


This article provides essential insights into pharmacy regulatory affairs tailored for leaders, underscoring the complexities of compliance, emerging trends, and the skills crucial for success in this domain. It highlights the significance of effective communication and a comprehensive understanding of global regulations, alongside the integration of digital technologies. These elements are vital for navigating the ever-evolving landscape of pharmacy regulatory affairs, ultimately ensuring successful product development and compliance.
Navigating the intricate landscape of pharmacy regulatory affairs is a critical endeavor for leaders aiming to ensure compliance and drive innovation. As the pharmaceutical industry evolves, understanding the nuances of global regulatory frameworks and the essential skills required for success becomes paramount. However, with the rapid pace of change and the increasing complexity of compliance requirements, pharmacy professionals must consider: how can they effectively adapt and thrive in this challenging environment? This article explores ten essential insights that not only highlight best practices but also equip leaders with the knowledge needed to overcome the hurdles in pharmacy regulatory affairs.
bioaccess® effectively utilizes its extensive knowledge in early-phase clinical research to enhance the compliance environment for healthcare professionals. By leveraging Colombia's competitive advantages—such as cost savings exceeding 30% compared to North America and Western Europe, review speeds of 90-120 days, and a high-quality healthcare system recognized among the best globally—bioaccess® not only guarantees compliance but also boosts the efficiency of clinical trials. This agility markedly reduces the time to market, enabling leaders in pharmacy regulatory affairs to introduce their products more swiftly, ultimately improving patient care and fostering advancements in medical knowledge.
The impact of compliance speed is evident; studies indicate that companies capitalizing on these advantages can achieve enrollment rates 50% faster than traditional markets, thereby increasing the likelihood of successful trial outcomes. Notably, through strategic partnerships, such as with the Caribbean Health Group, bioaccess® is positioning Barranquilla as a premier location for clinical trials in Latin America. This further illustrates that a focus on compliance efficiency can lead to significant improvements in both time and resources.

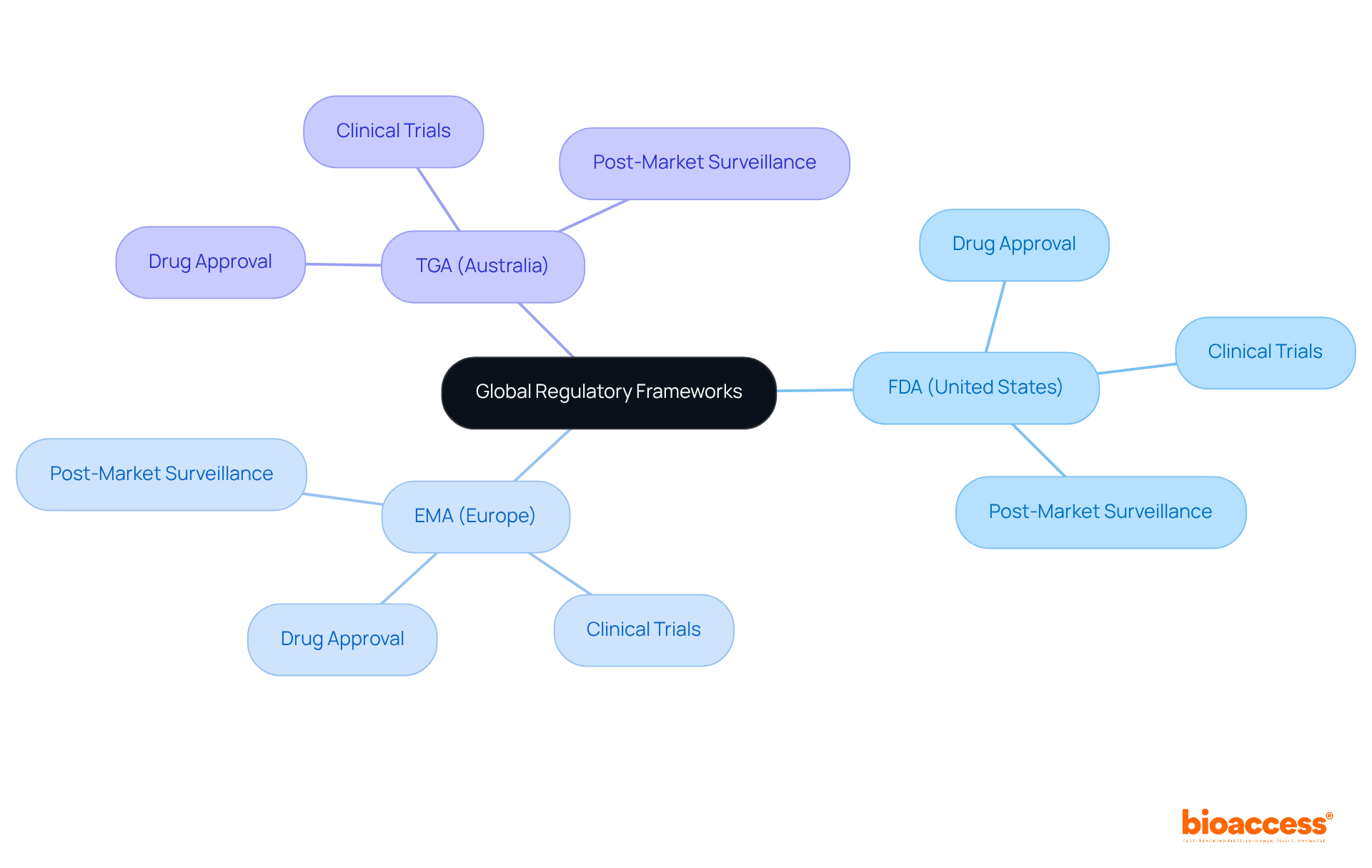
Pharmacy professionals must possess a comprehensive understanding of various global compliance frameworks, including:
Each regulatory body presents distinct requirements for drug approval, clinical trials, and post-market surveillance. It is imperative for leaders to prioritize staying informed about these regulations, as this knowledge ensures compliance and facilitates smoother product launches across diverse markets.
