

The article titled "10 Essential Features of Electronic Trial Master File (eTMF) Solutions" identifies key functionalities that render eTMF systems indispensable in clinical research. It emphasizes that features such as:
are critical for enhancing the efficiency and regulatory adherence of clinical trials. These functionalities streamline processes and significantly improve data integrity, underscoring the vital role of eTMF solutions in modern clinical research.
The landscape of clinical research is rapidly evolving, with electronic trial master file (eTMF) solutions at the forefront of this transformation. These advanced digital platforms serve as more than mere repositories for documents; they have emerged as essential tools that streamline operations, enhance compliance, and facilitate real-time collaboration among research teams.
As organizations navigate the complexities of regulatory requirements and strive for efficiency, a critical question arises: what are the key features that render eTMF solutions indispensable in today's clinical trials? This article delves into ten essential functionalities that can significantly impact the success of clinical research, offering insights into how these tools can optimize processes and drive better outcomes.
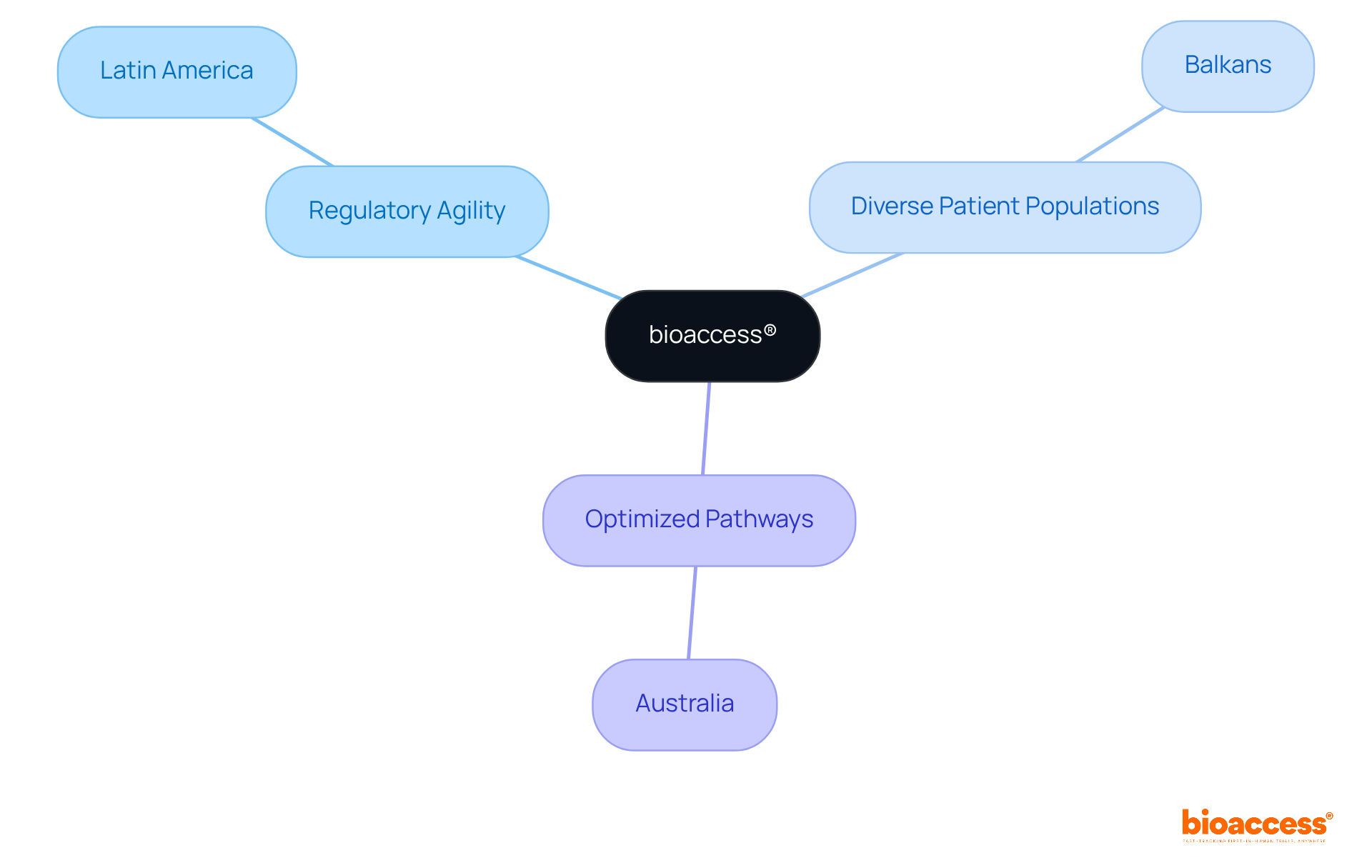
bioaccess® harnesses electronic trial master file eTMF solutions to accelerate research processes in clinical trials. By synergizing regulatory agility from Latin America with diverse patient populations in the Balkans and optimized pathways in Australia, bioaccess® ensures that trials are not only compliant but also remarkably efficient. This strategic integration of the electronic trial master file etmf facilitates expedited document management and fosters real-time collaboration, both of which are crucial for adhering to stringent timelines in clinical research.
