


Navigating the intricate landscape of clinical trials demands a solid understanding of Clinical Trial Agreements (CTAs), which are fundamental to the success of research initiatives. These vital legal documents not only outline responsibilities but also ensure adherence to ever-evolving regulations, significantly impacting the speed and efficiency of introducing new treatments to the market. With the continuous shifts in regulatory requirements and heightened scrutiny on compliance, clinical research directors face the pressing question: how can they effectively manage CTAs to mitigate risks and foster collaboration? This article delves into ten essential CTAs that every director should be familiar with, providing the insights necessary to streamline clinical research processes and propel innovation in medical technology.
bioaccess® harnesses the regulatory speed of Latin America, the diverse patient demographics of the Balkans, and the streamlined pathways in Australia to secure ethical approvals in just 4-6 weeks. This strategic combination empowers Medtech, Biopharma, and Radiopharma innovators to achieve enrollment rates that are 50% faster than traditional markets, significantly reducing the time to market for new medical technologies. By leveraging these regional strengths, bioaccess® enables clients to navigate complex healthcare environments effectively, ensuring prompt access to vital patient information and insights.
In 2023, Brazil experienced a notable double-digit percentage increase in new medical studies, particularly in oncology and infectious diseases. This trend underscores the growing significance of Latin America in global research. As the industry evolves, the focus on regulatory agility and diverse participant recruitment remains essential for enhancing the effectiveness and safety of treatments across various populations.
The collaboration between bioaccess® and its clients is crucial in addressing the challenges faced in cta in clinical research. By prioritizing these elements, bioaccess® not only streamlines the research process but also fosters innovation in medical technology, ultimately benefiting patients worldwide.

Clinical Research Agreements, or CTAs in clinical research, are essential legal contracts that delineate the responsibilities of all parties involved in clinical studies. With the updates in 2025, adherence to Good Clinical Practice (GCP) guidelines has become even more critical, emphasizing ethical conduct and data integrity.
How can directors ensure that the CTA in clinical research clearly defines roles, responsibilities, and obligations? This clarity is vital to mitigate risks associated with non-compliance, which can lead to significant legal repercussions and delays in trial execution.
Recent data indicates that poorly organized calls to action can attract increased scrutiny and enforcement measures. This underscores the necessity for careful drafting and regular audits. Legal specialists advocate for comprehensive agreements that incorporate clear indemnification clauses and publication rights, safeguarding all parties involved. Successful examples of well-defined CTAs in clinical research demonstrate how effective agreements can foster collaboration and ensure compliance with evolving regulations.
To maintain alignment with the latest legal modifications and uphold the integrity of medical research, it is recommended to frequently update the CTA in clinical research. Leveraging robust clinical trial management services, such as those provided by Bioaccess, can significantly enhance feasibility studies, compliance reviews, project management, and reporting processes. This ultimately leads to more successful trial outcomes. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, exemplifies the level of expertise available to navigate these complexities effectively.
