


The article titled "10 Essential eCRFs for Streamlined Clinical Research Success" underscores the pivotal role of electronic case report forms (eCRFs) in optimizing the efficiency and effectiveness of clinical research. It asserts that bespoke eCRF designs, which are meticulously aligned with regulatory standards and feature user-friendly interfaces, can profoundly enhance data quality, expedite participant enrollment, and ensure compliance. This, in turn, facilitates the successful execution of clinical trials, making the case for their critical importance in the research landscape.
In the rapidly evolving landscape of clinical research, the efficiency and accuracy of data collection are paramount. Electronic Case Report Forms (eCRFs) have emerged as essential tools that not only streamline the research process but also enhance compliance with regulatory standards. This article delves into ten crucial eCRFs that promise to revolutionize clinical trials, offering insights into their design, implementation, and the significant benefits they bring to Medtech innovators. However, as the demand for rapid innovation grows, research teams must consider:
bioaccess® excels in creating personalized eCRFs tailored to meet the unique requirements of Medtech innovators. By addressing the distinct challenges these firms face, bioaccess® simplifies information gathering, enhances adherence, and accelerates regulatory approvals. This customized approach allows Medtech companies to concentrate on innovation while bioaccess® manages the complexities of health information, facilitating enrollment 50% faster than traditional methods.
For instance, bioaccess® utilizes a collaborative design strategy, working closely with clients to ensure that electronic case report forms align with their study protocols and regulatory requirements. This collaboration not only improves information quality but also expedites the trial timeline by utilizing eCRFs, enabling quicker market entry for new medical devices. The emphasis on eCRFs has significantly influenced innovation success rates, as evidenced by successful applications in numerous clinical trials, where these tailored electronic case report forms have led to improved efficiency and outcomes.