The electronic trial master file (eTMF) has evolved significantly from traditional paper-based systems to sophisticated digital platforms, capturing the attention of clinical research professionals. Initially, eTMFs served merely as document repositories. However, advancements in technology have transformed the electronic trial master file (eTMF) into a dynamic system that facilitates real-time information access, compliance tracking, and automated workflows. This evolution is essential for addressing the growing demands of regulatory bodies and ensuring the integrity of the study.
With bioaccess®, studies can enroll participants 50% quicker due to its advanced electronic document management features. This results in significant savings of $25K per patient through FDA-ready data that eliminates rework and delays. These capabilities streamline testing procedures and assist startups in overcoming regulatory challenges, thereby accelerating approval processes and enhancing overall testing efficiency. The integration of such technology not only enhances operational efficiency but also positions organizations to meet the rigorous standards set by regulatory authorities.
In conclusion, the shift towards the electronic trial master file (eTMF) represents a critical advancement in clinical research. Collaboration with innovative platforms like bioaccess® is vital for navigating the complexities of regulatory compliance and optimizing study outcomes. The next steps involve embracing these technologies to drive efficiency and effectiveness in clinical trials.

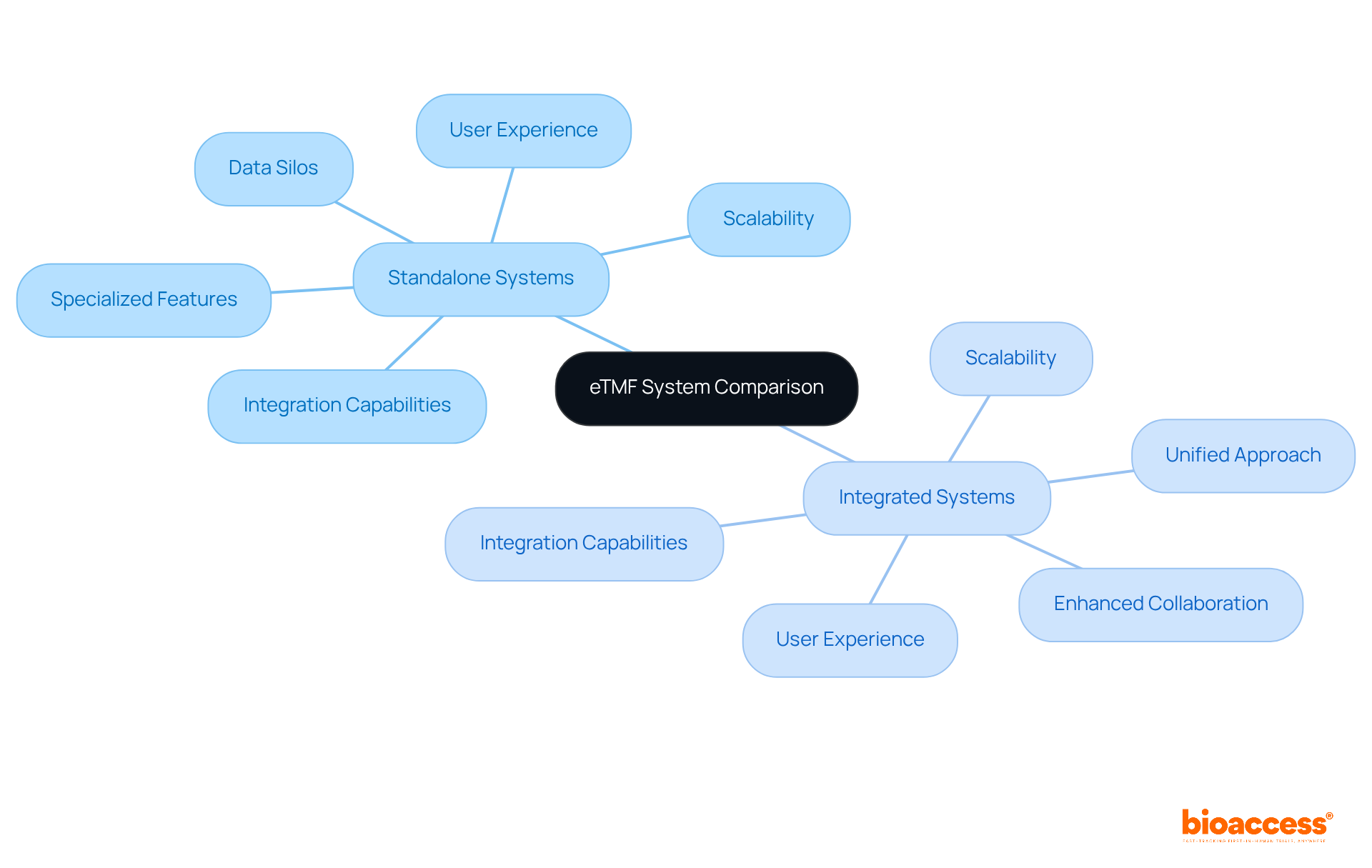
When organizations are faced with the decision between standalone and integrated electronic trial master file (ETMF) systems, it is crucial to consider several key factors:
Standalone systems may provide specialized features that cater to specific needs; however, they often result in data silos that can hinder overall efficiency. In contrast, integrated systems offer a unified approach, significantly enhancing collaboration and data sharing across various platforms. The ultimate choice will depend on the unique requirements and resources of the organization, underscoring the importance of a tailored electronic trial master file (ETMF) solution within the clinical research landscape.
Centralized document management, real-time collaboration tools, automated workflows, and robust compliance tracking are essential components of electronic trial master file (ETMF) systems that significantly enhance Clinical Trial Management System (CTMS) integration. These functionalities ensure that all related documents are readily accessible and consistently updated, streamlining communication among teams and boosting overall efficiency.
Modern electronic management solutions facilitate instant access to documents, allowing teams to collaborate effectively regardless of location, thereby accelerating decision-making and minimizing delays in research processes. Compliance tracking within the electronic trial master file (ETMF) systems is crucial for meeting regulatory standards, providing an organized approach to monitoring and documenting compliance activities.
By leveraging these features, organizations can optimize their clinical studies, ensuring they meet both operational and regulatory requirements efficiently. With bioaccess®'s solutions, organizations can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving substantial cost savings with FDA-ready data—no rework, no delays.

