


The title "10 Essential Features of a Clinical Trials Management System" poses a critical question: What are the key functionalities necessary for an effective clinical trials management system (CTMS)? This article outlines essential features, including:
These functionalities are not merely beneficial; they are crucial for enhancing the efficiency, accuracy, and overall success of clinical trials. By ensuring compliance, improving data integrity, and facilitating informed decision-making, a robust CTMS significantly contributes to the advancement of clinical research.
As the landscape of clinical research continues to evolve, the demand for effective management solutions becomes increasingly critical. A well-designed Clinical Trials Management System (CTMS) can streamline processes, enhance compliance, and ultimately accelerate the journey from concept to market. However, with numerous options available, what essential features set a CTMS apart from the rest? Understanding these key functionalities is vital for organizations aiming to optimize their clinical trials and ensure successful outcomes.
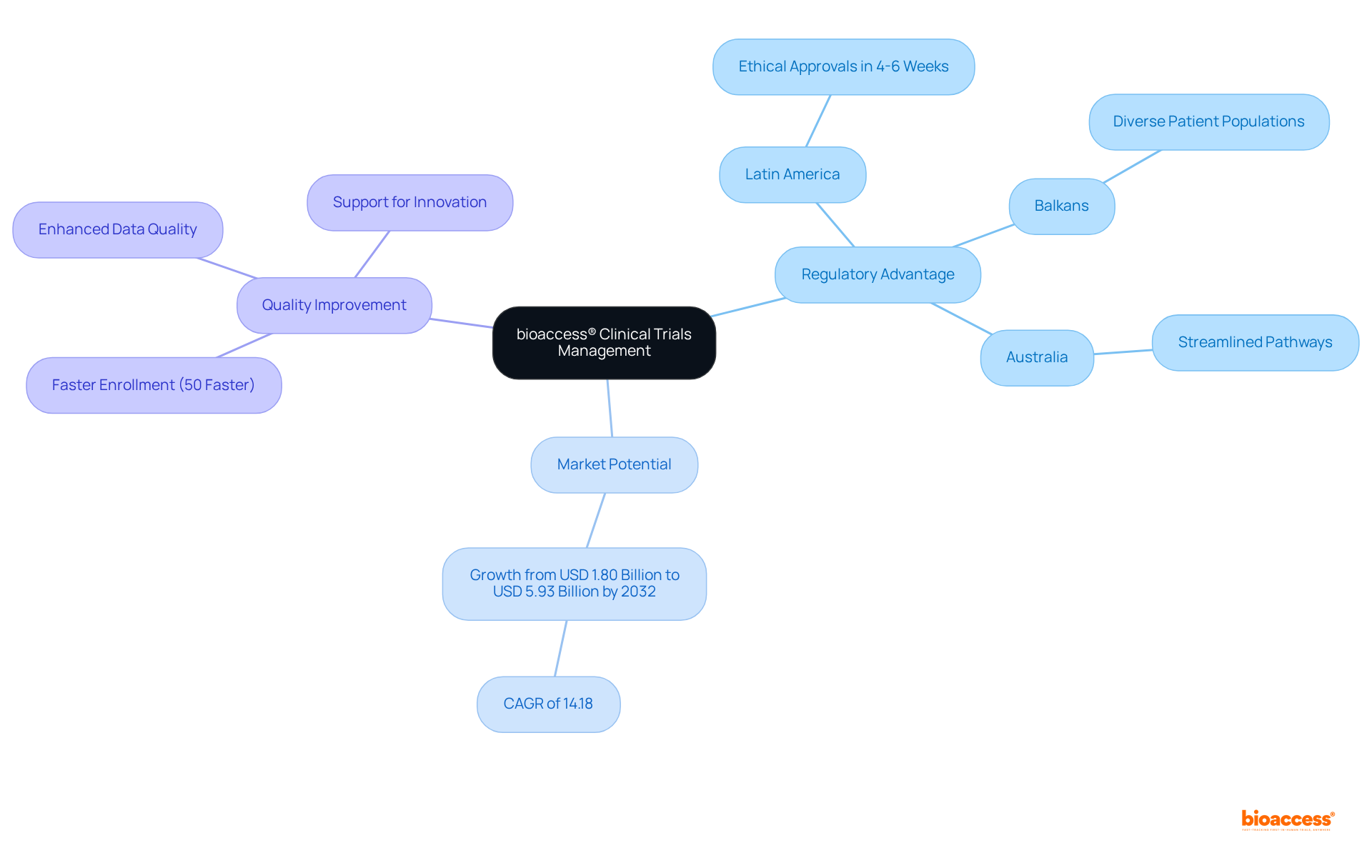
bioaccess® excels in providing expedited oversight of studies specifically tailored for Medtech innovators. By harnessing the regulatory speed of Latin America, the diverse patient populations in the Balkans, and the streamlined pathways in Australia, bioaccess® achieves ethical approvals in a remarkable 4-6 weeks. This rapid turnaround is crucial for Medtech companies aiming to expedite their innovations to market, significantly impacting their development timelines.
With the worldwide research management systems market expected to expand from USD 1.80 billion in 2023 to USD 5.93 billion by 2032, the need for effective management solutions is evident. Moreover, as the intricacy of medical studies rises, the requirement for sophisticated CTMS functionalities becomes essential. Successful instances from Latin America and Australia showcase how bioaccess®'s method not only speeds up the clinical research process but also improves the quality of information gathered, ultimately promoting innovation in the Medtech sector.