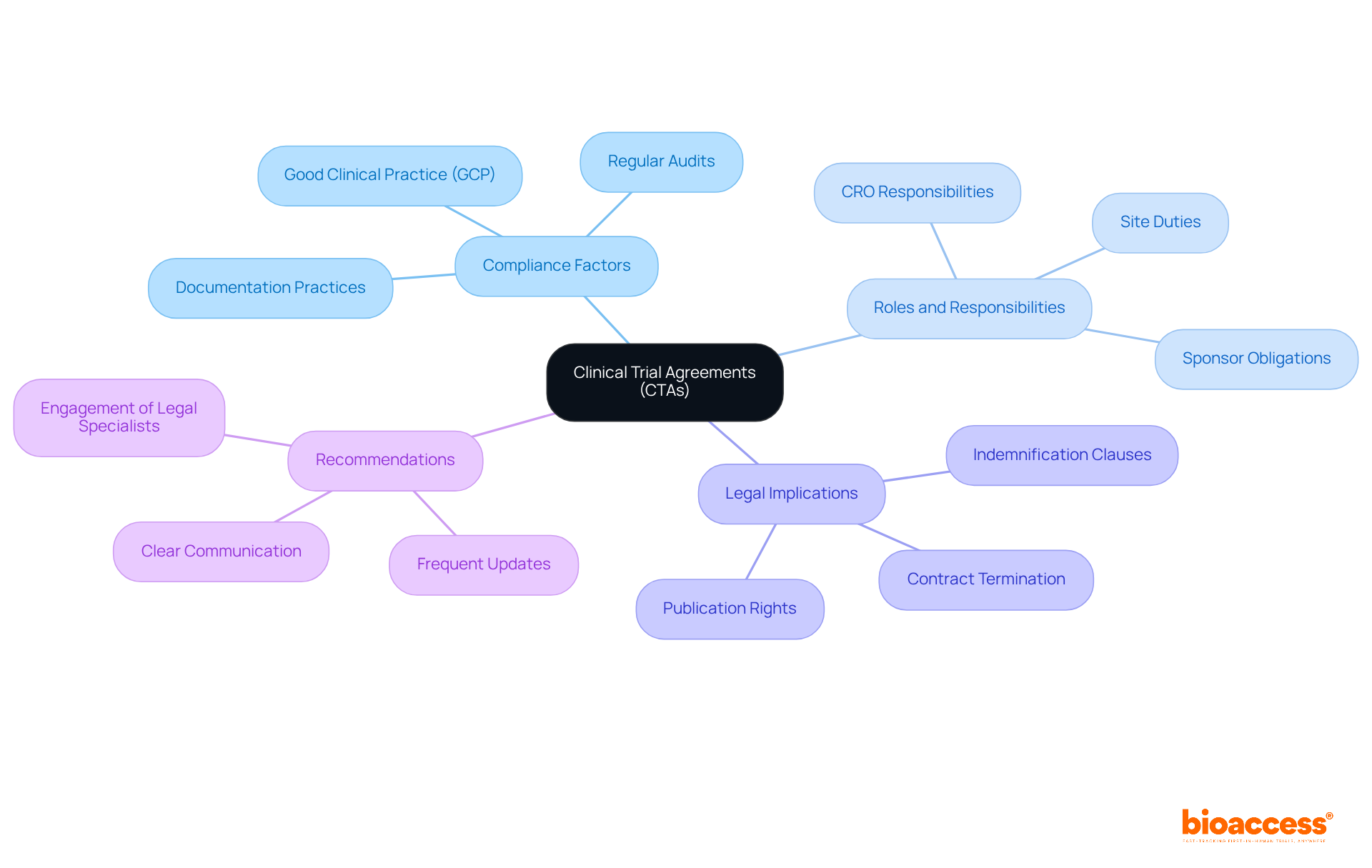
Successful negotiation of CTAs in clinical research is essential, demanding a strategic approach that prioritizes preparation, effective communication, and adaptability. Establishing a collaborative environment is crucial; it ensures that all parties feel valued and understood throughout the process. Key strategies include:
Understanding the motivations and needs of the other party can significantly enhance the negotiation experience, leading to more favorable outcomes. Regular training on negotiation tactics empowers teams, enabling them to navigate complex discussions with greater confidence and effectiveness. This training not only enhances personal skills but also positively influences overall research outcomes, underscoring the importance of investing in negotiation abilities.
Moreover, detailed budget and payment terms provide transparency in the financial aspects of the trial, ensuring that all parties are aligned on expectations. As John F. Kennedy aptly stated, 'Let us never negotiate out of fear. But let us never fear to negotiate,' emphasizing the importance of a confident approach in negotiations. Additionally, clinical trial assistants play a crucial role in ensuring compliance with relevant laws, regulations, and ethical standards, which is vital for the integrity of CTAs in clinical research.

