Overview
The article titled "10 Essential Insights for Every Clinical Study Volunteer" presents crucial information that potential volunteers must grasp before engaging in clinical trials. It underscores the significance of informed consent, the evaluation of risks and benefits, and the necessity for effective communication with research teams. Furthermore, it elucidates the overall clinical trial process, collectively empowering volunteers to make informed decisions and enriching their participation experience.
Introduction
Understanding the clinical trial process is crucial for anyone considering volunteering in medical research. With an increasing number of studies seeking participants, the opportunity to contribute to groundbreaking advancements in healthcare has never been more significant. However, navigating the complexities of clinical trials can be daunting, leaving potential volunteers with numerous questions and concerns. What essential insights can empower individuals to make informed decisions about their participation and enhance their overall experience?
bioaccess®: Accelerate Your Clinical Trial Experience with Expert Guidance
At bioaccess®, we excel in enhancing the trial process, ensuring that volunteers receive exceptional guidance throughout their journey. With over 15 years of experience in early-phase medical research, our team is adept at facilitating prompt ethical approvals and effective enrollment, leveraging our pre-qualified networks of more than 50 activated sites in under eight weeks.
Volunteers who serve as clinical study volunteers in studies managed by bioaccess® can expect a streamlined and organized experience, supported by a dedicated team committed to advancing medical innovation responsibly and ethically. Recent advancements in study management, including the integration of digital tools and patient-focused designs, further enhance the recruitment process, making participation more accessible and engaging.
Notably, around 80% of research studies are postponed or terminated due to recruitment challenges, underscoring the significance of our role in improving this process. Our FDA/EMA/MDR-ready datasets and centralized monitoring capabilities ensure that our expert guidance not only enhances the overall study experience but also significantly impacts outcomes, fostering a collaborative environment where volunteers feel valued and informed.
As Samruddhi Yardi aptly states, 'research studies are the cornerstone of medical advancement,' highlighting the essential nature of our efforts in this area.
Learn the Clinical Trial Process: Key Steps Every Volunteer Should Know
Learn the Clinical Trial Process: Key Steps Every Volunteer Should Know
Understanding the clinical trial process is essential for volunteers. Key steps include:
- Screening: Assessing eligibility based on specific criteria, which is crucial as many patients lack knowledge about the recruitment process.
- Informed Consent: Reviewing and signing documents that describe the project's purpose, procedures, risks, and benefits. This step is vital, as 93.6% of patients with chronic conditions want assurance they can complete the trial. Dr. Neal Thomas emphasizes, "The participant will have a full understanding of exactly what is expected, what the potential risks are, and what the potential benefits are."
- Randomization: Assigning participants to various research groups, if relevant, which helps ensure impartial results.
- Treatment: Receiving the intervention or placebo as part of the research, allowing researchers to understand how therapies function in healthy individuals.
- Follow-Up: Attending scheduled visits for monitoring and data collection, which is essential for maintaining participant engagement and ensuring accurate data. Notably, 70% of the population lives two hours or more from an academic medical center, highlighting the logistical challenges many face in participating.
To enhance your experience as a clinical study volunteer, it is important to familiarize yourself with these steps and consider managing your consent preferences effectively. Understanding your rights as a participant can empower you throughout the trial process. Furthermore, if you have any worries regarding your involvement, feel free to contact the coordinators for clarification.
Informed consent is an essential process that ensures volunteers are comprehensively informed about their participation in clinical research. As a volunteer, you possess the right to receive detailed information regarding the project's purpose, procedures, risks, and benefits. You are encouraged to ask questions and seek clarification on any aspect of the research. Importantly, you can opt out of the research at any moment without facing any penalties.
It is your responsibility to thoroughly read the consent form and discuss any concerns with the research team. This proactive engagement not only protects your rights but also fosters a trusting relationship with the researchers. Effective informed consent practices, including the use of clear language and multimedia tools, can significantly enhance your understanding and comfort level with the research. By being well-informed, you contribute to the integrity of the research process and ensure that your involvement aligns with your values and preferences.
Evaluate Benefits and Risks: What You Need to Consider Before Joining a Study
Before a clinical study volunteer participates in a clinical trial, it is essential to evaluate both the potential advantages and disadvantages involved. Benefits for a clinical study volunteer often include:
- Access to innovative treatments
- Close medical supervision
- The opportunity to contribute to vital medical research
For instance, involvement in randomized controlled trials (RCTs) has been linked to enhanced health outcomes, as research indicates that RCT participants frequently experience higher survival rates than non-participants.