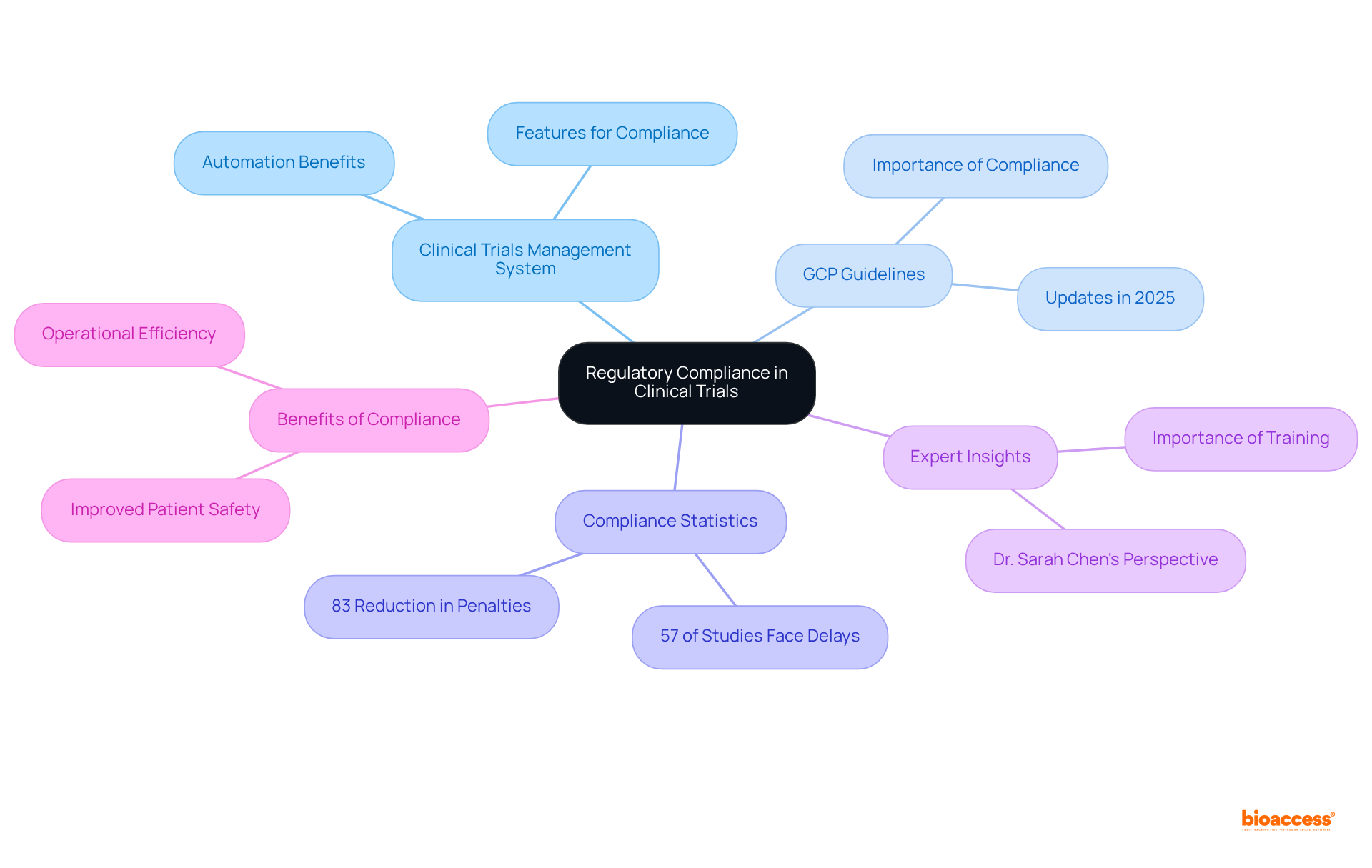
A robust clinical trials management system is crucial for ensuring compliance with both local and international regulatory standards. It must encompass features that effectively track approvals, maintain comprehensive documentation, and ensure that all activities align with Good Clinical Practice (GCP) guidelines. The updates to GCP in 2025 underscore the necessity for enhanced information integrity and patient safety, making compliance even more vital.
Statistics reveal that approximately 57% of medical studies encounter delays due to compliance issues, while 57% of medical researchers believe data problems lead to study delays. This highlights the significance of a clinical trials management system in mitigating these risks. By automating compliance checks and providing real-time updates, the clinical trials management system simplifies the oversight of study processes and aids organizations in avoiding the costly delays and penalties associated with non-compliance. Improved compliance processes can reduce regulatory penalties by 83%, showcasing the effectiveness of clinical trials management systems in enhancing compliance.
Expert opinions reinforce that adherence to GCP is not merely a regulatory obligation but a fundamental aspect of ethical research, safeguarding participants' rights and ensuring the integrity of study outcomes. For example, Dr. Sarah Chen pointed out that regulatory analytics transforms compliance from a reactive, checkbox exercise into a dynamic, intelligence-driven function capable of anticipating risks before they arise.
Entities that utilize sophisticated clinical trials management system solutions have reported significant improvements in compliance rates, further demonstrating the advantages of integrating technology into clinical study oversight.
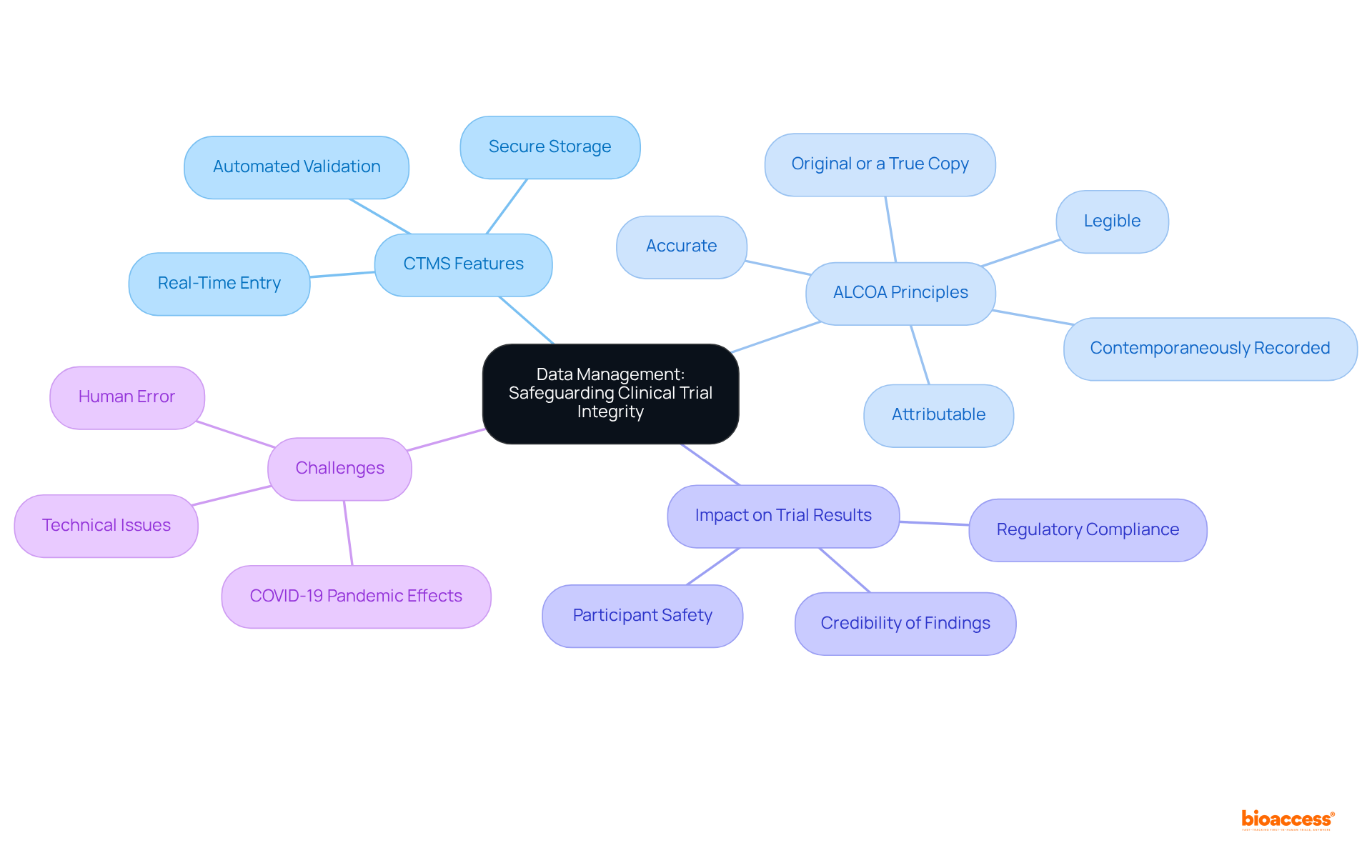