As highlighted by industry leaders, "Standardization allows our teams to get a jump-start and gives us a head start on programming and study startup activities." This statement underscores the importance of standardization in the development of eCRFs, which fosters efficiencies and enhances data quality. In today's fast-paced MedTech landscape, where rapid innovation is vital, eCRFs are not merely advantageous but essential for success. To maximize the effectiveness of electronic case report forms, Medtech innovators should consider incorporating standardized templates and processes into their clinical trials.
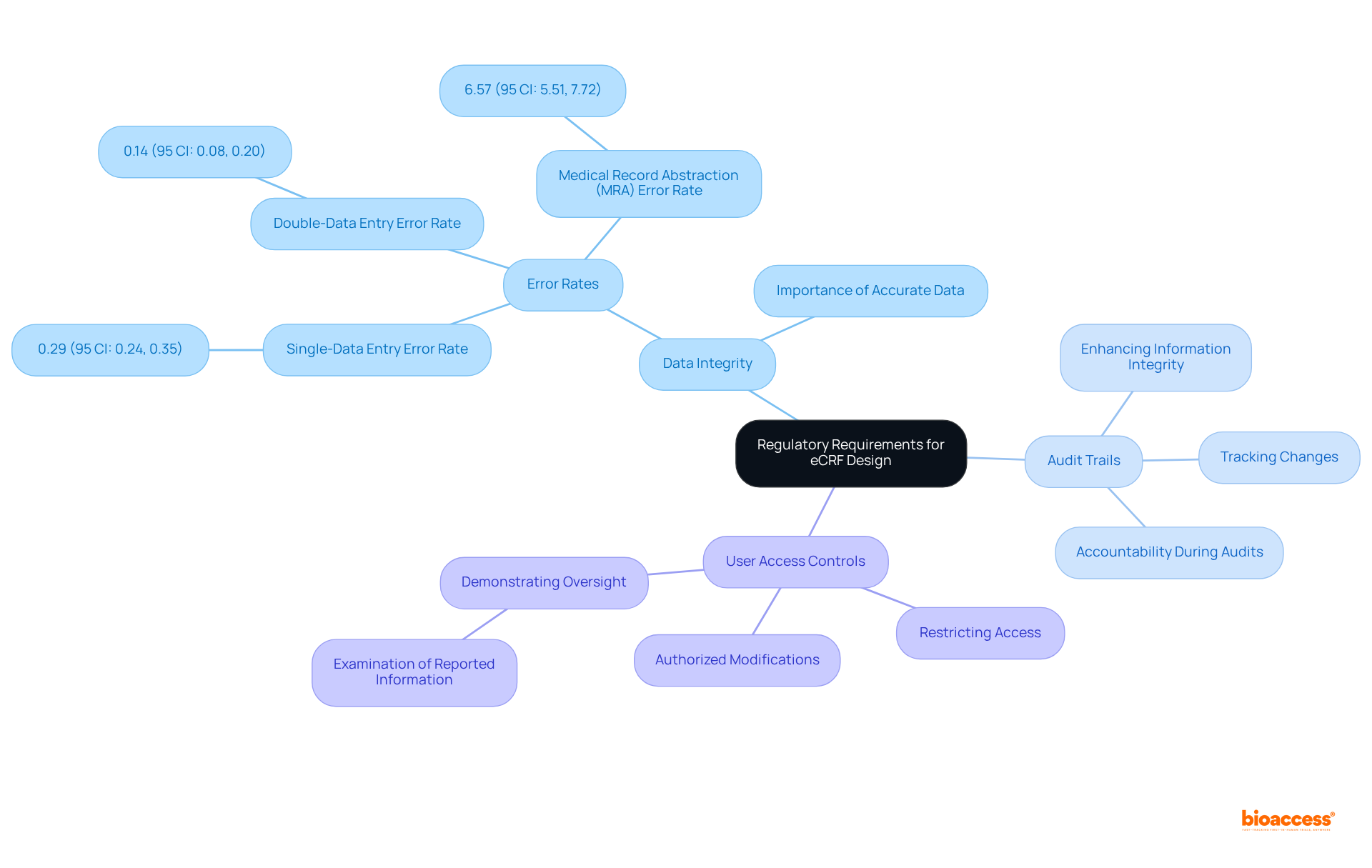
Creating electronic case report templates necessitates a comprehensive understanding of regulatory requirements, particularly the FDA guidelines and ICH GCP standards. Adhering to these regulations is crucial for ensuring that the information collected is valid and can withstand rigorous audits. Key considerations include:
By aligning eCRFs with these regulatory requirements, clinical research teams can enhance the credibility of their studies and streamline the regulatory submission process, ultimately facilitating faster approvals and improving the overall quality of clinical research. Furthermore, effective eCRFs designs that comply with FDA and ICH GCP standards serve as practical examples for teams aiming to improve their compliance and information integrity.
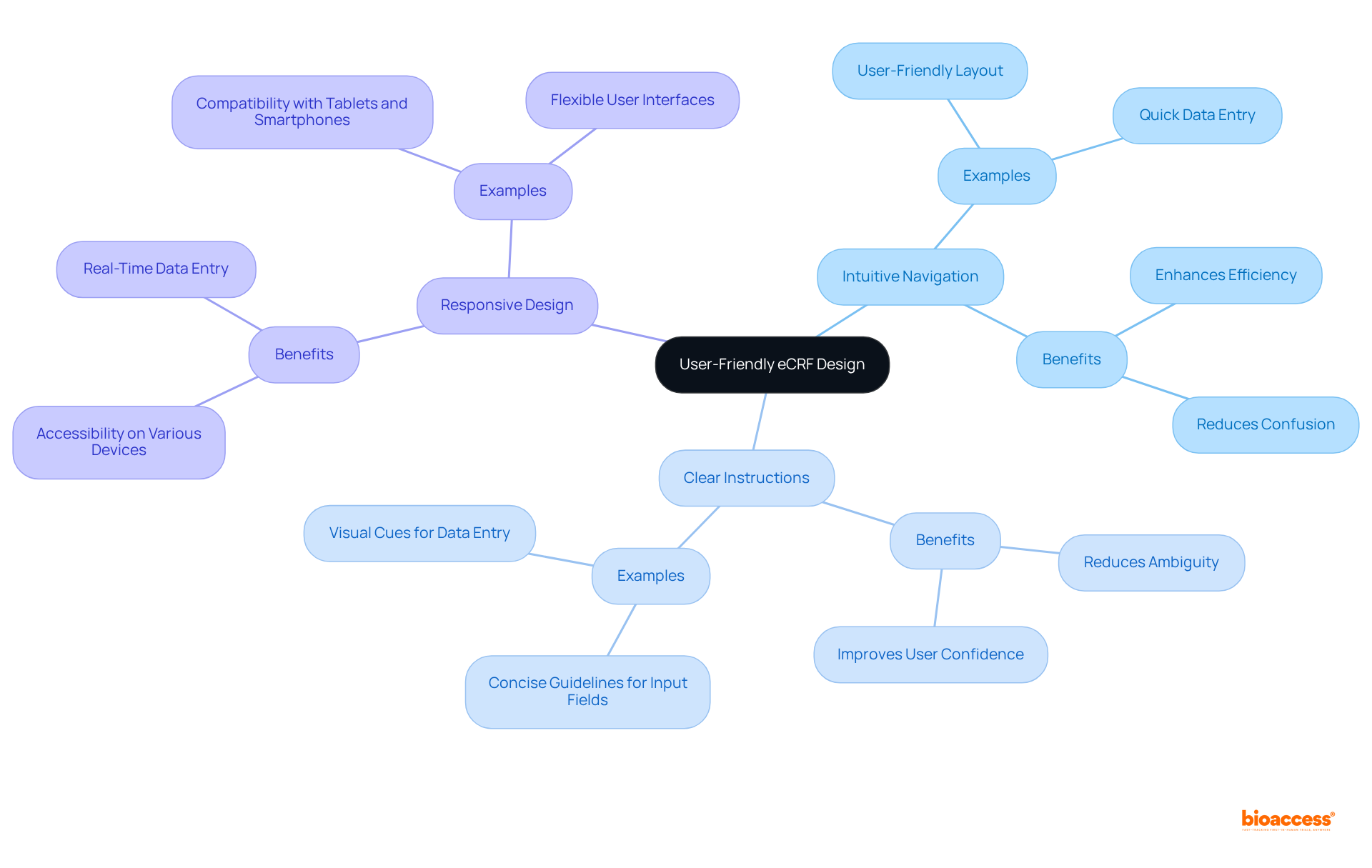
Designing intuitive interfaces for eCRFs is essential for enhancing accuracy and reducing entry mistakes. Key design principles include:
Responsive design ensures eCRFs are accessible on various devices, including tablets and smartphones, thereby enhancing usability for medical staff across diverse settings. This flexibility is essential, as it enables smooth information entry in real-time, regardless of location.