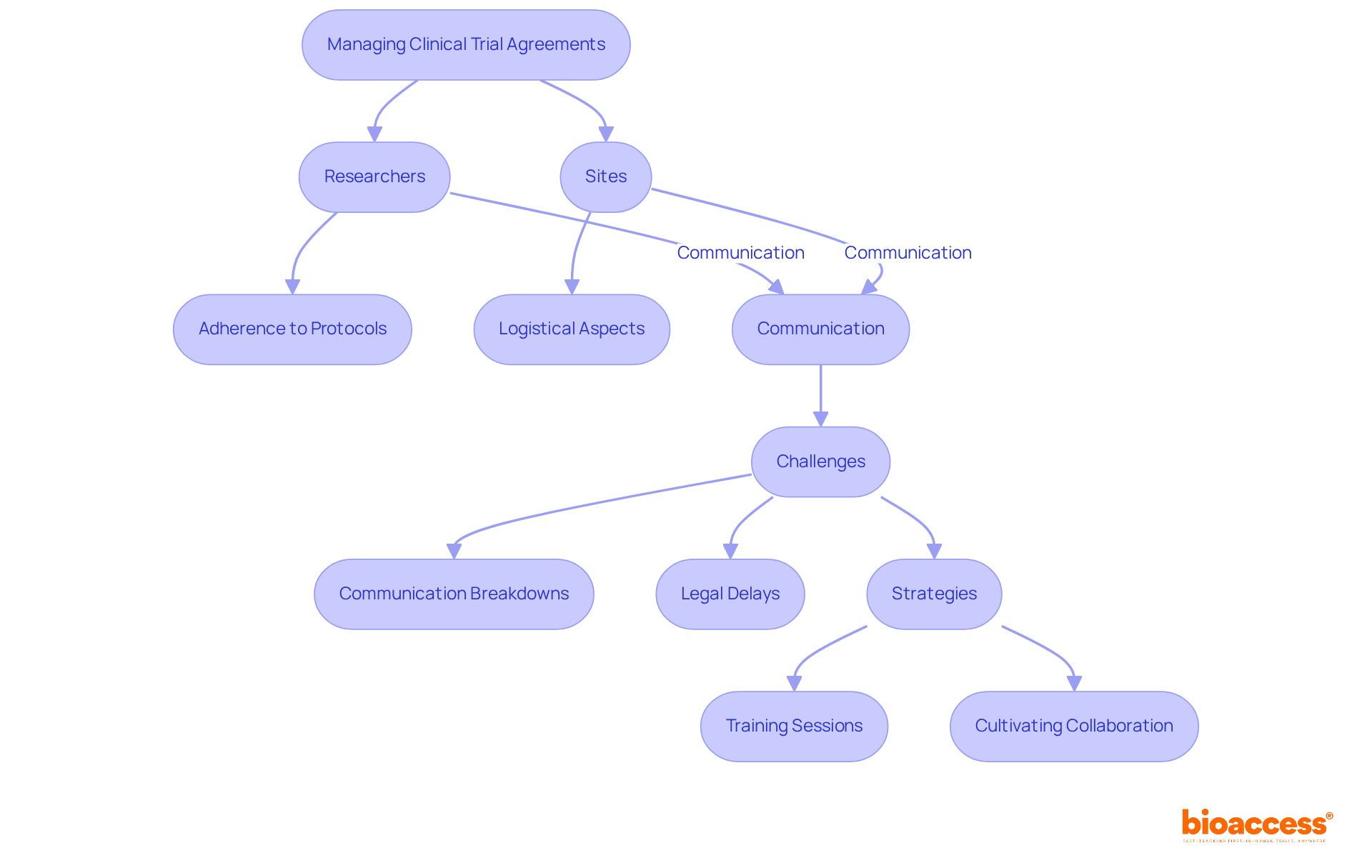
In managing CTAs in clinical research, the distinct yet interdependent roles of researchers and sites are crucial. Researchers ensure adherence to study protocols and compliance with regulatory standards, while sites focus on logistical aspects like patient recruitment and data collection. Effective communication between researchers and site staff is essential for swiftly addressing challenges and maintaining study momentum. Statistics reveal that communication breakdowns can significantly hinder legal proceedings, resulting in delays and increased costs.
To mitigate these risks, directors must cultivate a culture of collaboration and accountability. Implementing regular training sessions keeps all parties informed about current regulations and best practices. This proactive strategy not only enhances the oversight of the CTA in clinical research but also fosters a more efficient and effective research environment. By prioritizing communication and collaboration, stakeholders can navigate the complexities of clinical trials with greater confidence and success.