A clinical trials management system must encompass robust information management capabilities, including secure storage, real-time entry, and automated validation processes. These characteristics are crucial for guaranteeing that all information gathered during the experiment is precise, comprehensive, and easily obtainable for examination. By prioritizing information integrity, the clinical trials management system (CTMS) not only enhances the credibility of trial results but also supports compliance with regulatory submissions, which is essential for gaining approvals from agencies like the FDA and EMA.
Best practices for maintaining information integrity in clinical research include adhering to the ALCOA principles—ensuring records are:
Moreover, executing thorough information handling strategies (DMPs) that detail information gathering, storage, and analysis procedures is essential. For example, using electronic systems for information handling can greatly lessen human mistakes and enhance reliability.
The influence of efficient information management on clinical trial results cannot be emphasized enough; it directly affects the credibility of findings and the overall success of the trial. As Richard Zink observed, 'The quality of the information influences the validity and integrity of the final analysis, and our capacity to convey significant results.'
Furthermore, the COVID-19 pandemic has brought forth new challenges to information integrity, necessitating adaptive strategies to ensure participant safety and information reliability. Continuous training and education for all personnel involved in research trials are crucial for upholding high standards of information integrity. As the landscape of clinical research evolves, maintaining these standards remains a fundamental priority for sponsors and stakeholders alike.
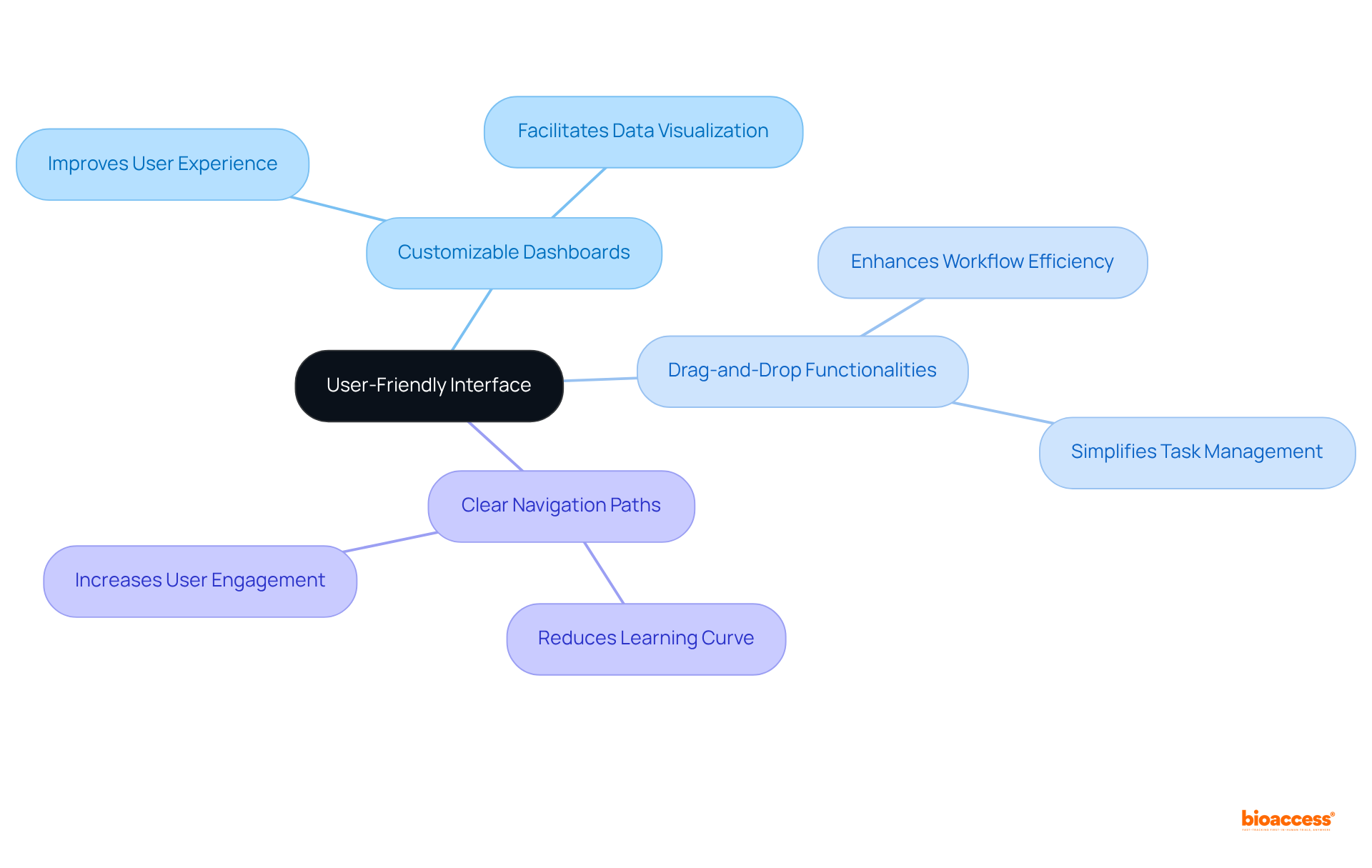
An intuitive user interface is paramount for a clinical trials management system, empowering research teams to navigate the system effortlessly. Features such as customizable dashboards, drag-and-drop functionalities, and clear navigation paths significantly enhance user experience. By minimizing the learning curve, a user-friendly interface allows teams to concentrate on their research activities rather than grappling with technology.