By emphasizing user-friendly design, research teams can simplify information collection processes, enabling staff to focus on their main duties instead of dealing with complicated interfaces. As one clinical researcher remarked, "Maximizing information precision in electronic case report form design is not solely about technology; it's about fostering an environment where users can execute their best work without unnecessary obstacles." Moreover, adherence to regulations such as HIPAA and GDPR is vital for ensuring information security, further highlighting the significance of careful eCRFs design.
Creating clear information gathering strategies is crucial for the success of eCRFs. To achieve this, consider the following approaches:
By implementing these strategies, research teams can significantly improve the quality of the information gathered, streamline the overall management process, and ultimately contribute to more reliable and impactful research results. Successful executions of standardized information entry have demonstrated that organizations can obtain ethical approvals in as little as 4-6 weeks and enroll participants 50% faster than traditional markets, emphasizing the essential role eCRFs play in contemporary research. As highlighted by specialists in the field, 'Standardized information entry is essential for ensuring patient safety, regulatory adherence, and cost efficiency in healthcare,' stressing the importance of rigorous information management practices.
Incorporating real-time information access into eCRFs significantly enhances the efficiency of clinical trials. This innovation not only streamlines processes but also yields several key advantages:
Furthermore, real-time monitoring can greatly affect results and guarantee regulatory adherence, which is crucial as worldwide trials are projected to exceed $70 billion by 2025. To further support these claims, Meri Beckwith notes, "Real-time monitoring serves as a means to maintain information integrity, offering a layer of confidence that the trials will yield reliable results."
To effectively implement real-time monitoring in clinical trials, eCRFs must be integrated with robust information management systems that support instant uploads and analytics. Leveraging advancements in AI and machine learning will enhance accuracy and operational efficiency, paving the way for more successful clinical research outcomes.