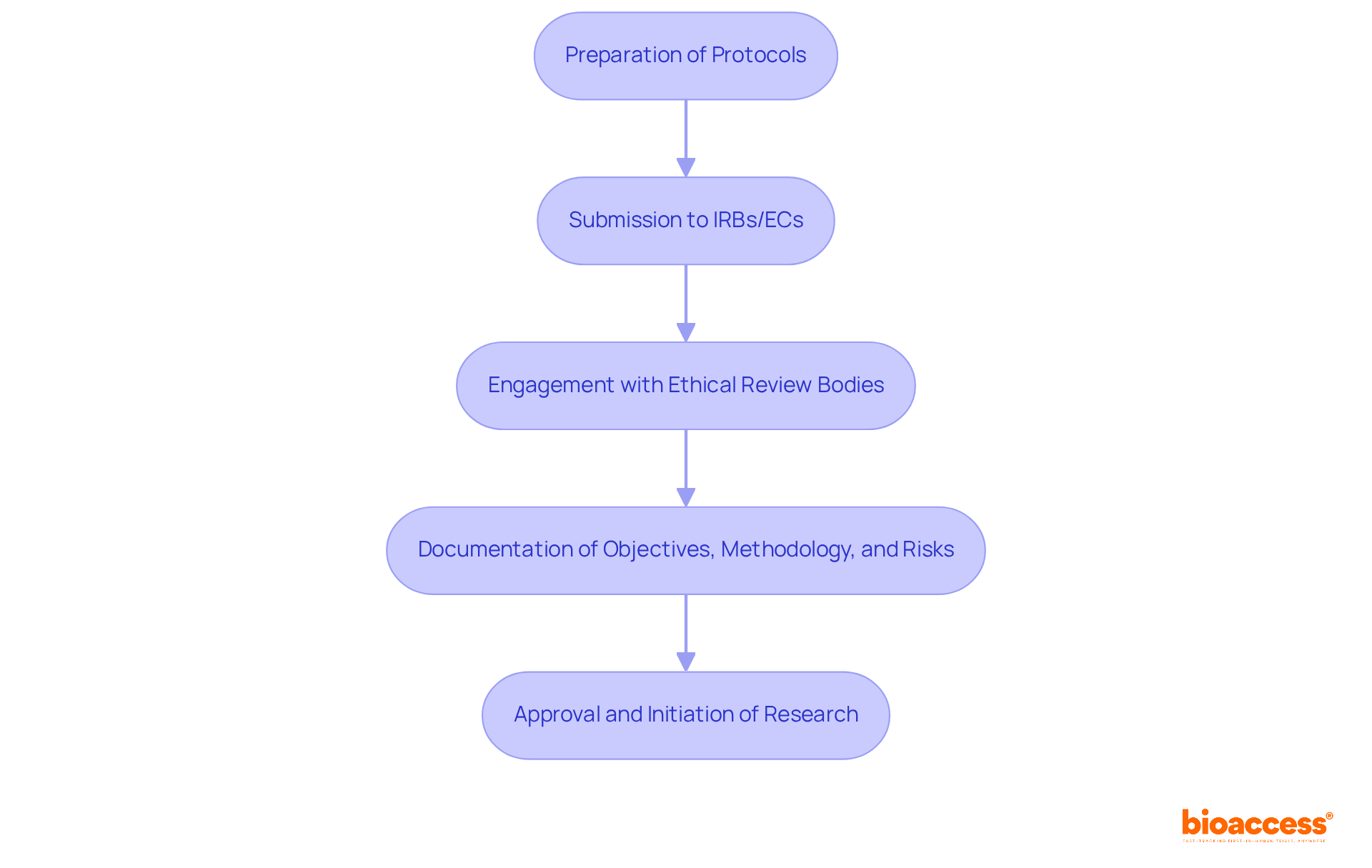
Navigating the ethical approval process is crucial for clinical research, requiring meticulous preparation and submission of detailed protocols to Institutional Review Boards (IRBs) or Ethics Committees (ECs). This essential step safeguards the rights and well-being of individuals involved in research studies. Directors must compile comprehensive documentation that clearly outlines the study's objectives, methodology, and potential risks.
