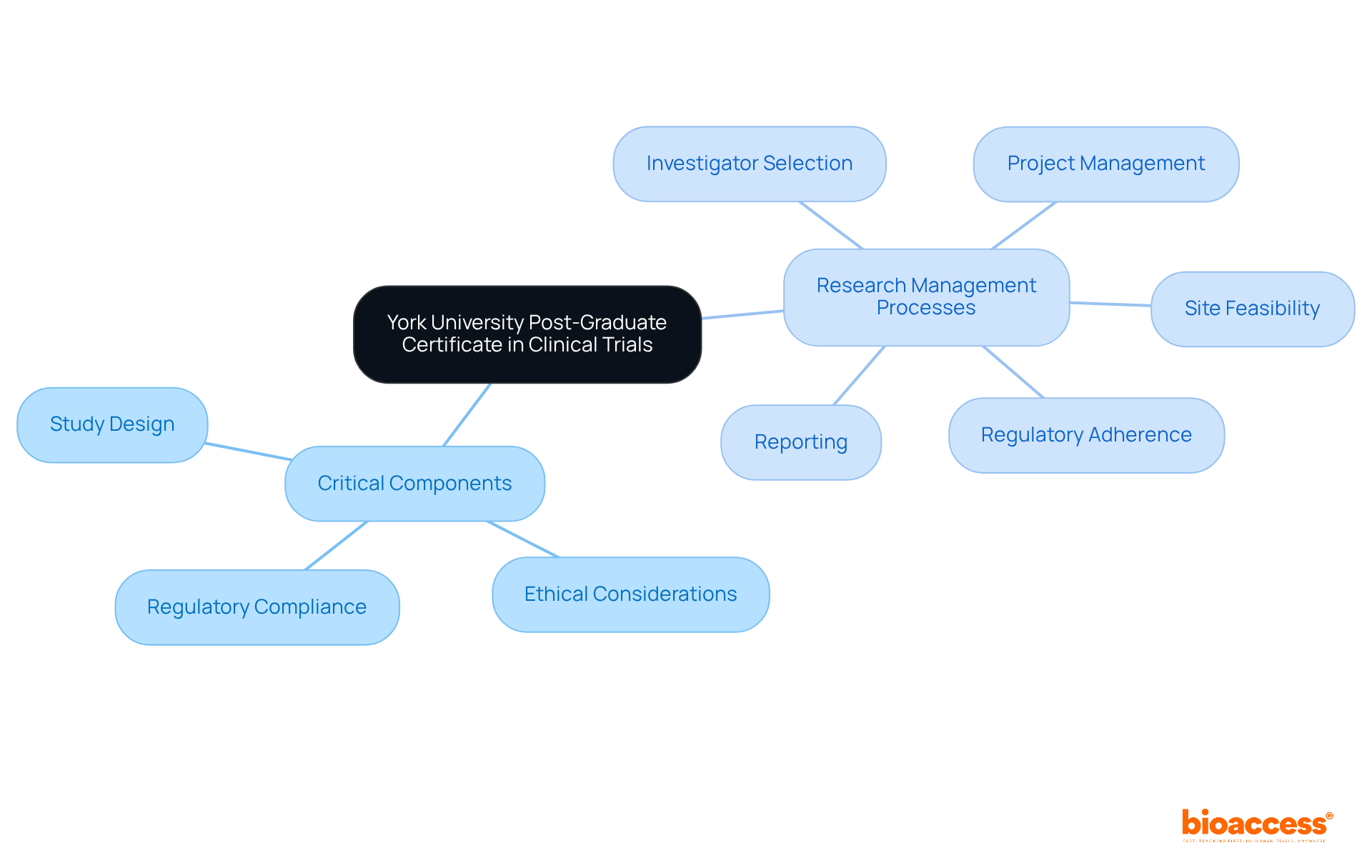
The York University Post-Graduate Certificate in Clinical Trials courses offers a comprehensive curriculum designed to equip students with essential skills for a successful career in trial management. This program offers clinical trials courses that address critical components such as:
This ensures that participants develop a thorough understanding of the medical investigation landscape. Emphasizing practical applications, students engage in clinical trials courses that prepare them to adeptly manage the complexities of research studies. This includes navigating extensive processes related to advancing medical device evaluations, such as:
This program presents significant advantages for individuals aiming to enhance their expertise and advance their careers within the dynamic field of research, particularly in overcoming the challenges faced by medical device startups.