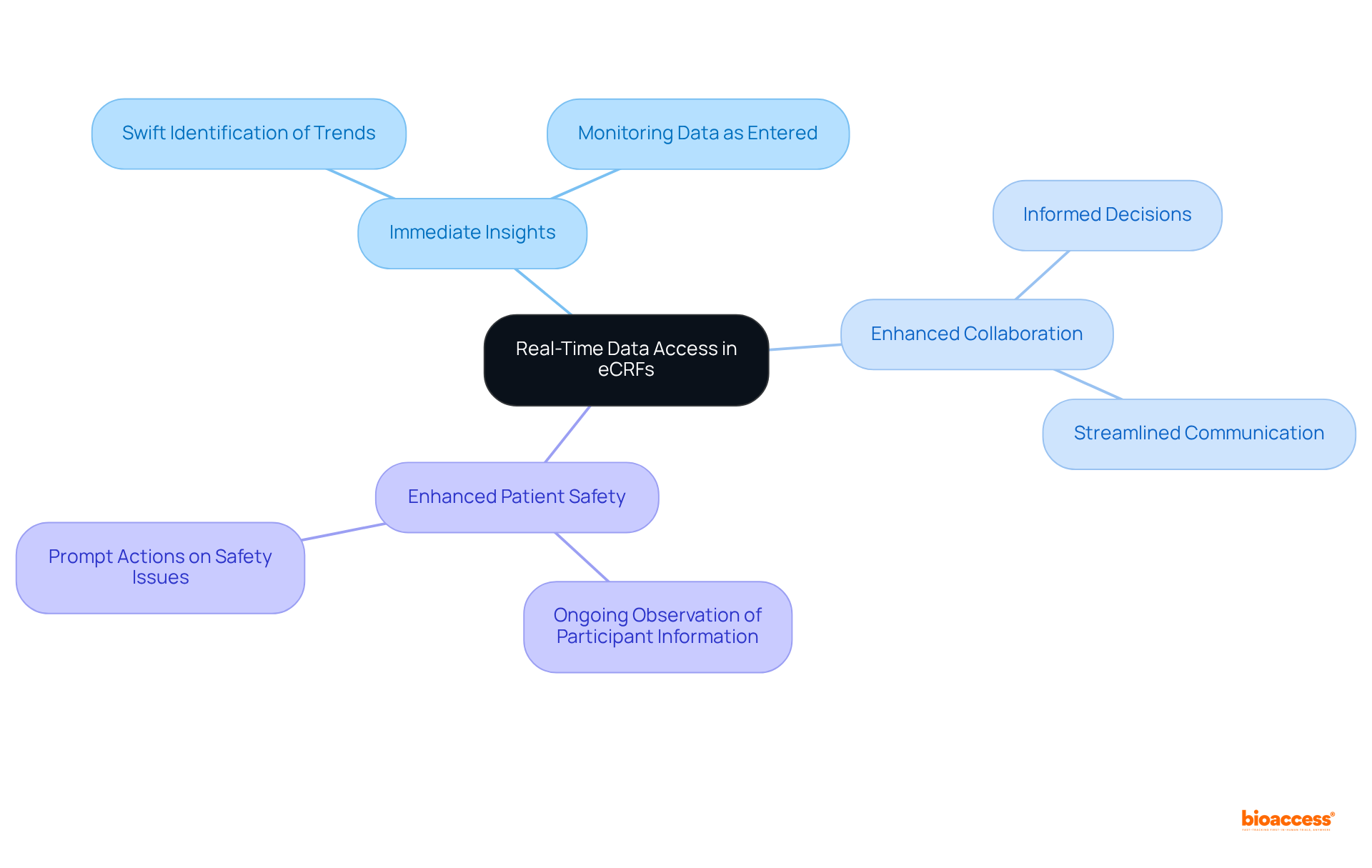
Ensuring information security and privacy in eCRFs design is essential for protecting sensitive patient details. Key measures include:
Data Encryption: Implementing robust encryption protocols, such as AES and RSA, for data both in transit and at rest is crucial to protect against unauthorized access. This practice not only secures sensitive information but also aligns with regulatory compliance requirements. As Diana Andrade, Founder & Managing Director at RD Privacy, states, "Data must be stored securely for regulatory compliance and potential future audits."
Access Controls: Role-based access restrictions guarantee that only permitted individuals can access sensitive information, effectively reducing the risk of breaches. This approach fosters a culture of accountability and security among team members.
Conducting regular audits of the eCRFs is vital for identifying and mitigating potential security vulnerabilities. These audits help preserve the integrity of the information and ensure adherence to evolving regulatory standards. Significantly, healthcare cyberattacks impacted more than 100 million individuals in 2023, underscoring the essential requirement for robust security measures.
By prioritizing these information security measures, research teams can effectively safeguard participant details and maintain the highest standards of regulatory compliance. Notably, the typical HIPAA fine has diminished since 2018, even as the number of breaches has risen, emphasizing the financial consequences of security failures.