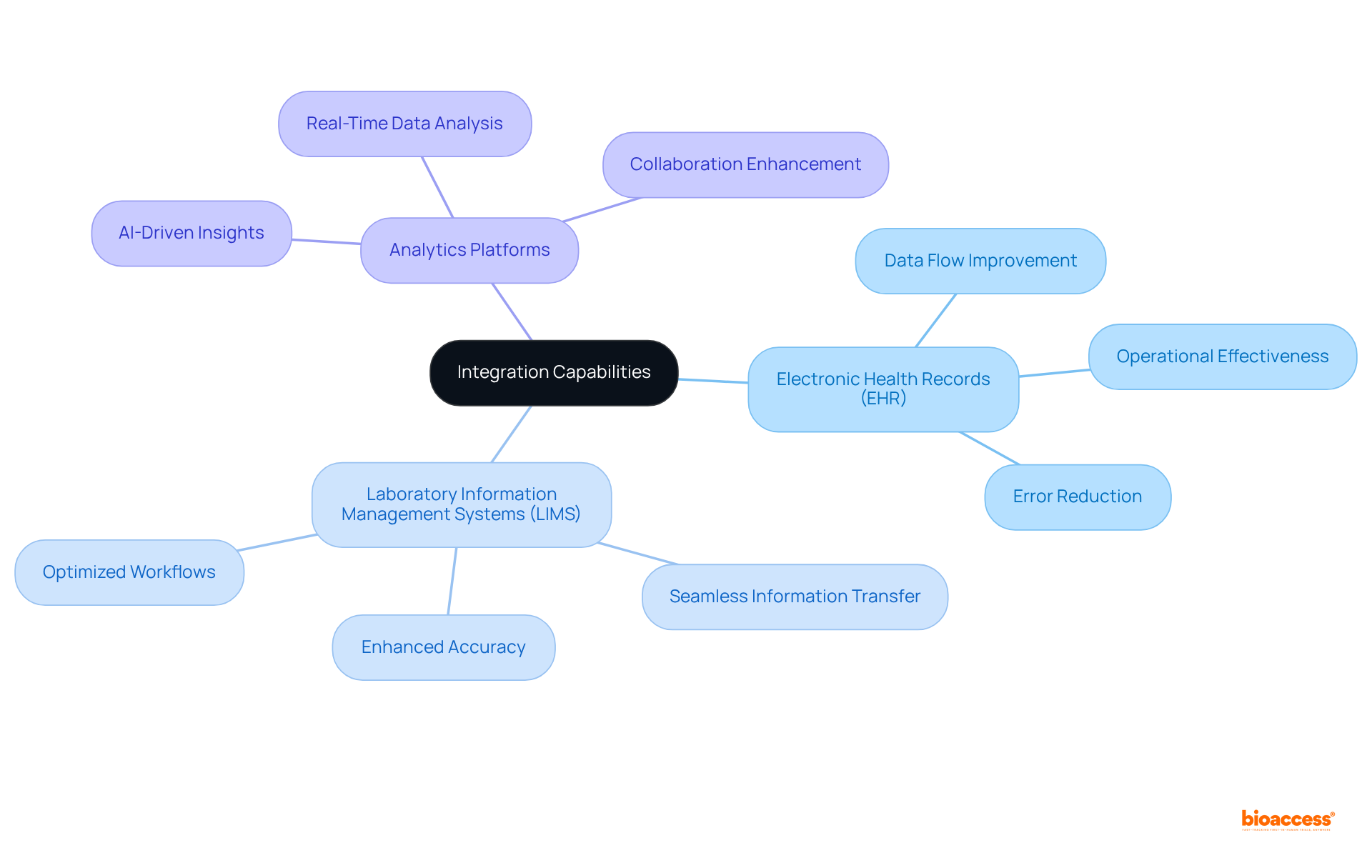
A clinical trials management system must provide robust integration features with systems such as Electronic Health Records (EHR), Laboratory Information Management Systems (LIMS), and analytics platforms. This interoperability facilitates seamless information transfer, significantly reducing manual input and minimizing the risk of errors. As research experiments increasingly adopt unified systems for data handling, operational effectiveness improves, leading to enhanced accuracy and optimized workflows. Notably, approximately 40% of global study initiations utilize integrated systems, reflecting a growing recognition of the importance of interoperability in research data management. Recent advancements in 2025, including the integration of AI-driven analytics into the clinical trials management system, highlight the urgent need for cohesive data environments that promote collaboration and improve overall study outcomes. Moreover, organizations like bioaccess® are at the forefront of this initiative, enabling faster enrollment and ethical approvals, which further enhances the efficiency of clinical studies.
An effective clinical trials management system is essential in today’s clinical research landscape, incorporating sophisticated reporting and analytics capabilities that empower managers to produce real-time reports on essential performance indicators (KPIs). These reports yield valuable insights into patient recruitment rates, information quality, and compliance metrics. By harnessing the power of analytics, organizations can make data-informed decisions that not only enhance performance but also proactively address potential challenges.