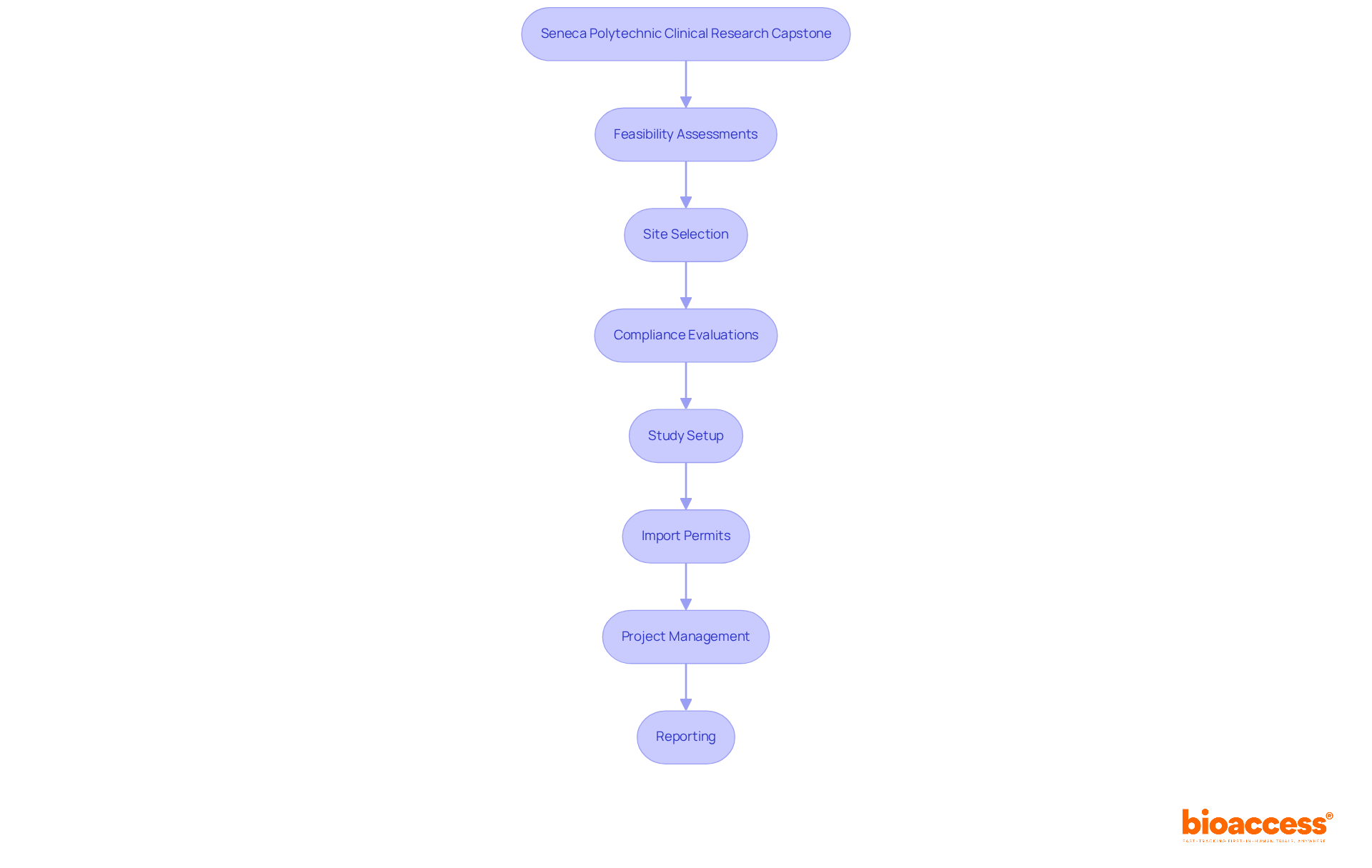
The Seneca Polytechnic Clinical Research Capstone immerses students in a real-world research project, providing a platform to apply theoretical knowledge within a practical environment. This experience is crucial for honing problem-solving skills and grasping the complexities of management, especially in navigating the Latin American Medtech landscape.
Participants collaborate closely with industry experts, including professionals from bioaccess®, a leading Contract Research Organization that supports medical device studies in Latin America. This partnership offers invaluable insights into comprehensive research study management services, encompassing:
As emphasized by industry leaders, hands-on training is vital for bridging the gap between academic knowledge and real-world applications, ensuring that future researchers are well-equipped to tackle the challenges inherent in research studies. Remarkably, 90% of students indicated a preference for hands-on learning with wireless devices, underscoring the demand for practical training.