However, the potential risks for a clinical study volunteer must not be overlooked. Participants may encounter:
- Side effects from experimental therapies
- The possibility of being assigned to a placebo group
- The significant dedication of time and effort required for research protocols
Statistics reveal that logistical barriers considerably impact involvement rates, with many potential volunteers living more than two hours from study centers. Moreover, the increasing complexity of research trial protocols necessitates that individuals fully understand what participation entails.
Carefully evaluating these factors will empower you to make an informed decision that aligns with your health goals and personal circumstances. As emphasized by medical researchers, a comprehensive understanding of the risks and benefits is crucial for potential clinical study volunteers, ensuring they are well-prepared for the journey ahead.
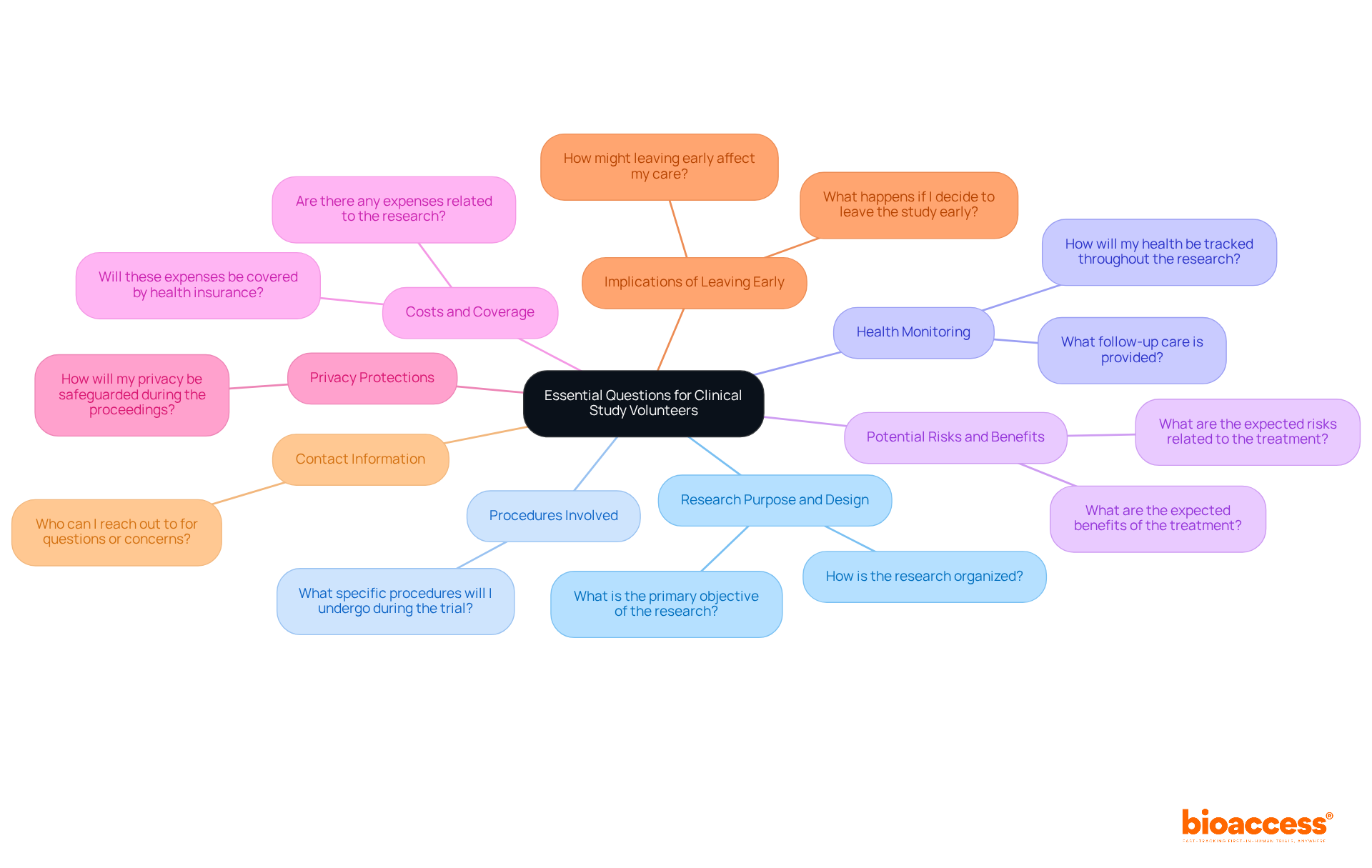
Ask the Right Questions: Essential Inquiries for Clinical Study Volunteers
As a clinical study volunteer, asking the right questions is crucial for understanding your role and the trial's implications. Consider inquiring about the following key aspects:
- Research Purpose and Design: What is the primary objective of the research, and how is it organized?