Engaging with ethical review bodies early in the planning phase is not just beneficial; it can significantly expedite approvals. Studies indicate that early engagement can reduce the time to ethics approval by up to 30%. This statistic underscores the importance of proactive communication with these bodies. Ongoing education on ethical standards is vital for all team members engaged in research, ensuring that everyone is aware of the latest guidelines and best practices.
Insights from ethics committee members emphasize the importance of clear communication and thorough documentation in facilitating faster review processes. By prioritizing these factors, organizations can enhance their chances of successful submissions and streamline the initiation of research studies. The path to ethical approval may be complex, but with the right strategies in place, it can be navigated effectively.
Key terms in Clinical Trial Agreements (CTAs) in clinical research, such as 'sponsor,' 'investigator,' 'site,' 'indemnification,' and 'confidentiality,' are crucial for directors navigating the complexities. The sponsor typically assumes financial responsibility for the study, ensuring that necessary resources are allocated effectively. Meanwhile, the investigator plays a pivotal role in overseeing the study's execution, maintaining compliance with regulatory standards and ethical guidelines.
Indemnification clauses are essential elements of contracts, designed to protect parties from legal responsibilities arising from the examination. These clauses can vary significantly, often stipulating that the sponsor will indemnify the investigator and site against claims related to the investigational product or study procedures. Understanding the frequency of indemnification claims in clinical studies highlights the importance of these clauses, as they can mitigate financial risks associated with unexpected occurrences.
Confidentiality agreements within clinical trial agreements are equally vital, safeguarding sensitive information exchanged among sponsors, investigators, and sites. These agreements ensure that proprietary data and participant information remain protected, fostering trust among all parties involved. By mastering these key terms, directors can enhance their communication with legal teams, ensuring that expectations are clearly defined and aligned, ultimately contributing to the trial's success.