One student noted, 'As we are moving more towards technology-based healthcare settings, hands-on practice for students is a good experience.' This underscores the importance of integrating practical training into medical studies, which not only enhances individual career trajectories but also contributes to job creation, economic development, and the improvement of healthcare in local communities.
The York University Clinical Research Operations course offers a comprehensive exploration of managing research studies from inception to completion. Participants will develop essential skills in project planning, resource allocation, and team management—skills crucial for overseeing complex study projects.
As the industry evolves towards hybrid outsourcing models and adaptable study designs, clinical trials courses prove to be particularly advantageous for those aspiring to leadership positions in medical research. Effective project management is paramount; studies reveal that organizations that provide substantial support for skill development experience significantly higher project performance rates.
By mastering these competencies, participants will be well-equipped to navigate the dynamic landscape of medical studies in 2025.
The Seneca Polytechnic clinical trials courses provide participants with a comprehensive understanding of design principles, which are essential for the effective management of medical studies. Students delve into various methodologies, statistical considerations, and ethical issues that are integral to study design. A pivotal element of this foundational knowledge is the importance of statistical power analysis, ensuring that studies are sufficiently powered to detect significant effects. The clinical trials courses further underscore the necessity of ongoing education in biostatistics for healthcare professionals, promoting a culture of continuous learning within medical research.
As Meri Beckwith, Co-CEO, articulates, 'An adequately powered study ensures that the results are not only valid but also generalizable.' Grasping these principles not only enhances the quality of medical studies but also promotes a culture of evidence-based practice in healthcare research. Additionally, students investigate the implications of measurement scales—nominal, ordinal, and interval—on the choice of statistical methods, providing a nuanced perspective on experimental design.
This understanding is complemented by insights into bioaccess's extensive management services for research studies, which encompass:
Each critical for successful research outcomes. Moreover, the impact of medtech research studies on local economies—spanning job creation, economic growth, healthcare improvements, and international collaboration—underscores the broader significance of well-structured trials in advancing public health and fostering economic development.

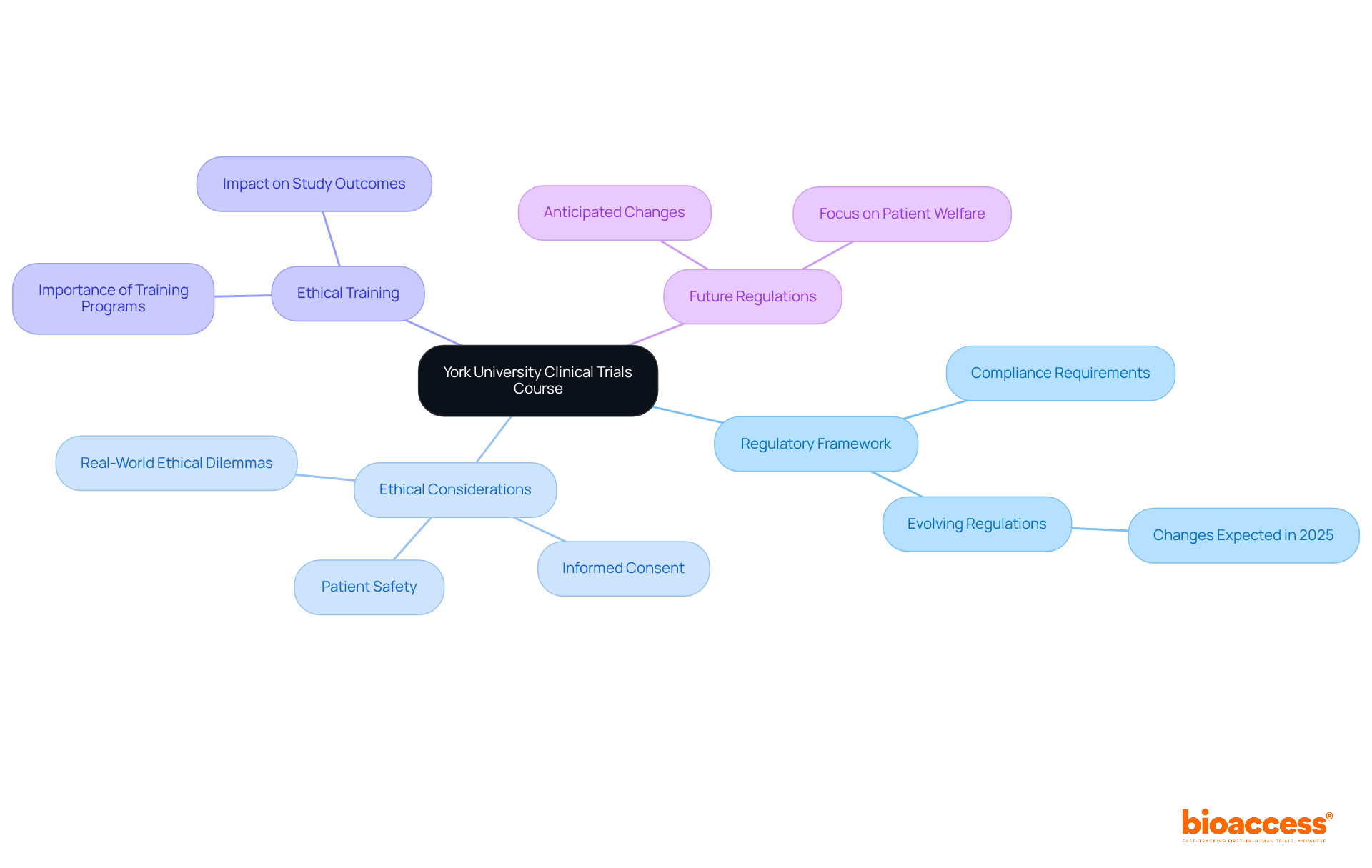
The Regulatory and Ethical Issues in Trials course at York University explores the intricate framework of regulations governing clinical trials courses in medical studies. Participants will examine critical ethical considerations, with a particular focus on the importance of informed consent and patient safety. Understanding these elements is vital for study leaders enrolled in clinical trials courses, as it ensures compliance and upholds the highest ethical standards in their research endeavors.
Real-world examples of ethical dilemmas faced in research highlight the necessity for robust ethical training, which has been shown to significantly enhance study outcomes. As regulations evolve in 2025, this course equips leaders with the knowledge necessary to navigate these changes effectively, ensuring that patient welfare remains paramount in medical research.