- Procedures Involved: What specific procedures will I undergo during the trial?
- Health Monitoring: How will my health be tracked throughout the research, and what follow-up care is provided?
- Potential Risks and Benefits: What are the expected risks and advantages related to the treatment drug or therapy?
- Costs and Coverage: Are there any expenses related to the research, including tests, procedures, or research drugs, and will these be covered by health insurance?
- Privacy Protections: How will my privacy and personal information be safeguarded during the proceedings?
- Implications of Leaving Early: What happens if I decide to leave the study early, and how might that affect my care?
- Contact Information: Who can I reach out to for questions or concerns during the testing period?
These questions not only clarify your responsibilities as a clinical study volunteer but also help you make informed choices about your involvement. Participating in open discussion with the research team can greatly improve your comprehension and ease, ultimately aiding in the study's success.
Research Trial Credibility: How to Identify Reputable Clinical Studies
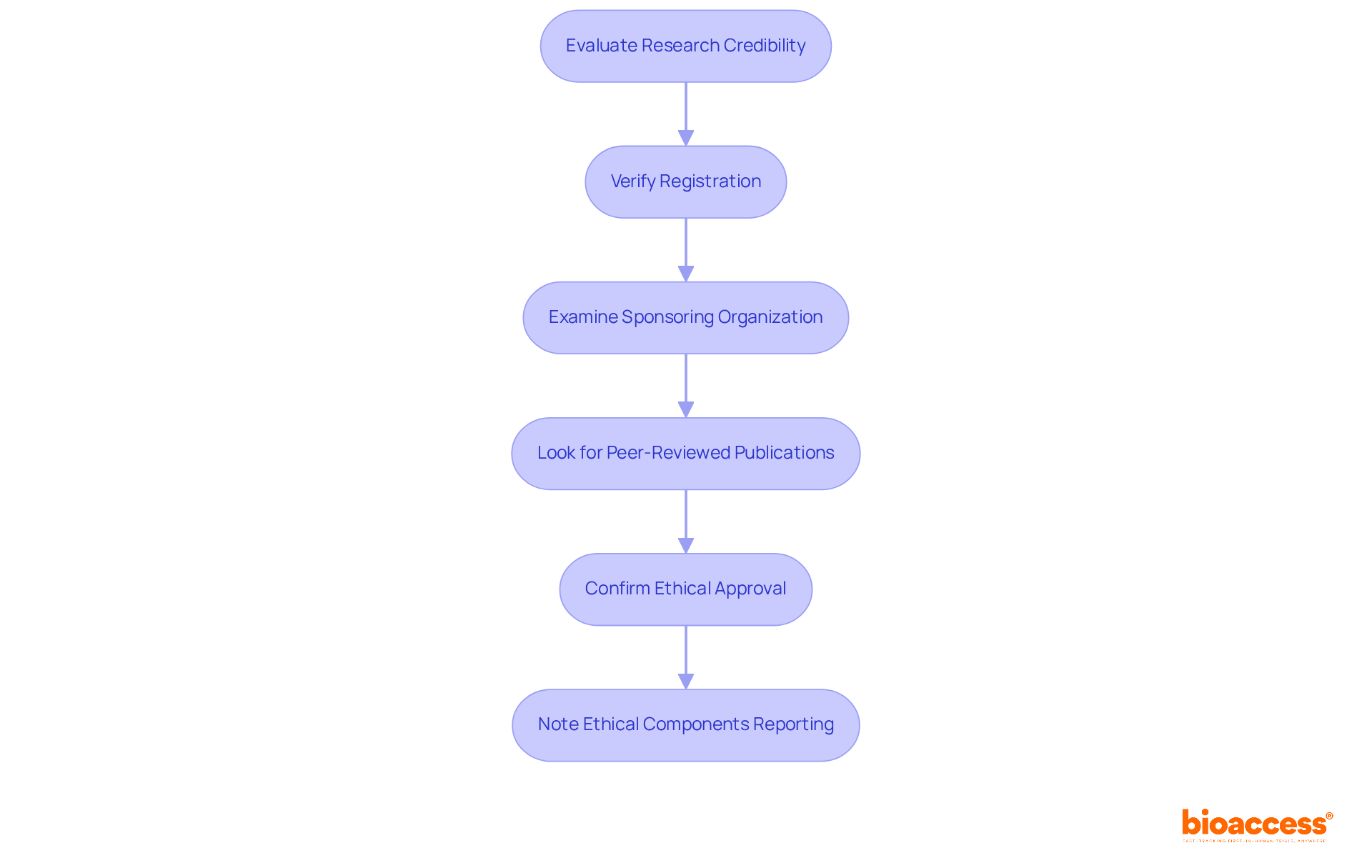
To ensure involvement in a trustworthy research initiative, consider the following steps:
- Verify the registration of the research on trial databases, such as ClinicalTrials.gov, which currently lists over 551,947 projects worldwide.