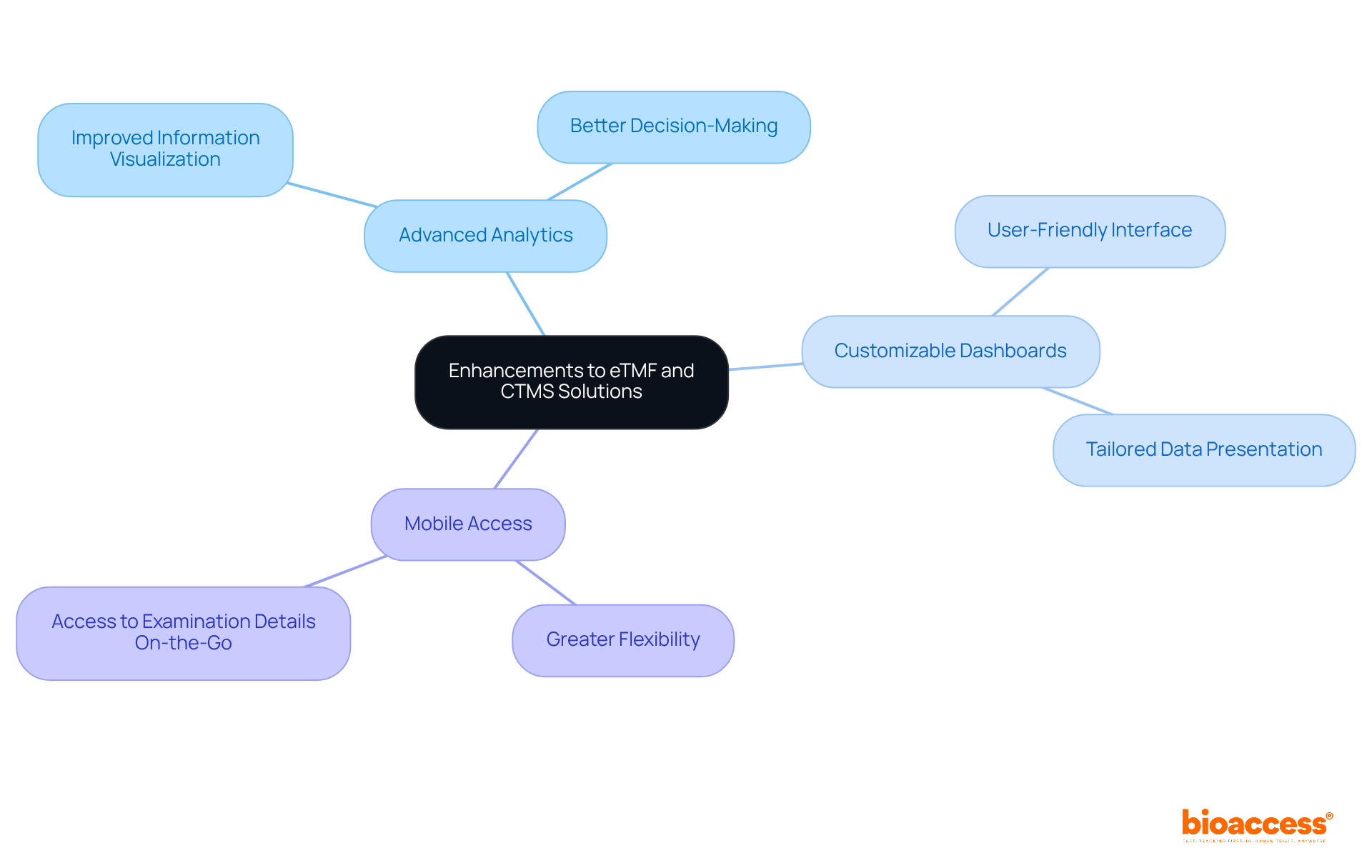
Beyond core functionalities, additional features such as advanced analytics, customizable dashboards, and mobile access significantly enhance the electronic trial master file eTMF and CTMS solutions. These characteristics not only enable improved information visualization but also facilitate better decision-making and greater flexibility for users who require access to examination details while on the go. Implementing these enhancements can result in more efficient management of experiments and improved compliance, underscoring their critical role in advancing clinical research.