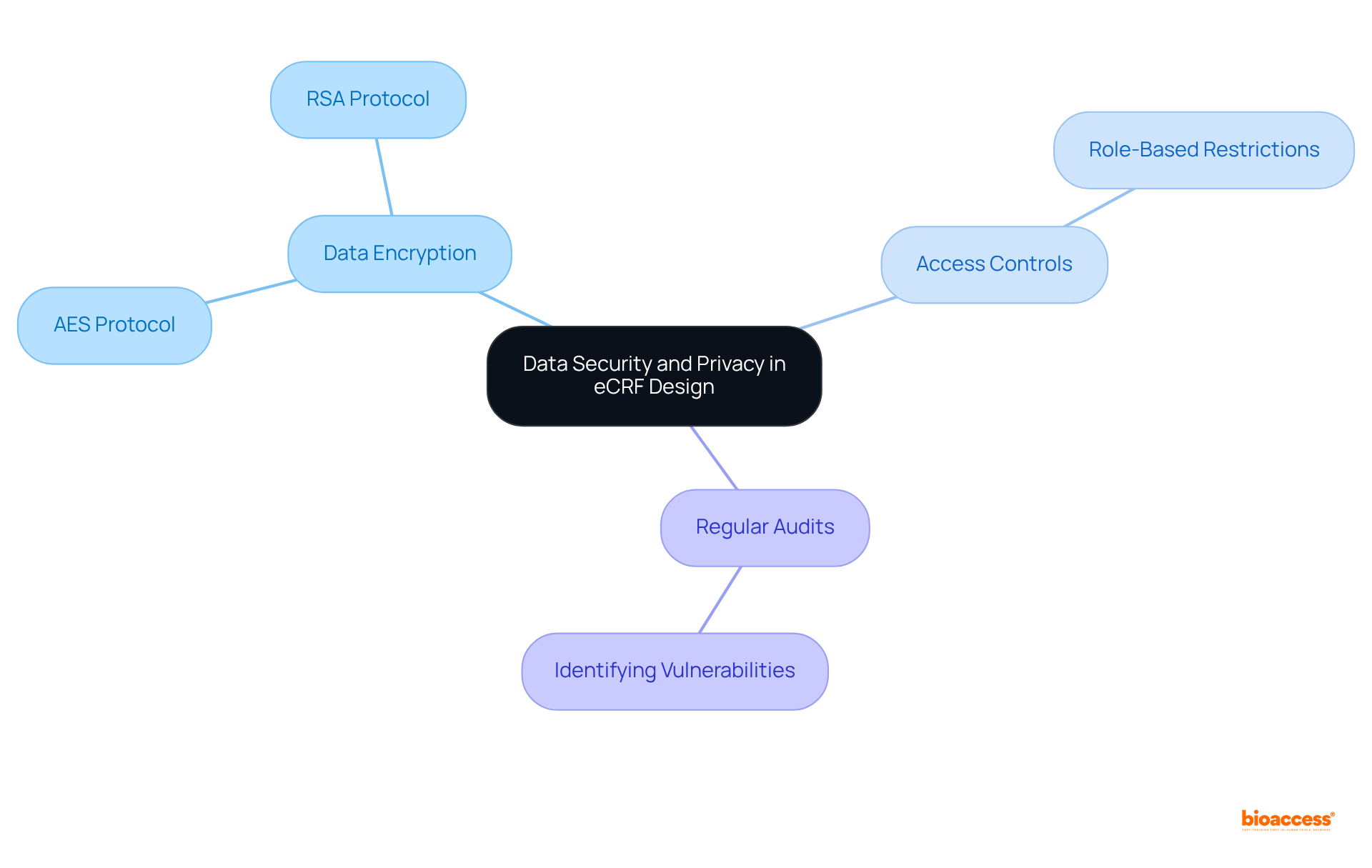
Automating eCRFs processes can significantly enhance efficiency in clinical trials. Here are key automation strategies to consider:
According to industry insights, around 60% of all jobs contain at least 30% of their constituent activities that can be automated, emphasizing the potential for efficiency improvements in trials. By utilizing these automation strategies, research teams can enhance productivity and reduce the burden of repetitive tasks in eCRFs, ultimately resulting in more efficient and successful trials.