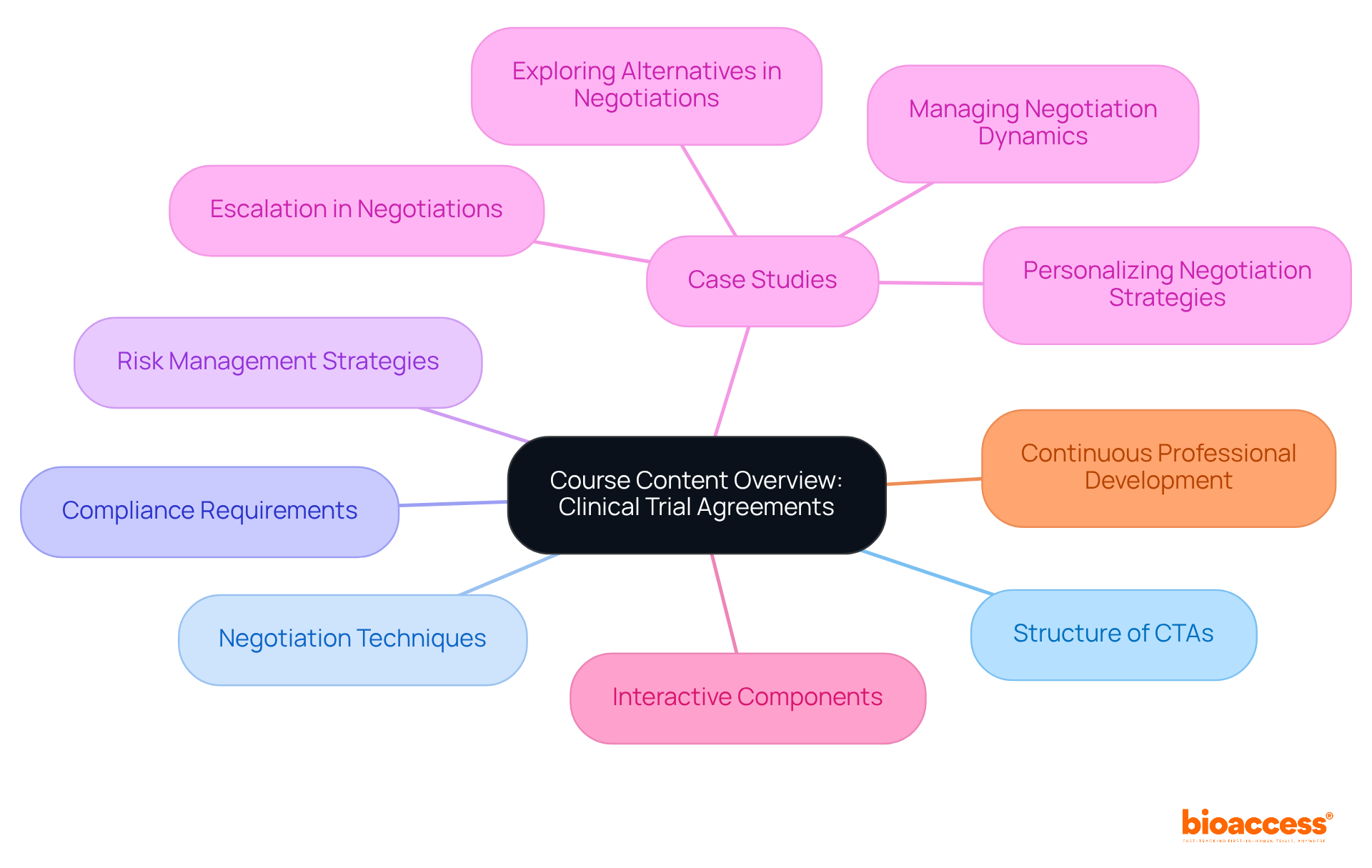
A comprehensive course on the cta in clinical research is essential for professionals, as it covers critical topics like the structure of CTAs, negotiation techniques, compliance requirements, and risk management strategies. By incorporating case studies that highlight common challenges faced during negotiations, participants gain invaluable practical insights, learning from real-world scenarios that resonate with their experiences.
Moreover, courses that include interactive components foster the application of learned concepts in practical contexts, significantly enhancing the overall learning experience. Continuous professional development is vital in this field; it ensures that professionals stay informed about evolving industry standards and regulatory changes. As industry leaders emphasize, ongoing education is crucial for sustaining a competitive advantage and effectively managing the complexities of research in this dynamic landscape.
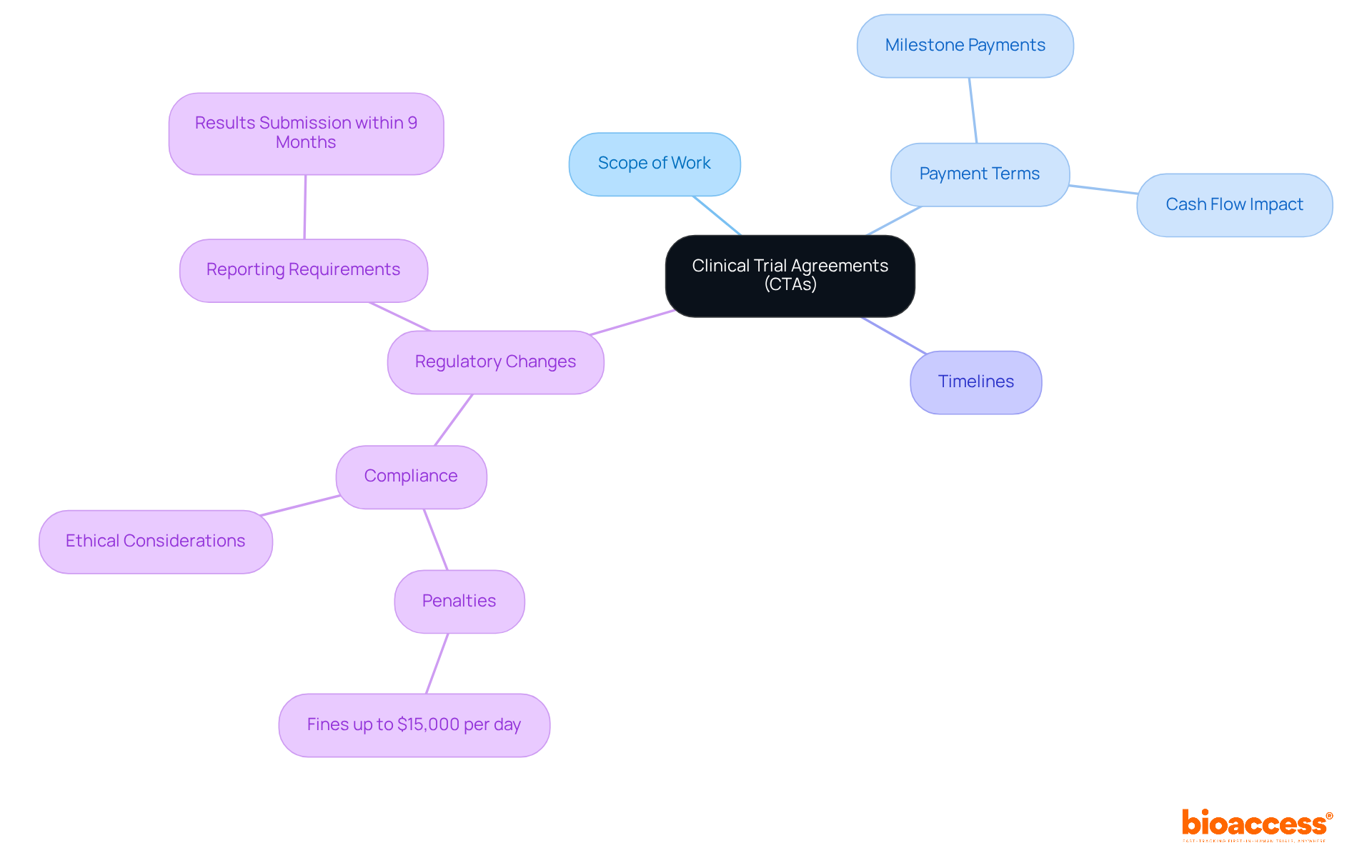
Clinical Trial Agreements (CTAs in clinical research) are vital to the success of trials, establishing the legal and operational framework necessary for them. Directors must understand the essential components of the CTA in clinical research, which encompass the scope of work, payment terms, and timelines. Recent changes in oversight requirements now mandate that sponsors submit results within nine months of the primary completion date, reduced from twelve months. This shift underscores the critical need for timely reporting and compliance.
Key payment terms often include milestone payments tied to specific deliverables, significantly impacting cash flow and project timelines. Grasping these terms is crucial for effective negotiation and resource management. Furthermore, the evolving regulatory landscape demands a strong focus on ethical considerations and compliance. Non-compliance can lead to severe penalties, including fines of up to $15,000 per day for ongoing violations.