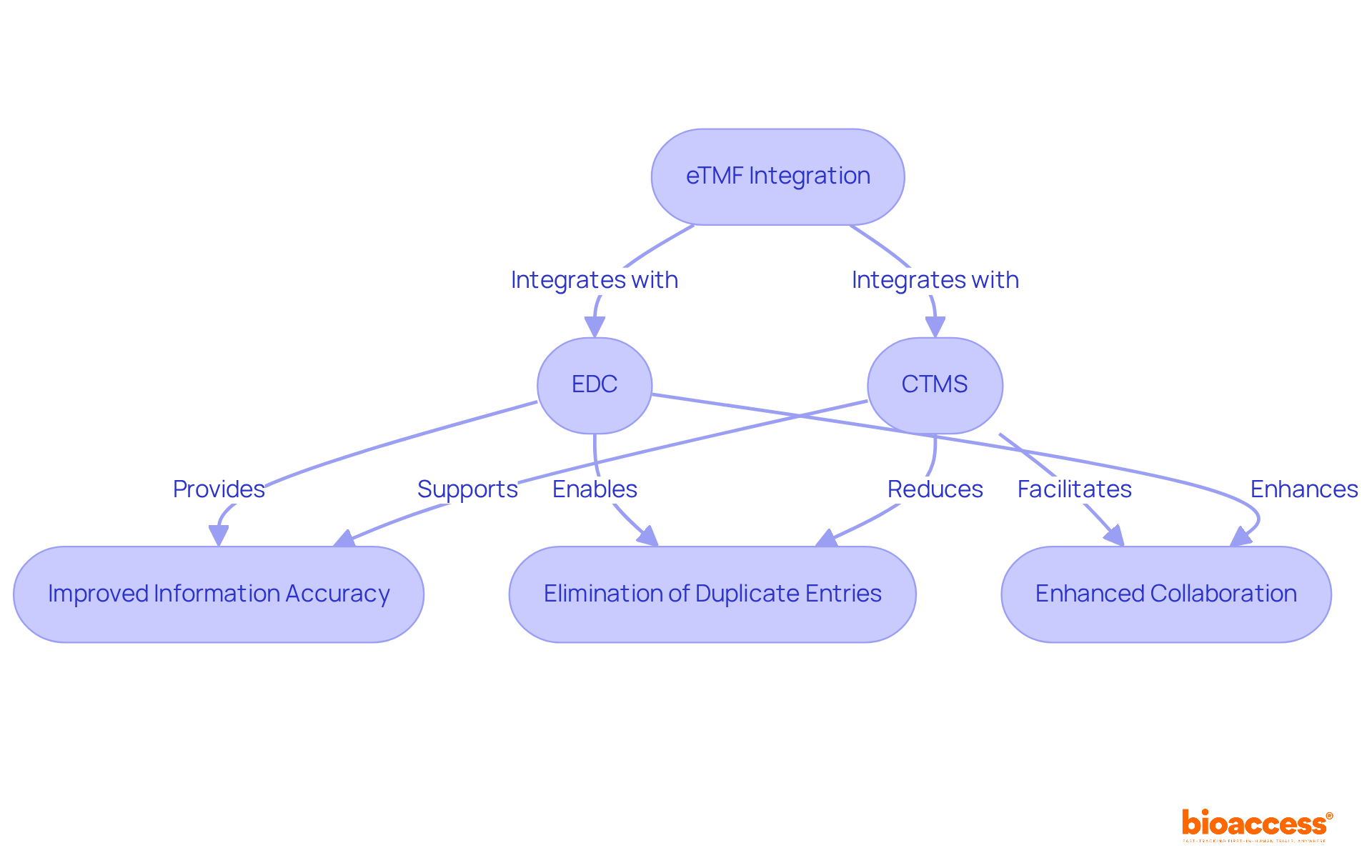
Integrating the electronic trial master file eTMF with essential systems like EDC (Electronic Capture of Information) and CTMS (Clinical Management Systems) is vital for establishing a cohesive clinical research information ecosystem. This integration facilitates seamless information sharing and real-time updates, providing all stakeholders with instant access to the latest details. By creating an integrated environment, organizations can significantly enhance operational effectiveness, ensuring compliance with regulatory standards and improving information integrity throughout the process.
For instance, EDC systems increase information accuracy by minimizing transcription errors, while streamlined workflows eliminate duplicate data entry and manual transfers. Successful implementations of the electronic trial master file etmf with EDC and CTMS have showcased improved collaboration among study teams, leading to timely task completion and better overall management.
However, it is essential to acknowledge that change management may encounter resistance from teams adapting to new workflows, which can present challenges during the integration process. As Medha Datar observes, "The research landscape is constantly changing, with technology playing an essential role in optimizing procedures, handling information, and enhancing efficiency.
As clinical studies increasingly embrace decentralized and hybrid models, Electronic Data Capture (EDC) systems must adapt to meet these evolving requirements. Key characteristics essential for facilitating hybrid studies in 2025 include:
These advancements enhance patient involvement and streamline information collection processes, significantly improving overall study efficiency. For instance, bioaccess enables treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than Western sites, resulting in $25K savings per patient with FDA-ready data—no rework, no delays.
The integration of wearable devices allows for continuous monitoring of vital signs, enabling researchers to gather real-time information directly from participants. This shift towards remote information gathering is crucial, as research indicates that 80% of medical studies fail to meet their recruitment schedules, underscoring the necessity for innovative solutions.