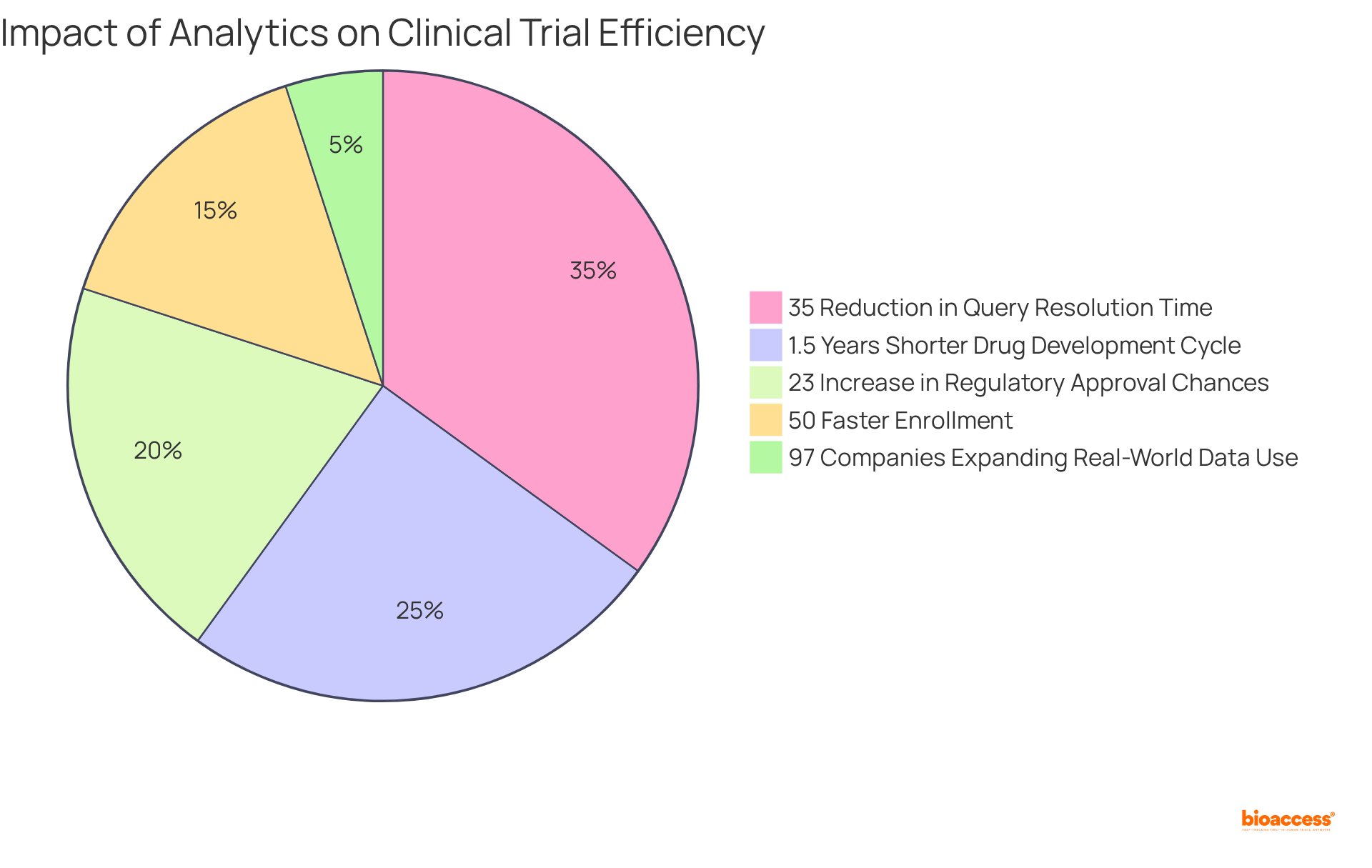
Notably, clinical studies that utilize a clinical trials management system for integrated information handling solutions have demonstrated a remarkable 35% reduction in query resolution time, significantly boosting overall efficiency. Moreover, research indicates that pharmaceutical firms utilizing a clinical trials management system can shorten their drug development cycle by an average of 1.5 years and enhance their chances of regulatory approval by 23%.
Furthermore, bioaccess® enables enrollment 50% faster than conventional markets, underscoring the vital role of analytics in refining testing processes. As we approach 2025, the demand for real-time reporting tools is projected to escalate, with 97% of companies planning to expand their use of real-world patient data to enhance decision-making accuracy. This shift towards data-driven decision-making is crucial for improving study outcomes and ensuring patient safety.
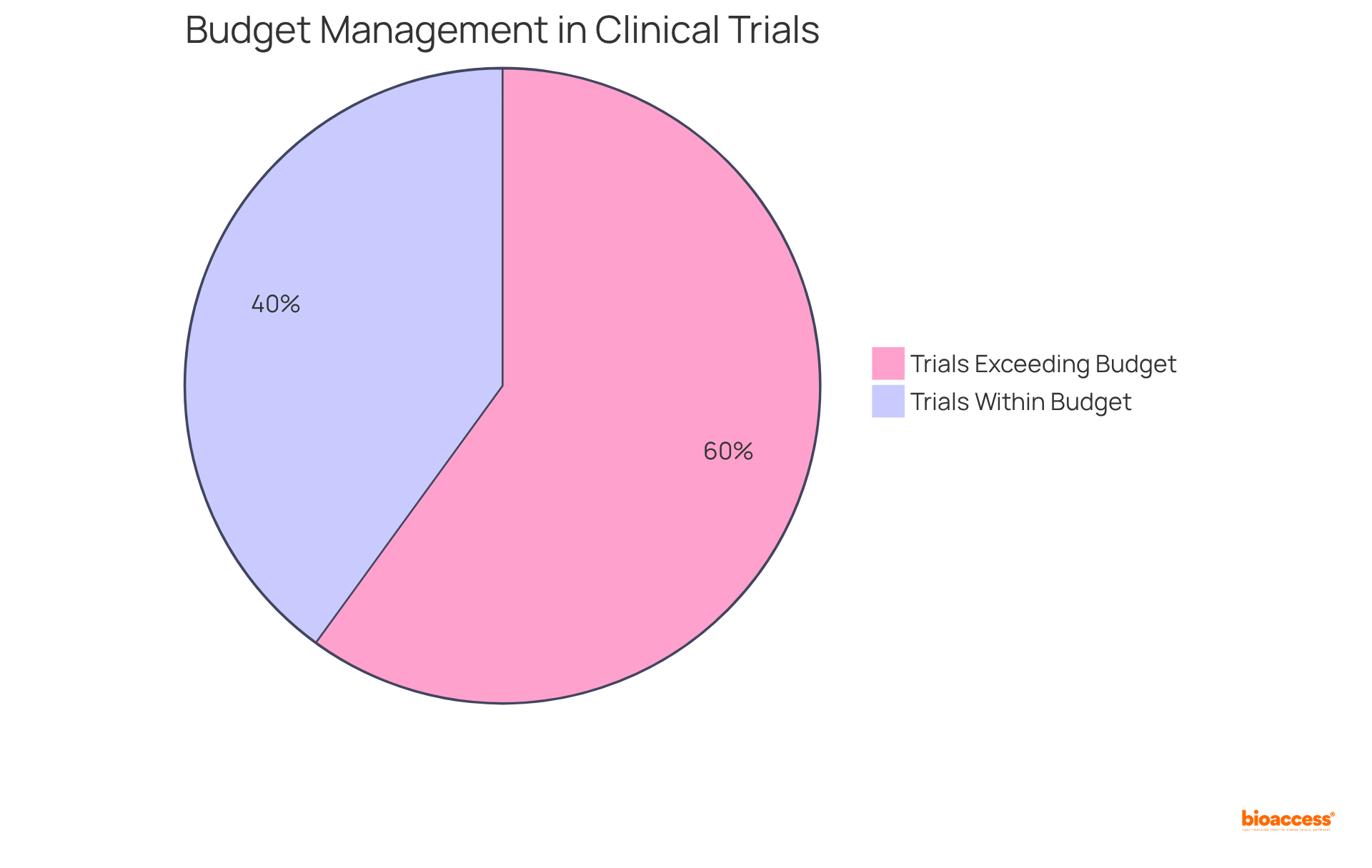
A robust clinical trials management system is essential for integrating comprehensive financial management tools that streamline budgeting, expense tracking, and invoicing processes. Key features, such as budget forecasting, expense reporting, and financial reconciliation, are vital for maintaining the financial viability of research studies. Notably, budget overruns are prevalent in clinical trials, with 60% exceeding their budgets by an average of 30%. This underscores the necessity for precise financial oversight.