By fostering strong connections with sponsors and research locations, directors can streamline negotiations and enhance the overall effectiveness of clinical studies. This collaboration ultimately results in quicker access to innovative treatments for patients. As you navigate the complexities of clinical research, consider how these insights can help you address your own challenges and improve your trial outcomes.
In early-phase studies, the cta in clinical research plays a crucial role in addressing significant challenges such as participant recruitment, safety monitoring, and data management. Statistics reveal that successful participant recruitment rates in early-phase trials can dramatically influence study timelines and outcomes. In fact, some organizations have achieved enrollment rates up to 50% faster than traditional markets. To optimize recruitment, directors should implement targeted strategies, leveraging local patient pools and utilizing digital outreach methods, which have proven effective in enhancing participant engagement.
Clear communication with all stakeholders is paramount for aligning expectations and fostering collaboration. Flexibility within the agreement is essential, allowing for adjustments that reflect the dynamic nature of early-phase research and the requirements of the cta in clinical research. This adaptability is vital in addressing unexpected challenges, such as policy changes or shifts in participant availability.
Moreover, understanding the unique regulatory requirements for early-phase studies is critical for ensuring compliance and safeguarding participant safety. By proactively addressing these factors, directors can significantly enhance the success rates of early-phase clinical trials, ultimately leading to more efficient pathways for bringing innovative therapies to market.

Directors seeking to enhance their grasp of Clinical Trial Agreements (CTAs) should consider a range of valuable resources, such as:
Organizations like the Association of Clinical Research Professionals (ACRP) and the Clinical Trials Transformation Initiative (CTTI) offer essential training opportunities that keep professionals informed about best practices and regulatory changes. By engaging with professional networks and attending industry conferences, directors not only foster knowledge sharing but also strengthen collaboration among peers.