- Examine the reputation and history of the sponsoring organization; established entities are more likely to adhere to ethical standards and provide comprehensive clinical trial management services, including feasibility assessments, site selection, compliance reviews, and trial setup.
- Look for peer-reviewed publications related to the research, as these indicate a level of scrutiny and validation by the scientific community.
- Confirm that the research has received ethical approval from an Institutional Review Board (IRB), which protects participant rights and ensures research integrity.
- Note that only 5.4% of studies report all three ethical components (IRB approval, Declaration of Helsinki, and informed consent), highlighting significant gaps in ethical practices.
By conducting this thorough research, including understanding the regulatory functions of INVIMA, you can confidently evaluate the legitimacy of the study and its potential contributions to medical knowledge.
Communicate Effectively: Building a Relationship with Your Clinical Research Team
Establishing a strong relationship with your clinical research team is paramount for a fulfilling study experience. Open communication regarding your health history, concerns, and feedback throughout the study cultivates a collaborative environment. Regular updates and check-ins from the research team not only keep you informed about the study's progress but also reinforce your role as a valued participant.
Research indicates that patients who feel connected to their study coordinators are more likely to adhere to protocols and accurately report their symptoms. For example:
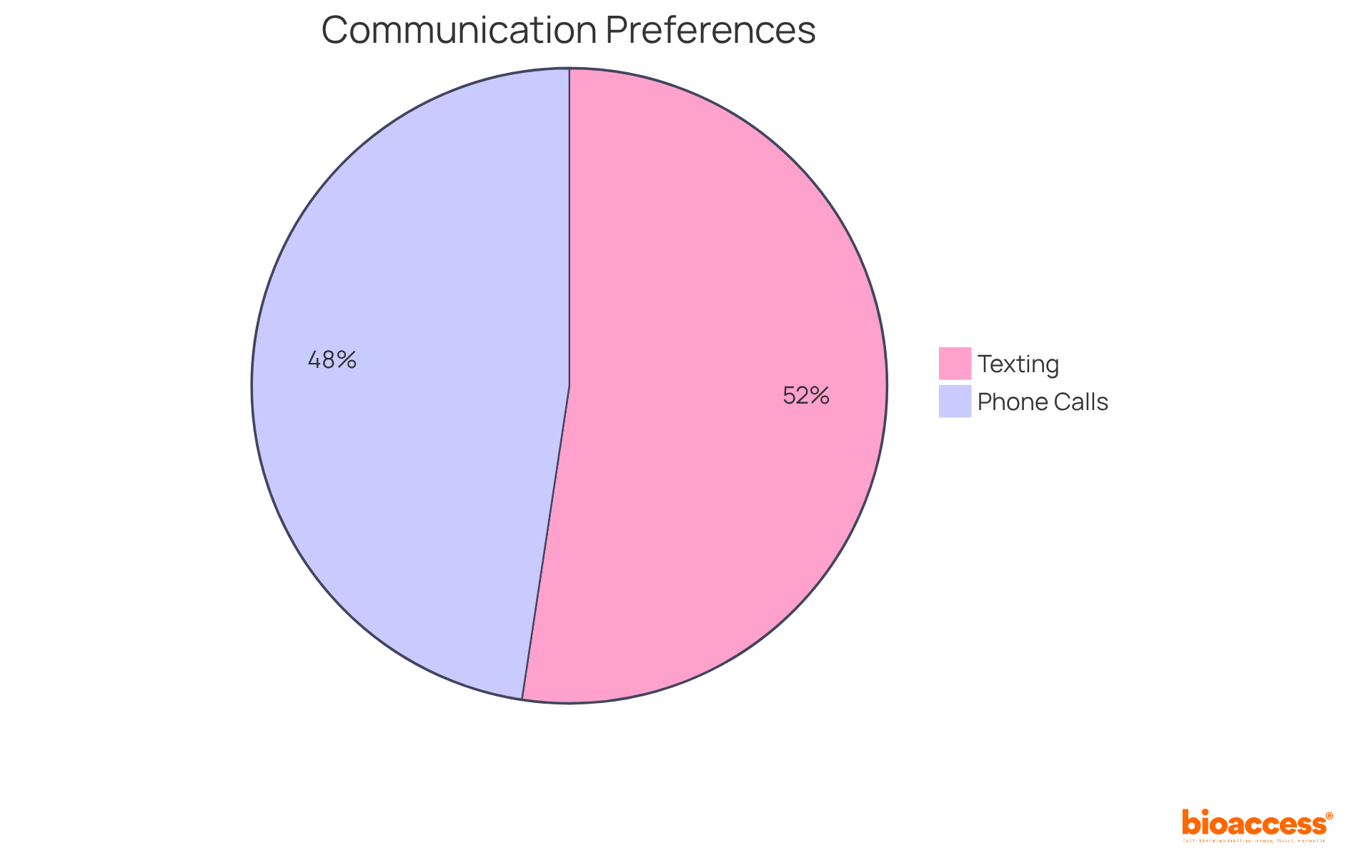
- 80% of participants prefer texting for communication, which can streamline interactions and enhance engagement.