By offering clear visibility into financial performance, a clinical trials management system enables organizations to make informed strategic decisions regarding resource allocation and cost management. Firms utilizing analytics are 35% more likely to achieve their study goals and can decrease cost overruns by up to 20%. In 2025, employing sophisticated budgeting tools can significantly enhance the precision of financial forecasts.
It is particularly noteworthy that the median projected expense of crucial studies was $48 million per approved medication. Optimal methods in budgeting and expense monitoring involve consistent financial assessments and the use of analytics to pinpoint cost factors. This approach can assist in reducing the risk of budget excesses and guaranteeing positive outcomes.
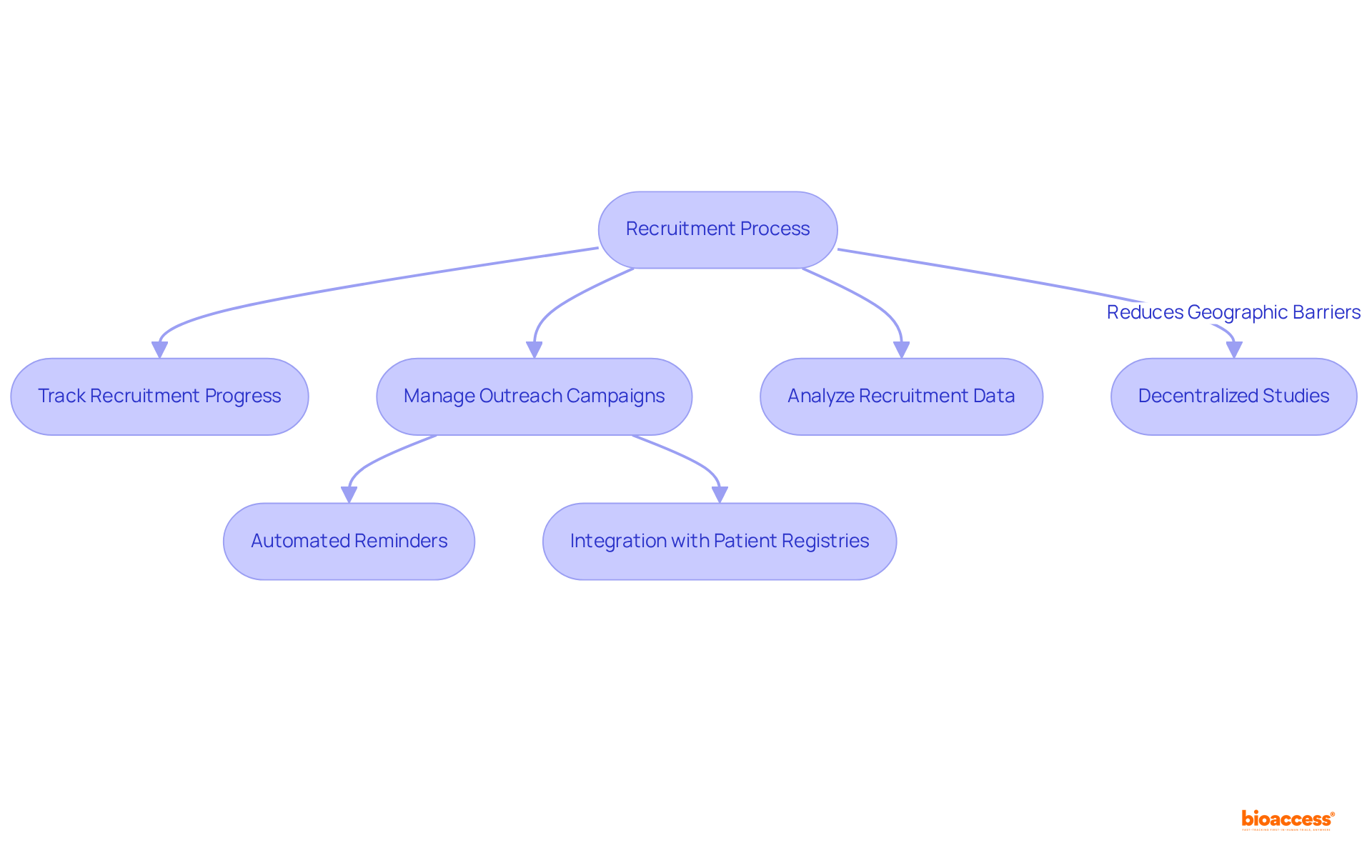
A clinical trials management system is crucial for optimizing participant recruitment by providing tools that track recruitment progress, manage outreach campaigns, and analyze recruitment data. Features such as automated reminders for follow-ups and seamless integration with patient registries significantly enhance recruitment efficiency. Notably, bioaccess® achieves enrollment 50% faster than traditional markets, underscoring the critical nature of timely enrollment. Delays in clinical studies can cost sponsors between $600,000 and $8 million per day, making effective recruitment strategies essential for maintaining study timelines and enhancing the chances of successful outcomes.