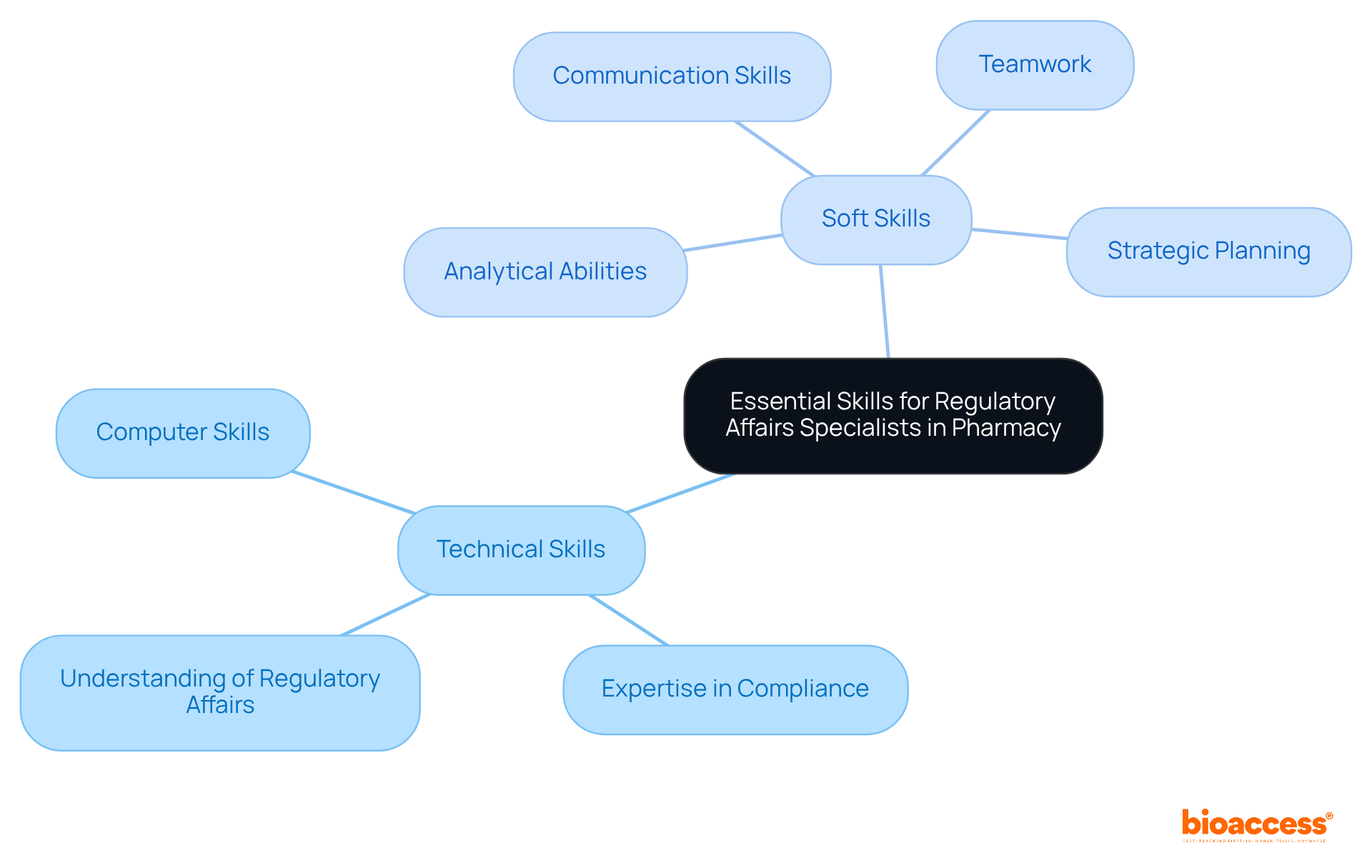
Specialists in pharmacy regulatory affairs are required to integrate technical expertise with essential soft skills to excel in their roles. These essential skills include:
Statistics indicate that compliance experts significantly benefit from these soft skills, as they foster efficient collaboration and negotiation. For example, the average annual salary for a Regulatory Pharmacist in the United States is $99,793, underscoring the value placed on these professionals.
Additionally, expertise in pharmacy regulatory affairs is crucial, enabling specialists to navigate the complexities of compliance submissions effectively. Successful project management examples in pharmacy regulatory affairs often emphasize the importance of:
Furthermore, the expertise of leaders like Ana Criado, who possesses extensive experience with INVIMA and serves as a consultant for global companies, highlights the necessity of having professionals with a strong background in biomedical engineering and health economics. This diverse skill set not only enhances adherence but also contributes to the overall success of pharmaceutical innovations within pharmacy regulatory affairs.
Moreover, proficiency in computer skills is essential for mastering elements related to submissions and adherence, further illustrating the varied skill set required in this field.