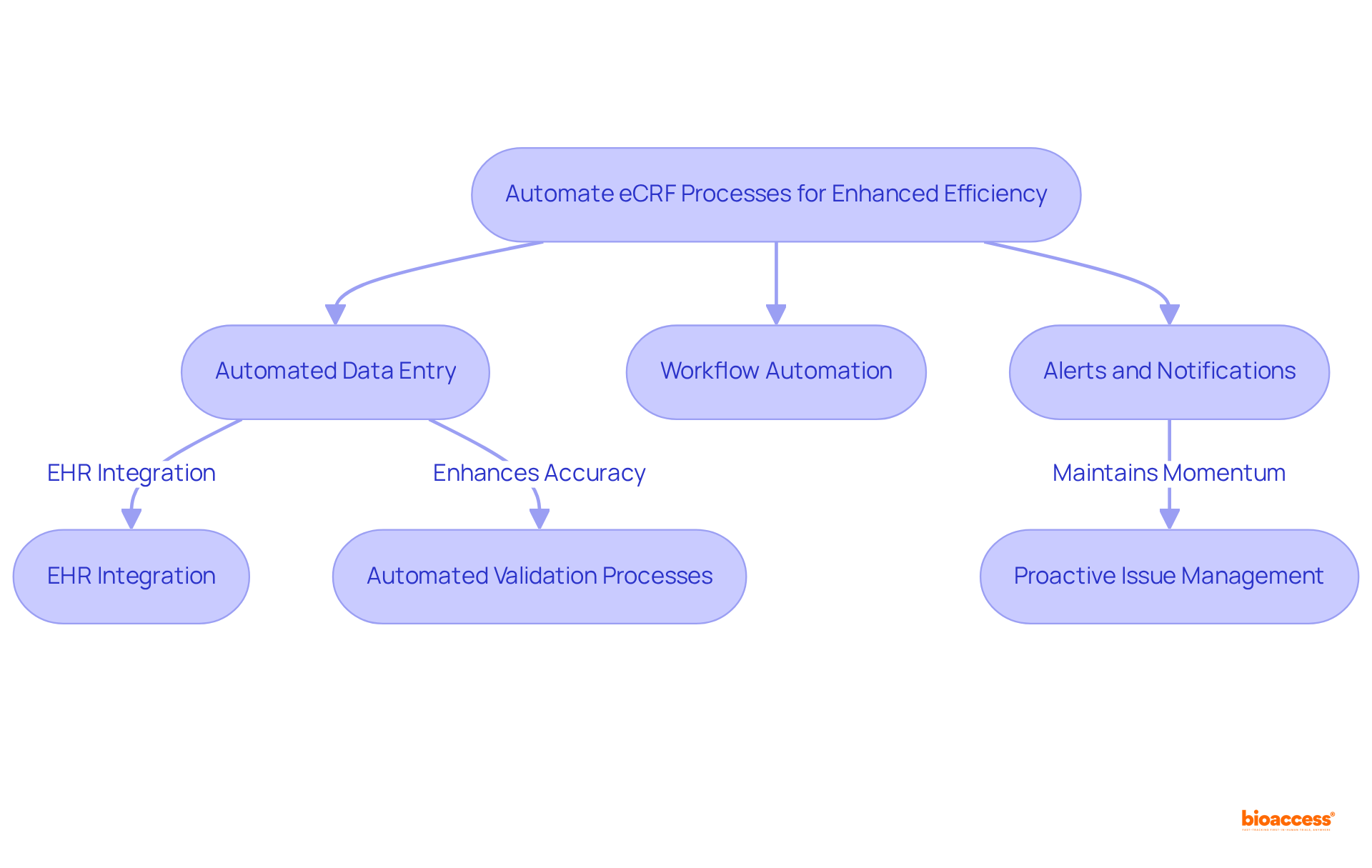
Training personnel on eCRFs is essential for enhancing their effectiveness in clinical research. Comprehensive training programs must encompass key elements that ensure success:
Including statistics, such as the fact that EDC systems significantly reduce the time and resources needed for entry, monitoring, and reporting, underscores the importance of effective training programs. Additionally, quotes from thought leaders, like Albert Einstein's assertion that "Intellectual growth should commence at birth and cease only at death," can inspire staff to embrace continuous learning.
Moreover, citing case studies such as "Insights on Transitioning to EDC" illustrates the practical effects of effective training on electronic case report form systems.