Mobile technology can foster participation from typically underrepresented groups, enriching the diversity of research data. As Yvonne Chan, MD, states, 'Mobile technology and connected devices could facilitate valuable multi-dimensional, detailed, real-world medical data capture.'
By leveraging these features, EDC systems are poised to transform the landscape of clinical research, making studies more efficient and patient-centered.

Essential features of the electronic trial master file (ETMF) encompass:
These features are crucial for effective management of experiments and adherence to regulatory standards. Organizations must also contemplate additional functionalities, such as:
to enhance their electronic trial master file (ETMF) and Clinical Trial Management System (CTMS) solutions. A cohesive information environment is vital for improving research procedures and achieving superior results.

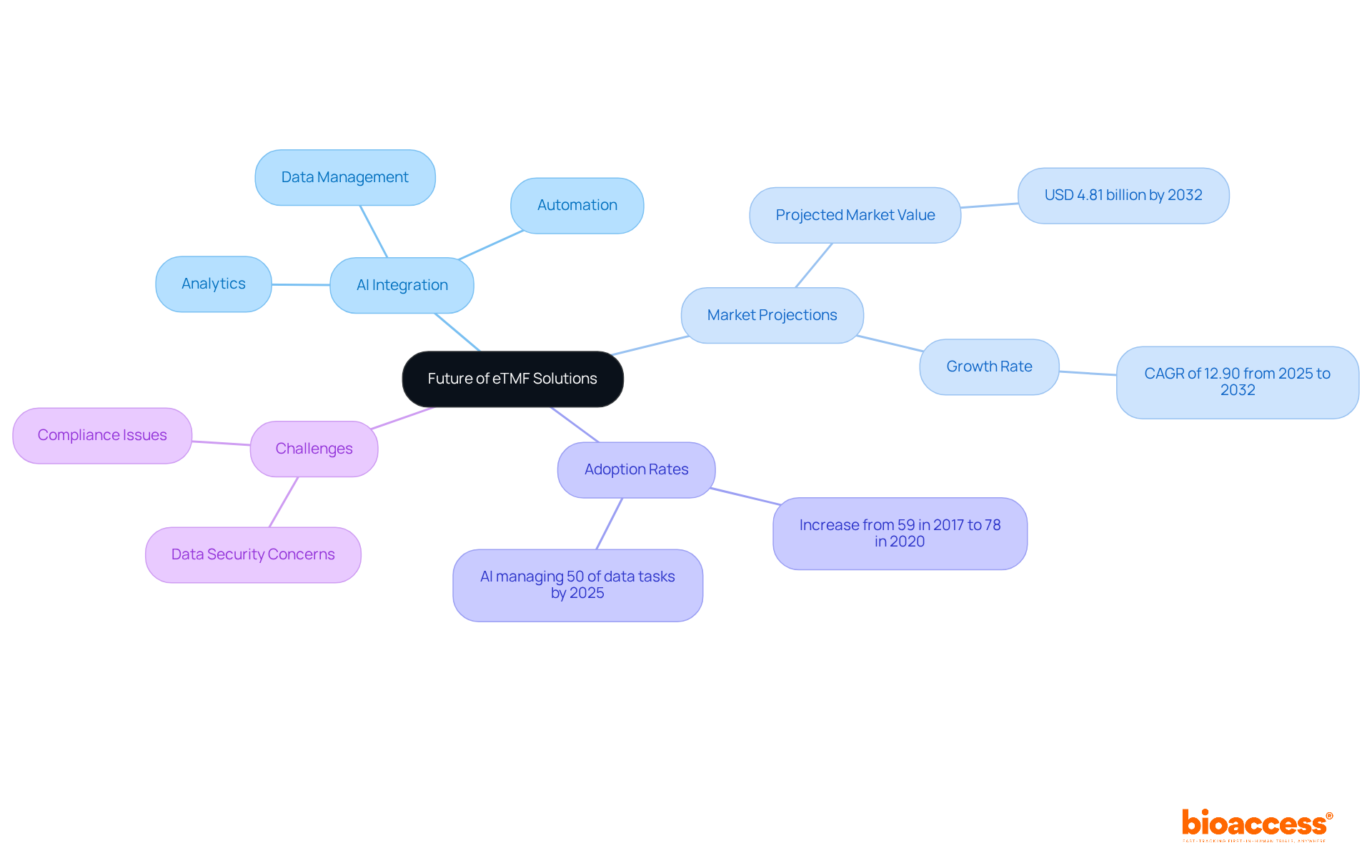
The future of the electronic trial master file (eTMF) solutions in clinical research is poised for remarkable advancements, particularly through the integration of AI-driven analytics and automation. These innovations are expected to greatly simplify testing procedures, enhance information quality, and ensure compliance with evolving regulatory standards. Notably, AI technologies are projected to manage up to 50% of research data tasks by 2025, improving data accuracy and reducing timelines by 20%.