- 72.7% of participants favored phone calls when communicating with healthcare providers, underscoring the significance of direct communication methods.
Your perspectives are vital to the project's success; thus, a proactive approach to communication can significantly enhance both your experience and the outcomes.
Consider Future Medical Care: How Participation May Affect Your Health Options
Becoming a clinical study volunteer can profoundly impact your future medical treatment. It is crucial to evaluate whether the research offers ongoing health monitoring or access to innovative treatments post-trial.
Studies indicate that clinical study volunteers involved in randomized controlled trials (RCTs) often experience improved long-term health outcomes compared to non-participants. Investigations reveal that:
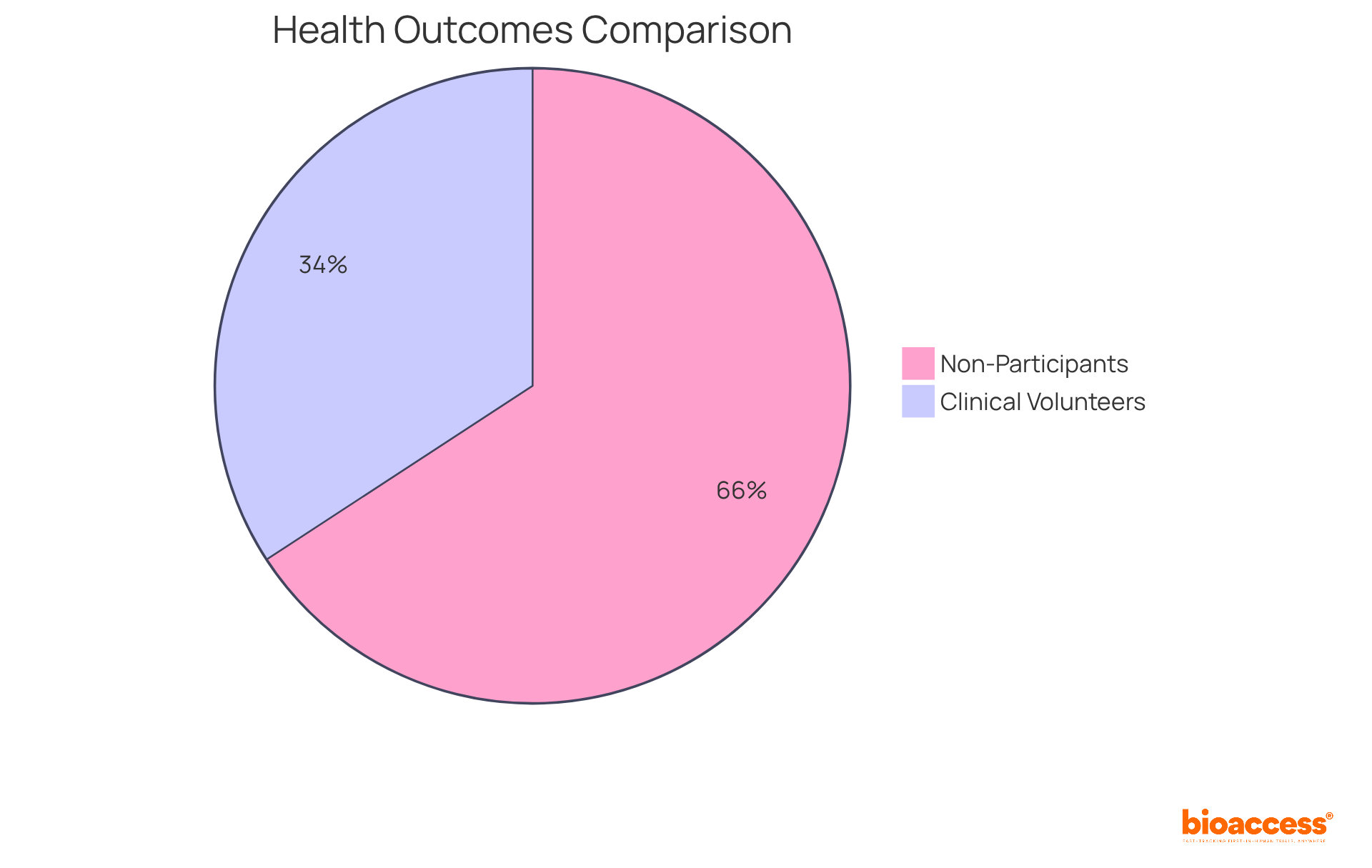
- 50% of non-participants faced mortality or cancer recurrence
- Only 26% of clinical study volunteers involved in RCTs experienced similar outcomes
Discussing your involvement with your healthcare provider is vital, as it may influence your treatment options moving forward. By proactively addressing these considerations, you empower yourself to make informed decisions about your health, ultimately enhancing your overall care experience.