Decentralized studies have emerged as an effective recruitment approach, allowing participants to engage in study activities locally or virtually, thus minimizing geographic barriers. As Drozd observed, "Decentralized studies reduce geographic obstacles and mobility constraints." As the landscape of research studies evolves, utilizing a clinical trials management system not only streamlines the recruitment process but also fosters a more patient-focused approach, ultimately leading to improved study outcomes.
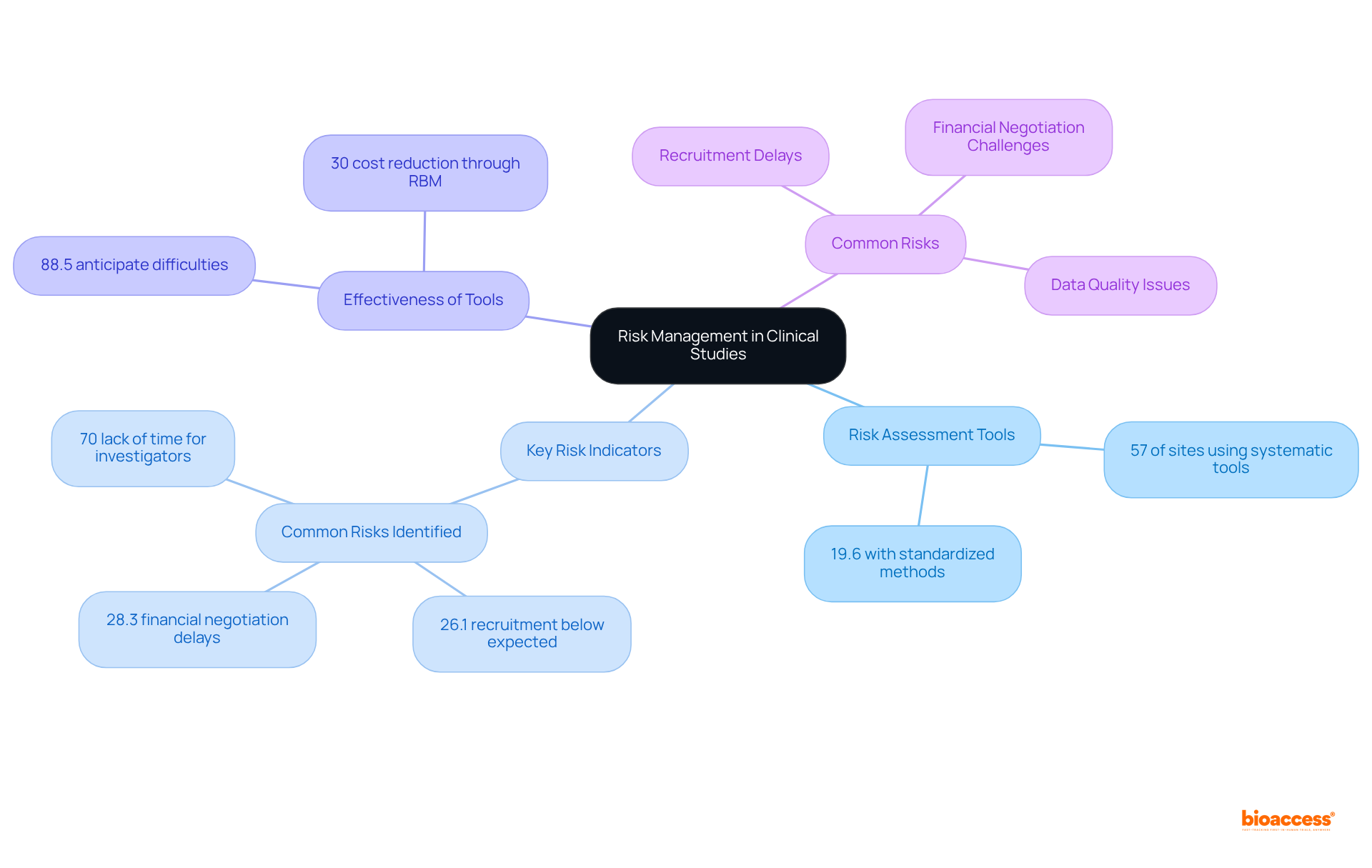
A Clinical Studies Oversight System (CSOS) is crucial for incorporating robust risk control features, empowering study managers to identify, evaluate, and mitigate potential risks throughout the study lifecycle. Effective tools for conducting risk assessments, monitoring Key Risk Indicators (KRIs), and implementing mitigation strategies are not just beneficial; they are essential.
Notably, 57% of research sites reported utilizing systematic risk assessment tools, yet only 19.6% had a standardized method for risk analysis. Furthermore, a striking 88.5% of sites employing a risk management tool indicated they could anticipate possible difficulties, underscoring the effectiveness of these tools in foreseeing challenges. Additionally, 26.1% of locations reported recruitment below anticipated levels as a common risk, illustrating the significant hazards encountered in research studies.
By actively managing risks, organizations can minimize disruptions and enhance the overall success of medical studies. The integration of advanced analytics and technology, such as Central Statistical Monitoring (CSM), facilitates real-time data oversight, enabling the detection of anomalies and potential misconduct. This proactive stance is vital, especially considering that a substantial portion of medical studies faces major risks, including frequent issues such as recruitment delays and financial negotiation challenges.
By leveraging these tools and methodologies, research teams can optimize resource allocation and improve study outcomes, potentially reducing expenses by up to 30% through Risk-Based Monitoring (RBM).