Furthermore, the adoption of AI-powered analytics in medical studies has surged, with the utilization rates of the electronic trial master file (eTMF) increasing from 59% in 2017 to 78% in 2020. As Debashish Niyogi, Ph.D., noted, "the industry is requesting more efficient regulatory-compliant processes to create and manage essential research content."
Organizations such as bioaccess, which offer comprehensive research management services—including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—are strategically positioned to leverage these advancements. By proactively embracing these trends, they will be better equipped to navigate the complexities of modern clinical trials, establishing themselves at the forefront of the industry.
Additionally, the electronic trial master file market is projected to reach USD 4.81 billion by 2032, underscoring its growing significance. However, challenges such as data security and compliance concerns remain critical factors influencing the adoption of the electronic trial master file (eTMF) systems.
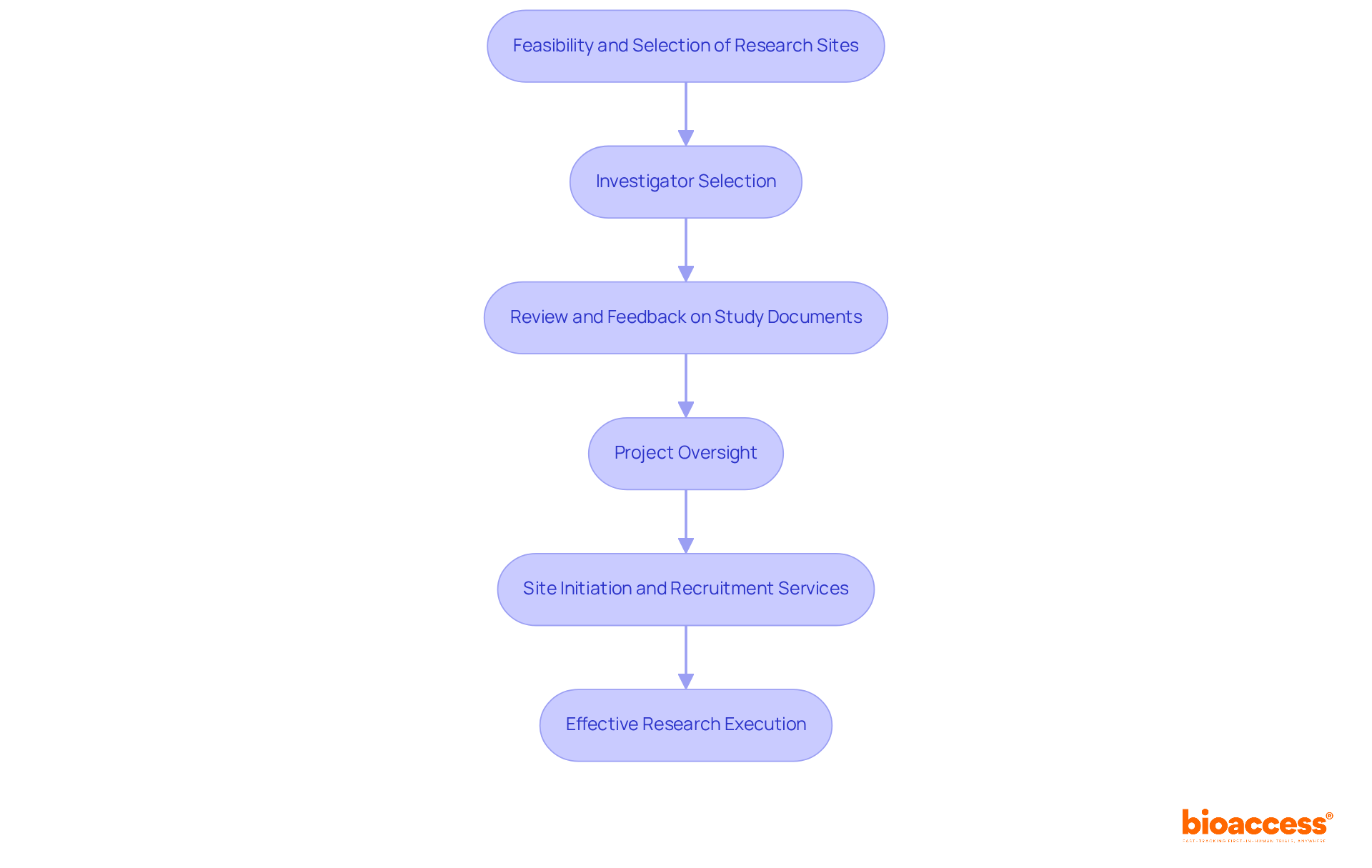
For organizations aiming to enhance their research procedures, collaborating with bioaccess® presents invaluable assistance. Our comprehensive approach encompasses:
Bioaccess® is adept at helping Medtech, Biopharma, and Radiopharma innovators streamline their research efforts.
Our expertise in project oversight and documentation guarantees effective study execution, complemented by expedited site initiation and participant recruitment services tailored to the unique challenges of medical research in LATAM, Eastern Europe, and Australia. The importance of collaboration in clinical trials cannot be overstated.
Book a meeting with bioaccess® to explore how we can support your clinical trials effectively.
The transformation of electronic trial master file (eTMF) solutions signifies a pivotal shift in the clinical research landscape, enhancing both efficiency and compliance. Transitioning from traditional paper-based systems to advanced digital platforms enables organizations to leverage real-time collaboration, automated workflows, and centralized document management. This evolution allows stakeholders to navigate the complexities of regulatory requirements while streamlining research processes effectively.
Key insights reveal essential features of eTMF solutions, such as:
The evolution of eTMF technology, particularly through platforms like bioaccess®, not only accelerates participant enrollment but also significantly reduces costs associated with clinical trials. Moreover, the integration of advanced analytics and mobile access is poised to enhance decision-making and optimize research outcomes.