Investing in these comprehensive training strategies not only enhances data quality but also empowers research teams to utilize eCRFs with confidence and efficiency, ensuring adherence to data validation plans and regulatory standards.
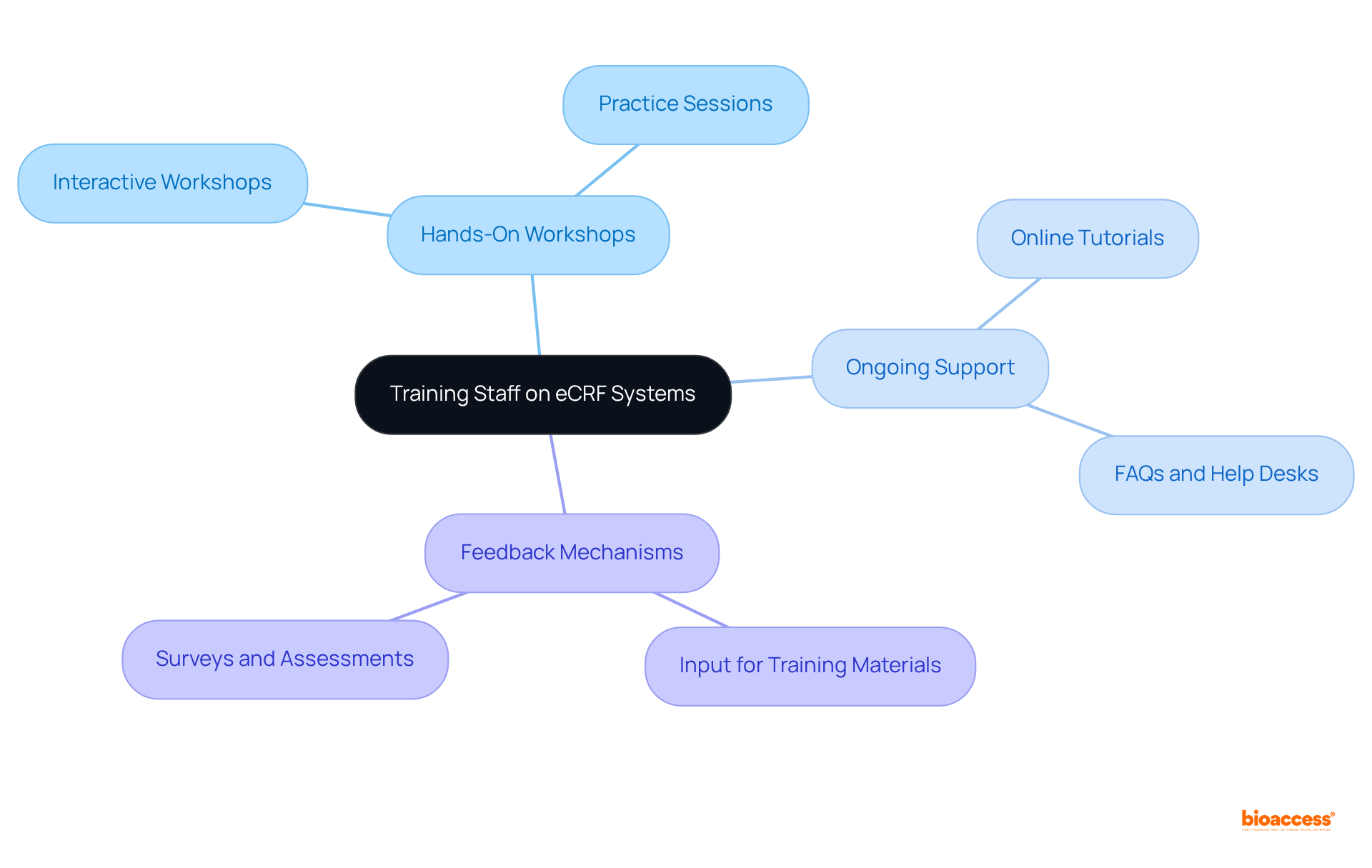
Implementing feedback loops is vital for the continuous improvement of eCRFs. Regular user surveys are essential; conducting surveys to gather input from clinical staff on their experiences with the eCRFs can mirror successful implementations seen in organizations like HealthVerity, which utilizes verified clinical narratives to enhance data accessibility and usability. Additionally, arranging focus groups with key stakeholders allows for discussions on challenges and possible improvements to the design. Engaging stakeholders in this manner can lead to innovative solutions that enhance the overall system.