Prepare Emotionally: Understanding the Psychological Impact of Clinical Trials
Being a clinical study volunteer can evoke a spectrum of emotions, ranging from excitement to anxiety. Notably, research indicates that approximately 72% of clinical study volunteers seek emotional support during their involvement, underscoring the critical need to address these feelings.
Emotional preparation entails recognizing these responses and actively pursuing support. Engaging in conversations with friends, family, or mental health professionals proves beneficial. Initiatives such as peer support groups and counseling services specifically designed for research participants provide essential emotional assistance.
Mental health experts emphasize that understanding emotional reactions is a fundamental aspect of the experience for a clinical study volunteer, significantly enhancing their ability to navigate the challenges they may face. As we approach 2025, cultivating emotional resilience will be paramount for the clinical study volunteers, ensuring they remain supported and informed throughout their journey.
Reflect on Your Motivation: Why You Want to Participate in a Clinical Trial
Before becoming a clinical study volunteer, it is essential to consider your motivations. Are you driven by a desire to contribute to medical research, gain access to innovative treatments, or assist others in need? Comprehending your motives for involvement not only offers clarity and purpose but also enhances your experience.
Research indicates that a significant percentage of clinical study volunteers are motivated by altruism, with many of them expressing a strong desire to help advance medical knowledge and improve patient outcomes. However, motivations can vary significantly among participants, influenced by personal characteristics such as health status and spirituality.
As one clinical investigator observed, 'Understanding elements that enhance patients' willingness to engage may assist research teams in enlisting the widest and most representative cohort of patients.' This self-reflection can guide your interactions with the research team, fostering a more engaged and meaningful participation in the study.
Conclusion
Engaging in clinical studies as a volunteer represents a vital contribution to medical research and innovation. The insights shared throughout this article underscore the importance of understanding the clinical trial process, ensuring informed consent, evaluating risks and benefits, and fostering effective communication with research teams. Each step in this journey is essential for enhancing the experience of volunteers and advancing healthcare outcomes.
Key points discussed emphasize:
- The significance of being well-informed about the clinical trial process.
- The necessity of understanding one’s rights and responsibilities.
- The importance of evaluating the credibility of studies before participation.
Volunteers are encouraged to ask pertinent questions and communicate openly with their research teams, as these actions foster trust and improve overall engagement. Furthermore, reflecting on personal motivations can lead to a more meaningful and fulfilling experience.
Ultimately, participation in clinical trials not only offers the potential for personal health benefits but also plays a crucial role in shaping the future of medical care. By understanding the intricacies of clinical studies and preparing emotionally for the journey, individuals can empower themselves to make informed decisions that align with their health goals. Taking these steps enhances the volunteer experience and contributes to the collective advancement of medical knowledge, underscoring the profound impact that each volunteer can have on the future of healthcare.
Frequently Asked Questions
What is bioaccess® and what services do they provide for clinical trials?
bioaccess® specializes in enhancing the clinical trial process by providing expert guidance to volunteers. With over 15 years of experience, they facilitate prompt ethical approvals and effective enrollment through a network of more than 50 activated sites, ensuring a streamlined experience for participants.
What can volunteers expect when participating in studies managed by bioaccess®?
Volunteers can expect a well-organized experience supported by a dedicated team focused on advancing medical innovation ethically. Recent advancements in study management, including digital tools and patient-focused designs, make participation more accessible and engaging.
What challenges do clinical trials face regarding recruitment?
Approximately 80% of research studies are postponed or terminated due to recruitment challenges, highlighting the importance of bioaccess®'s role in improving this process.