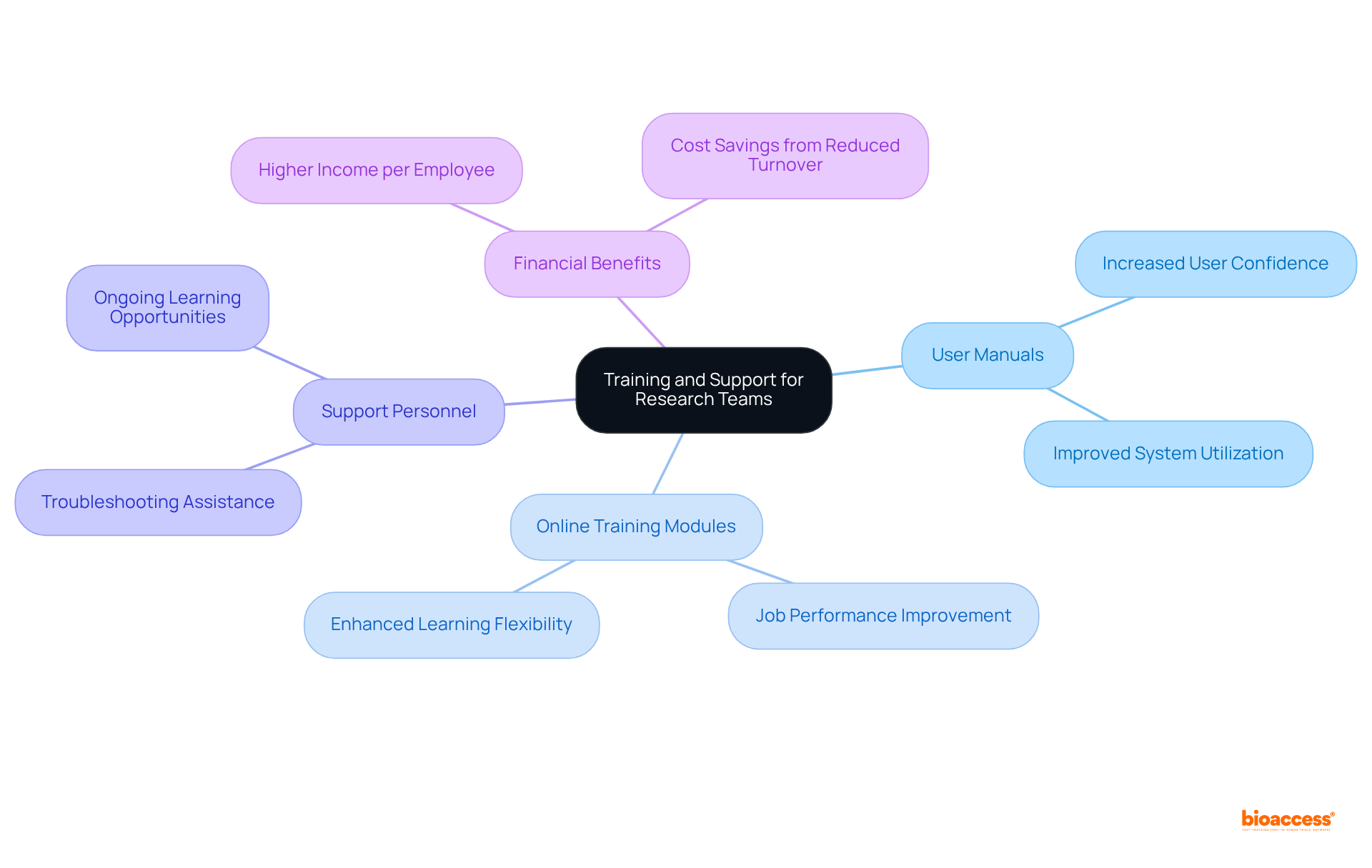
A robust clinical trials management system must prioritize training and support resources to empower research teams in maximizing system effectiveness. Essential components include:
Investing in thorough training not only boosts user adoption rates but also greatly enhances system utilization, resulting in more efficient management of tests and improved outcomes. For instance, organizations that implement structured training programs often see a marked increase in user confidence and competence, with 59% of employees stating that training improves their overall job performance.
Furthermore, as 94% of employees express a desire for ongoing learning opportunities, aligning training initiatives with user needs is crucial for fostering a culture of continuous improvement within clinical research teams. Additionally, companies with comprehensive corporate training programs have been shown to have 218% higher incomes per employee than those without formalized training, underscoring the financial benefits of investing in training.
By ensuring that staff are well-equipped to navigate the clinical trials management system, organizations can mitigate risks associated with underutilization and enhance the overall success of trials.
The exploration of essential features within a clinical trials management system (CTMS) underscores the critical role such systems play in enhancing the efficiency and success of clinical research. A well-structured CTMS not only streamlines operations but also ensures compliance, data integrity, and effective participant recruitment, all fundamental to advancing medical innovations.
Key insights from this discussion highlight the importance of:
The integration capabilities of a CTMS facilitate seamless data flow, while advanced reporting and analytics empower informed decision-making. Furthermore, effective financial management and risk mitigation strategies are essential for maintaining the viability of clinical studies. Training and support for research teams are equally crucial, as they enhance system utilization and foster a culture of continuous improvement.
In light of these insights, investing in a sophisticated clinical trials management system emerges as a necessity for organizations aiming to thrive in the competitive landscape of clinical research. Embracing these features will not only mitigate risks and enhance compliance but also drive innovation and improve patient outcomes. As the industry evolves, prioritizing these elements will be vital for ensuring the success of future clinical trials and ultimately advancing healthcare solutions.
What is bioaccess® and how does it benefit Medtech innovators?
bioaccess® provides expedited oversight of clinical trials specifically for Medtech innovators, achieving ethical approvals in just 4-6 weeks by leveraging regulatory speed in Latin America, diverse patient populations in the Balkans, and streamlined pathways in Australia. This rapid turnaround significantly impacts development timelines for Medtech companies.
What is the projected growth of the worldwide research management systems market?
The worldwide research management systems market is expected to grow from USD 1.80 billion in 2023 to USD 5.93 billion by 2032, indicating a clear need for effective management solutions.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is crucial to ensure adherence to both local and international standards, safeguard participants' rights, and maintain the integrity of study outcomes. Compliance issues can lead to significant delays in medical studies.
What are the consequences of non-compliance in clinical trials?
Approximately 57% of medical studies experience delays due to compliance issues, which can result in costly penalties. A robust clinical trials management system can help mitigate these risks by automating compliance checks and providing real-time updates.
What are the ALCOA principles in data management for clinical trials?
The ALCOA principles ensure that records are: - Attributable - Legible - Contemporaneously recorded - Original or a true copy - Accurate
How does a clinical trials management system (CTMS) enhance information integrity?
A CTMS enhances information integrity through secure storage, real-time entry, and automated validation processes, ensuring that all information gathered is precise, comprehensive, and readily available for examination.
What impact does information management have on clinical trial results?
Efficient information management directly influences the credibility of trial findings and the overall success of the trial, as the quality of information affects the validity and integrity of the final analysis.
What challenges to information integrity arose during the COVID-19 pandemic?
The COVID-19 pandemic introduced new challenges requiring adaptive strategies to ensure participant safety and information reliability, emphasizing the need for continuous training and education for all personnel involved in research trials.