Looking ahead, the proactive adoption of innovative eTMF solutions is crucial for organizations striving to remain competitive in the dynamic clinical research environment. As industry demands for efficiency and regulatory compliance continue to rise, embracing integrated eTMF systems will empower stakeholders to overcome challenges and achieve superior trial management. Engaging with expert solutions, such as those provided by bioaccess®, equips organizations with the necessary support to navigate this evolving landscape, ensuring that clinical trials are executed effectively and efficiently.
What is bioaccess® and how does it enhance clinical research?
bioaccess® utilizes electronic trial master file (eTMF) solutions to accelerate research processes in clinical trials by integrating regulatory agility from Latin America, diverse patient populations in the Balkans, and optimized pathways in Australia. This ensures trials are compliant and efficient, facilitating expedited document management and real-time collaboration.
How has the electronic trial master file (eTMF) evolved over time?
The eTMF has evolved from traditional paper-based systems to advanced digital platforms that allow for real-time information access, compliance tracking, and automated workflows. This evolution is essential for meeting regulatory demands and ensuring study integrity.
What are the benefits of using bioaccess® for clinical trials?
bioaccess® enables studies to enroll participants 50% faster and provides FDA-ready data that eliminates rework and delays, resulting in significant cost savings of $25K per patient. These features streamline testing procedures and help startups overcome regulatory challenges, enhancing overall testing efficiency.
What factors should organizations consider when choosing between standalone and integrated eTMF systems?
Organizations should consider scalability, user experience, and integration capabilities with other clinical trial management systems (CTMS). Standalone systems may offer specialized features but can create data silos, while integrated systems enhance collaboration and data sharing.
Why is the shift towards electronic trial master file (eTMF) systems important in clinical research?
The shift to eTMF systems represents a critical advancement in clinical research, enabling organizations to navigate regulatory compliance complexities and optimize study outcomes. Embracing these technologies is essential for driving efficiency and effectiveness in clinical trials.