Staying updated on the latest trends and developments in clinical research is crucial for effective CTA in clinical research management. This knowledge empowers directors to navigate the complexities of the field with confidence, ensuring they can address challenges head-on. As the landscape of clinical research continues to evolve, the commitment to ongoing education and professional development becomes increasingly vital.

In the realm of clinical research, understanding Clinical Trial Agreements (CTAs) is paramount for directors aiming to navigate the complexities of trial management effectively. This article highlights the essential CTAs that every director should be familiar with, emphasizing their role in ensuring compliance, fostering collaboration, and expediting the research process. A robust grasp of these agreements not only facilitates smoother negotiations but also enhances the overall efficiency of clinical trials.
Key insights discussed include:
The collaboration between researchers and sites, as well as the proactive approach to ethical approvals, are critical components that contribute to the success of clinical trials. Moreover, the emphasis on continuous education and the utilization of available resources serves as a reminder of the dynamic nature of this field.
Ultimately, embracing the principles outlined in this article empowers clinical research directors to enhance trial outcomes and accelerate the delivery of innovative therapies. By prioritizing education, collaboration, and compliance, directors can navigate the evolving landscape of clinical research with confidence, ensuring that they are well-equipped to meet the challenges ahead.
What is bioaccess® and what advantages does it offer for clinical trials?
bioaccess® accelerates clinical trials by utilizing the regulatory speed of Latin America, diverse patient demographics in the Balkans, and streamlined pathways in Australia, allowing for ethical approvals in just 4-6 weeks. This strategic combination enables Medtech, Biopharma, and Radiopharma innovators to achieve enrollment rates that are 50% faster than traditional markets, reducing the time to market for new medical technologies.
How has the landscape of clinical studies in Latin America changed recently?
In 2023, Brazil saw a significant increase in new medical studies, particularly in oncology and infectious diseases, highlighting the growing importance of Latin America in global research.
What role does collaboration play in the bioaccess® process?
Collaboration between bioaccess® and its clients is crucial for addressing challenges in clinical trial agreements (CTAs) and streamlining the research process, ultimately fostering innovation in medical technology and benefiting patients worldwide.
What are Clinical Trial Agreements (CTAs) and why are they important?
CTAs are legal contracts that outline the responsibilities of all parties involved in clinical studies. They are essential for ensuring compliance with Good Clinical Practice (GCP) guidelines, which emphasize ethical conduct and data integrity.
What key factors should directors consider when drafting CTAs?
Directors should ensure that CTAs clearly define roles, responsibilities, and obligations to mitigate risks associated with non-compliance. This includes careful drafting, regular audits, and incorporating clear indemnification clauses and publication rights.
How can organizations maintain compliance with evolving regulations in CTAs?
Organizations should frequently update CTAs to align with the latest legal modifications and can leverage robust clinical trial management services to enhance feasibility studies, compliance reviews, project management, and reporting processes.
What strategies are recommended for successfully negotiating CTAs?
Successful negotiation strategies include utilizing standardized templates, prioritizing essential terms, remaining open to compromise, and understanding the motivations of the other party. Regular training on negotiation tactics is also beneficial.
Why is transparency in budget and payment terms important in CTAs?
Detailed budget and payment terms provide transparency in the financial aspects of the trial, ensuring that all parties are aligned on expectations, which is crucial for successful collaboration and compliance.
2025 FDAAA 801 Final Rule Changes Impacting Clinical Trial (https://prorelixresearch.com/2025-fdaaa-801-clinical-trial-rule-impact)
Compliance Challenges for Clinical Research Sites - Food and Drug Law Institute (FDLI) (https://fdli.org/2025/02/compliance-challenges-for-clinical-research-sites)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
FDA in Flux — July 2025 Newsletter (https://mintz.com/insights-center/viewpoints/2791/2025-07-08-fda-flux-july-2025-newsletter)
Changes in Industry Marketing and Research Payments to US Physicians and Teaching Hospitals During the COVID-19 Pandemic - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC9526091)