What are the key steps in the clinical trial process that volunteers should know?
Key steps include:
- Screening: Assessing eligibility based on specific criteria.
- Informed Consent: Reviewing and signing documents outlining the trial's purpose, procedures, risks, and benefits.
- Randomization: Assigning participants to different research groups to ensure unbiased results.
- Treatment: Receiving the intervention or placebo.
- Follow-Up: Attending scheduled visits for monitoring and data collection.
Why is informed consent important for clinical trial volunteers?
Informed consent ensures that volunteers are fully informed about the project, including its purpose, procedures, risks, and benefits. It allows participants to ask questions, seek clarification, and opt out at any time without penalties.
What rights do volunteers have during the informed consent process?
Volunteers have the right to receive detailed information about the study, ask questions, and discuss any concerns with the research team. They can also opt out of the research at any time without facing penalties.
How can volunteers enhance their understanding and comfort level with the research?
Volunteers can enhance their understanding by thoroughly reading the consent form, asking questions, and engaging proactively with the research team. Effective informed consent practices, including clear language and multimedia tools, can also improve comprehension.
List of Sources
- bioaccess®: Accelerate Your Clinical Trial Experience with Expert Guidance
- Clinical Trials Statistics and Facts (2025) (https://media.market.us/clinical-trials-statistics)
- Rebooting the Statistic That 5% of Eligible Patients Participate in Clinical Trials | Applied Clinical Trials Online (https://appliedclinicaltrialsonline.com/view/rebooting-the-statistic-that-5-of-eligible-patients-participate-in-clinical-trials)
- An auto-ethnographic study of co-produced health research in a patient organisation: unpacking the good, the bad, and the unspoken - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC11265487)
- 25+ useful clinical trial recruitment statistics for better results (https://antidote.me/blog/25-useful-clinical-trial-recruitment-statistics-for-better-results)
- 10 surprising medical research statistics for Clinical Trials Day (https://antidote.me/blog/medical-research-volunteers-clinical-trial-day-statistics)
- Learn the Clinical Trial Process: Key Steps Every Volunteer Should Know
- How researchers are enlisting volunteers to help shape clinical trials (https://news.vumc.org/2025/02/13/how-researchers-are-enlisting-volunteers-to-help-shape-clinical-trials)
- What clinical trial statistics tell us about the state of research today (https://antidote.me/blog/what-clinical-trial-statistics-tell-us-about-the-state-of-research-today)
- Rebooting the Statistic That 5% of Eligible Patients Participate in Clinical Trials | Applied Clinical Trials Online (https://appliedclinicaltrialsonline.com/view/rebooting-the-statistic-that-5-of-eligible-patients-participate-in-clinical-trials)
- 25+ useful clinical trial recruitment statistics for better results (https://antidote.me/blog/25-useful-clinical-trial-recruitment-statistics-for-better-results)
- The Medical Minute: Healthy volunteers vital to clinical trials (https://pennstatehealthnews.org/2018/09/medical-minute-healthy-volunteers-clinical-trials)
- Understand Informed Consent: Your Rights and Responsibilities as a Volunteer
- Court Ruling Alters Informed Consent Process (https://generalsurgerynews.com/In-the-News/Article/04-21/Court-Ruling-Alters-Informed-Consent-Process/63144)
- Informed Consent - StatPearls - NCBI Bookshelf (https://ncbi.nlm.nih.gov/books/NBK430827)
- FDA Works to Make Informed Consent Easier to Understand (https://fda.gov/news-events/fda-voices/fda-works-make-informed-consent-easier-understand)
- Informed consent (https://researchsupport.admin.ox.ac.uk/governance/ethics/resources/consent)
- Evaluate Benefits and Risks: What You Need to Consider Before Joining a Study
- Rebooting the Statistic That 5% of Eligible Patients Participate in Clinical Trials | Applied Clinical Trials Online (https://appliedclinicaltrialsonline.com/view/rebooting-the-statistic-that-5-of-eligible-patients-participate-in-clinical-trials)
- Clinical Research: Benefits, Risks, and Safety (https://nia.nih.gov/health/clinical-trials-and-studies/clinical-research-benefits-risks-and-safety)
- 25+ useful clinical trial recruitment statistics for better results (https://antidote.me/blog/25-useful-clinical-trial-recruitment-statistics-for-better-results)
- Benefits of Participation in Clinical Trials: An Umbrella Review (https://mdpi.com/1660-4601/19/22/15368)
- Clinical Trials - Dementia Statistics Hub (https://dementiastatistics.org/statistics/clinical-trials)
- Ask the Right Questions: Essential Inquiries for Clinical Study Volunteers
- Questions to Ask (https://hhs.gov/ohrp/education-and-outreach/about-research-participation/questions-to-ask)