The Seneca Polytechnic Course on Monitoring Research Studies equips attendees with essential skills for the efficient management and assessment of research projects. By emphasizing monitoring methods, information integrity, and adherence to regulatory standards, this clinical trials course is indispensable for individuals tasked with ensuring that clinical trials uphold the highest levels of integrity. Preserving information integrity is paramount, as inaccuracies can lead to patient harm and regulatory penalties. For instance, a Transcelerate analysis revealed that conventional monitoring techniques yield a low return on investment, with only 3.7% of electronic information capture (EDC) entries amended post-initial submission. This highlights the urgent need for robust monitoring practices.
Moreover, the integration of Key Risk Indicators (KRIs) can significantly enhance information quality by identifying potential issues early. An analysis of 212 studies demonstrated that 83% of site KRIs improved after addressing risk signals, showcasing the effectiveness of proactive monitoring strategies. Experts assert that central statistical monitoring serves as a cost-effective alternative to comprehensive onsite monitoring, facilitating the prompt detection of inconsistencies that could compromise study integrity.
In the domain of medical studies, ensuring information integrity transcends mere compliance; it is a fundamental aspect of ethical research. The repercussions of neglecting data integrity can be dire, resulting in study rejection, fines, or even criminal charges. Therefore, extensive training programs for medical personnel in clinical trials courses are crucial to uphold Good Clinical Practice (GCP) standards and ensure the reliability of study results. This course is vital for project leaders committed to advancing medical studies with integrity.
At bioaccess, our service capabilities encompass:
These comprehensive medical study management services are designed to assist project leaders in fostering medical investigations with integrity, ultimately contributing to job creation, economic growth, and healthcare enhancement within local communities.

The Seneca Polytechnic Advanced Concepts in Clinical Drug Development course provides a comprehensive exploration of the intricate journey involved in bringing a drug to market. Participants will delve into each phase of the drug development process, encompassing:
This advanced training empowers leaders in the field with critical insights essential for fostering innovation and enhancing the success of pharmaceutical products in a competitive landscape.

The Seneca Polytechnic Fundamentals of Information Management in Medical Research course, as part of the clinical trials courses, equips attendees with essential skills for managing information throughout the trial process. This includes comprehensive instruction on:
These are crucial elements for navigating the complexities of clinical study information. Maintaining information integrity is paramount, as high-quality information must be precise and suitable for statistical analysis, thereby ensuring reliable research outcomes. As Binny Krishnankutty from Dr. Reddy's Laboratories asserts, 'High-quality information should be absolutely precise and suitable for statistical analysis.'
In 2025, the importance of effective information management practices is greater than ever, focusing on minimizing errors and maximizing data utility for analysis. The clinical trials courses prepare participants to implement robust information management strategies that are vital for achieving compliance and enhancing the overall quality of research trials.