Furthermore, using feedback to make iterative updates to the eCRFs ensures that they evolve to meet the changing needs of the study. This iterative process is crucial for sustaining relevance in a fast-paced medical research environment. By promoting a culture of ongoing enhancement, clinical research teams can improve the usability and effectiveness of their eCRFs, ultimately leading to more efficient and successful clinical trials. Incorporating insights from leaders in privacy-protecting technologies, such as HealthVerity, can further strengthen the impact of these feedback loops.
Conducting pilot tests is a crucial step in the implementation of eCRFs. It is essential to develop realistic test scenarios that closely mimic actual data entry and usage during the trial. Gathering user feedback during the pilot phase is vital to identify usability issues and areas for improvement. Based on the results of the pilot tests, necessary adjustments to the eCRFs should be made prior to full-scale deployment.
By thoroughly testing eCRFs before implementation, clinical research teams can ensure a smoother rollout and significantly enhance the overall quality of data collection using eCRFs.
The integration of electronic case report forms (eCRFs) into clinical research is not merely a trend; it has solidified its status as a cornerstone for achieving streamlined success in the Medtech industry. By leveraging tailored eCRF design services, such as those offered by bioaccess®, organizations can expertly navigate the complexities of data collection, regulatory compliance, and user interaction. This strategic approach empowers Medtech innovators to concentrate on their core mission of advancing healthcare solutions while ensuring that their clinical trials are both efficient and compliant.
Throughout this discussion, key strategies for optimizing eCRF implementation have been highlighted, including:
The significance of real-time data access, automation, and robust security measures has also been underscored, illustrating how these elements contribute to enhanced data integrity and improved trial outcomes. Moreover, the necessity of training staff and implementing feedback loops ensures that eCRFs remain relevant and effective in a rapidly evolving research landscape.
In conclusion, the successful adoption of eCRFs is paramount for enhancing the efficiency and quality of clinical trials. As the demand for innovative medical solutions continues to escalate, so too does the need for effective data management practices. Embracing these best practices not only positions organizations for success but also reinforces the imperative of continuous improvement in clinical research. By prioritizing the development and implementation of high-quality eCRFs, stakeholders can significantly impact patient safety, regulatory adherence, and overall research outcomes.
What services does bioaccess® offer for Medtech innovators?
bioaccess® specializes in creating tailored electronic case report forms (eCRFs) that address the unique challenges faced by Medtech innovators, simplifying information gathering, enhancing adherence, and accelerating regulatory approvals.
How does bioaccess® improve the enrollment process for clinical trials?
bioaccess® facilitates enrollment 50% faster than traditional methods by managing the complexities of health information and utilizing a collaborative design strategy that aligns eCRFs with study protocols and regulatory requirements.
What role do eCRFs play in clinical trials?
eCRFs improve information quality and expedite trial timelines, leading to quicker market entry for new medical devices and significantly influencing innovation success rates in clinical trials.
Why is standardization important in eCRF development?
Standardization fosters efficiencies and enhances data quality, allowing teams to initiate programming and study startup activities more effectively, which is essential in the fast-paced MedTech landscape.
What regulatory requirements must be considered in eCRF design?
eCRF design must comply with FDA guidelines and ICH GCP standards, ensuring data integrity, establishing robust audit trails, and implementing user access controls to safeguard information integrity.
What is the significance of data integrity in eCRFs?
Data integrity ensures that the information collected is accurate, complete, and verifiable, which is crucial for passing audits and maintaining the credibility of clinical research.
How do audit trails contribute to eCRF compliance?
Audit trails track changes and provide a clear history of modifications, enhancing information integrity and accountability during audits, which is vital for regulatory compliance.
What design principles should be prioritized in eCRF development?
Key design principles include intuitive navigation, clear instructions, and responsive design to enhance user experience, reduce entry mistakes, and ensure accessibility across various devices.
How does user-friendly design impact data entry accuracy?
Intuitive interfaces and clear guidelines reduce confusion and errors, leading to higher accuracy in data entry, which ultimately improves the quality of the information collected.
Why is adherence to regulations like HIPAA and GDPR important in eCRF design?
Compliance with regulations such as HIPAA and GDPR is essential for ensuring information security, protecting sensitive data, and maintaining the trust of participants and stakeholders in clinical trials.