- Volunteering for research studies: 15 questions to ask (https://antidote.me/blog/research-studies-questions)
- Common statistical concerns in clinical trials - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC3059317)
- Questionnaires in clinical trials: guidelines for optimal design and administration - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC2823735)
- Research Trial Credibility: How to Identify Reputable Clinical Studies
- Why bad research is worse than no research (https://alasdairmunro.substack.com/p/why-bad-research-is-worse-than-no)
- Reporting ethical approval in case reports and case series in 12 consecutive years: A systematic review (https://onlinelibrary.wiley.com/doi/10.1002/hcs2.113)
- Trends and Charts on Registered Studies | ClinicalTrials.gov (https://clinicaltrials.gov/about-site/trends-charts)
- Ethics reporting practices in clinical research publications: A review of four Indian journals - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC4073550)
- The future of antibody-based HIV prevention (https://iavi.org/iavi-report/the-future-of-antibody-based-hiv-prevention)
- Communicate Effectively: Building a Relationship with Your Clinical Research Team
- Five Medical Communication Strategies Backed by Patient Insights | PPD (https://ppd.com/blog/medical-communication-strategies-patient-insights)
- Effectiveness and acceptability of methods of communicating the results of clinical research to lay and professional audiences: protocol for a systematic review - Systematic Reviews (https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-019-1065-x)
- Preferences and Attitudes Towards Digital Communication and Symptom Reporting Methods in Clinical Trials - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC11789515)
- Improving Communication in Clinical Research - SOCRA Blog (https://socra.org/blog/improving-communication-in-clinical-research)
- Consider Future Medical Care: How Participation May Affect Your Health Options
- Long-term Study of Bariatric Surgery for Obesity: LABS - NIDDK (https://niddk.nih.gov/about-niddk/research-areas/obesity/longitudinal-assessment-bariatric-surgery)
- Benefits of Participation in Clinical Trials: An Umbrella Review (https://mdpi.com/1660-4601/19/22/15368)
- Long-Term Health Effects of Participation in Project SHAD (Shipboard Hazard and Defense) (https://nap.nationalacademies.org/catalog/11900/long-term-health-effects-of-participation-in-project-shad-shipboard-hazard-and-defense)
- The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies (https://ascopubs.org/doi/10.1200/EDBK_156686)
- Impact of remission from type 2 diabetes on long-term health outcomes: findings from the Look AHEAD study - Diabetologia (https://link.springer.com/article/10.1007/s00125-023-06048-6)
- Prepare Emotionally: Understanding the Psychological Impact of Clinical Trials
- Association between mental health, psychological characteristics, and motivational functions of volunteerism among Polish and Ukrainian volunteers during the Russo-Ukrainian War - Scientific Reports (https://nature.com/articles/s41598-023-47840-z)
- The emotional side of taking part in a cancer clinical trial (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0284268)
- How to Make Clinical Trials More Feasible for Patients (https://clinicaltrialvanguard.com/clinicaltrials/reducing-patient-burden-in-clinical-trials-how-to-make-trials-more-feasible-for-patients)
- The emotional side of taking part in a cancer clinical trial - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC10124833)
- Who volunteers for phase I clinical trials? Influences of anxiety, social anxiety and depressive symptoms on self-selection and the reporting of adverse events | Request PDF (https://researchgate.net/publication/5532475_Who_volunteers_for_phase_I_clinical_trials_Influences_of_anxiety_social_anxiety_and_depressive_symptoms_on_self-selection_and_the_reporting_of_adverse_events)
- Reflect on Your Motivation: Why You Want to Participate in a Clinical Trial
- Patient involvement in clinical trials: motivation and expectations differ between patients and researchers involved in a trial on urinary tract infections - Research Involvement and Engagement (https://researchinvolvement.biomedcentral.com/articles/10.1186/s40900-019-0145-3)
- Exploring reasons for recruitment failure in clinical trials: a qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada - Trials (https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05818-0)
- Understanding Motivations to Participate in an Observational Research Study: Why do Patients Enroll? - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC4870048)
- Excluding People With Disabilities From Clinical Research: Eligibility Criteria Lack Clarity And Justification | Health Affairs Journal (https://healthaffairs.org/doi/10.1377/hlthaff.2022.00520)
- “At first, I was very afraid”—a qualitative description of participants’ views and experiences in the first Human Infection Study in Malawi - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC8825950)