Furthermore, it highlights the critical role of the Data Manager in overseeing the Clinical Information Management (CIM) process, ensuring that all information handling adheres to the Society for Clinical Information Management (SCIM) guidelines for effective practices. Participants will also delve into effective information handling techniques, such as:
These techniques aim to improve accuracy and tackle the challenges faced in management systems.
The We Can Train Competency-Based Clinical Trials Courses program is dedicated to cultivating practical skills through immersive, hands-on learning experiences. Participants engage in simulations and real-world situations that significantly enhance their ability to manage studies efficiently, particularly in overcoming recruitment challenges faced by Medtech and Biopharma startups, including the difficulties in securing healthcare provider involvement.
As emphasized by the Biopharma Institute, "To genuinely prepare, you need practical experience: practicing consent conversations, entering study information, managing protocols, and monitoring patients—all with expert assistance." This approach not only sharpens individual skills but also fosters essential leadership qualities vital for success in medical studies.
By focusing on practical competencies such as experiment setup, observation, and data management, the program prepares participants for clinical trials courses, ultimately leading to improved outcomes in their investigative endeavors. Furthermore, the statistic that '30% of research sites had record-keeping deficiencies' underscores the necessity of effective training in research management, especially given the challenges in securing site participation and ensuring patient eligibility.
bioaccess® offers comprehensive clinical trial management services that can further assist startups in overcoming these hurdles.
The landscape of clinical research is rapidly evolving, and the importance of well-structured training programs cannot be overstated. The courses highlighted in this article serve as essential tools for research leaders seeking to enhance their expertise and navigate the complexities of clinical trials. By engaging in these specialized programs, participants can develop the necessary skills to lead innovative research endeavors, ensuring that they are well-prepared for the challenges ahead.
Key insights from the discussed courses include:
These elements are crucial for fostering a culture of continuous learning and adaptability in the medical research field. Programs such as those offered by bioaccess®, York University, and Seneca Polytechnic not only equip individuals with the foundational knowledge required for successful trial management but also emphasize the significance of hands-on experience and real-world applications.
Ultimately, investing in clinical trials training is not just about personal career advancement; it is about contributing to the broader goals of improving healthcare outcomes and fostering innovation within the industry. As the field of clinical research continues to expand, the commitment to ongoing education and skill development will be vital for leaders aiming to drive progress and uphold the highest standards of integrity in their work. Embracing these educational opportunities will empower research professionals to make meaningful contributions to the future of medical science.
What is the purpose of the bioaccess® clinical trials courses program?
The bioaccess® clinical trials courses program is designed to enhance investigative skills through hands-on, real-world applications, providing participants with insights into regulatory frameworks, patient recruitment strategies, and ethical considerations.
How quickly can bioaccess® facilitate regulatory approvals compared to traditional processes?
Bioaccess® can achieve a sped-up regulatory approval process of 6-8 weeks, in contrast to the usual 6-12 months typically seen in the US/EU.
What advantages does bioaccess® offer in terms of patient recruitment for clinical trials?
Bioaccess® can enroll treatment-naive cardiology or neurology groups 50% quicker than locations in the Western regions, helping leaders navigate recruitment challenges efficiently.
What key skills does the York University Post-Graduate Certificate in Clinical Trials program aim to develop?
The program focuses on study design, ethical considerations, and regulatory compliance, ensuring participants gain a thorough understanding of the medical investigation landscape.
What practical applications do students engage in during the York University program?
Students engage in managing complexities related to medical device evaluations, including site feasibility, investigator selection, regulatory adherence, project management, and reporting.
What is the focus of the Seneca Polytechnic Clinical Research Capstone?
The capstone immerses students in a real-world research project to apply theoretical knowledge practically, honing problem-solving skills and understanding management complexities in the Medtech landscape.
How does the Seneca Polytechnic program enhance student learning?
Participants collaborate with industry experts, including those from bioaccess®, to gain insights into research study management services such as feasibility assessments, compliance evaluations, and project management.
What is the significance of hands-on training in clinical trials education?
Hands-on training is vital for bridging the gap between academic knowledge and real-world applications, ensuring future researchers are well-prepared to tackle challenges in research studies.
What do students prefer regarding their learning experience in clinical trials education?
90% of students indicated a preference for hands-on learning with wireless devices, highlighting the demand for practical training in medical studies.
How does practical training in clinical trials contribute to broader societal benefits?
Integrating practical training enhances individual career trajectories, contributes to job creation, economic development, and improves healthcare in local communities.