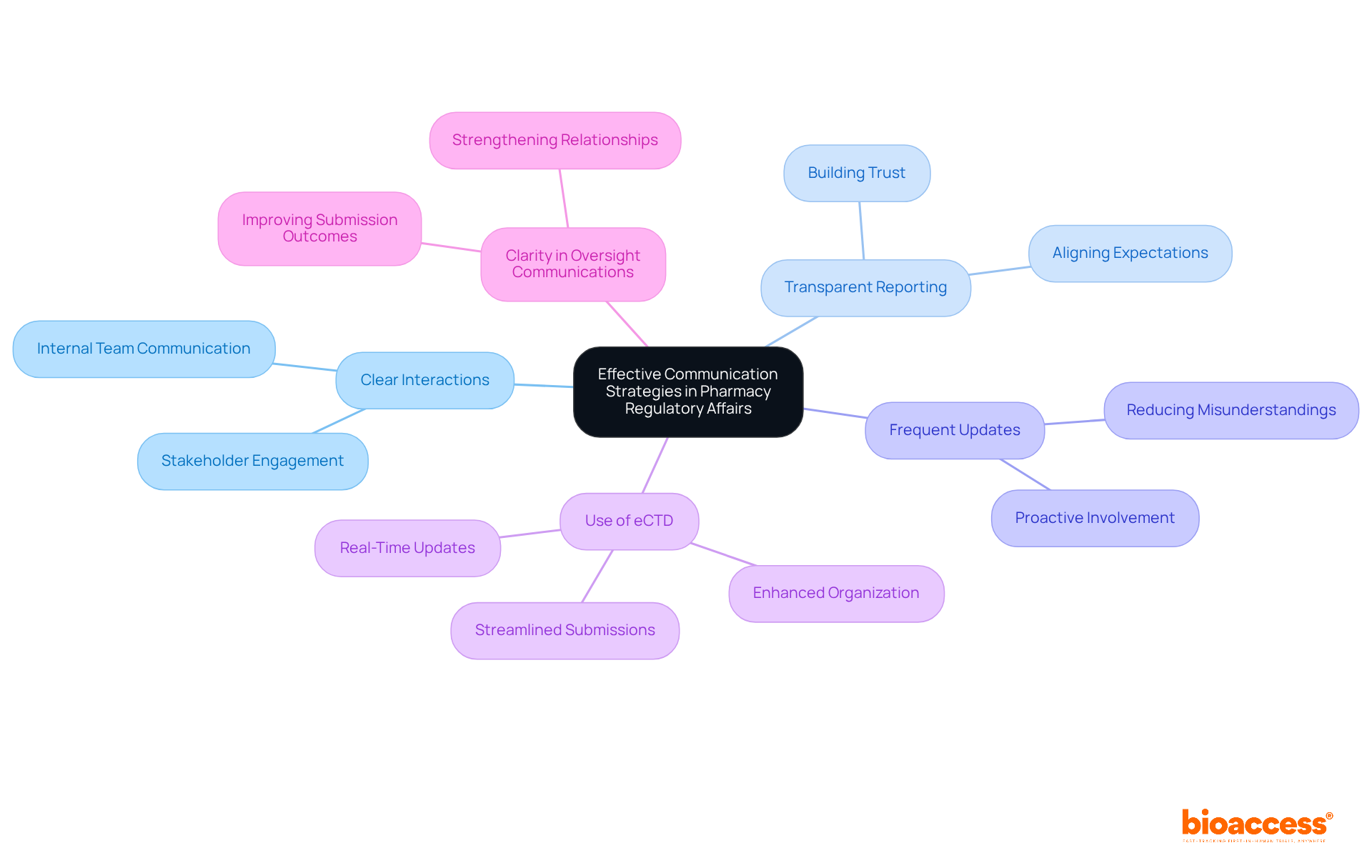
Professionals in pharmacy regulatory affairs encounter significant challenges, including rapidly evolving regulations, intricate submission processes, and the necessity for timely communication with regulatory bodies. The complexity of managing various projects while ensuring adherence can be overwhelming.
To effectively navigate these hurdles, leaders must adopt robust systems for pharmacy regulatory affairs that streamline operations and enhance oversight. For example, companies that have implemented continuous process verification have achieved a 23% reduction in waste and virtually eliminated quality investigations related to process variability.
Furthermore, promoting open communication channels with oversight organizations is essential for ensuring timely updates and feedback. This proactive strategy in pharmacy regulatory affairs not only mitigates compliance risks but also empowers organizations to respond swiftly to legal changes, ultimately enhancing their operational efficiency and market competitiveness.
By prioritizing these strategies, healthcare leaders can navigate the complexities of compliance matters more effectively and achieve successful results in their projects.

In the realm of pharmacy regulatory affairs, effective communication strategies are paramount. Experts must prioritize clear and concise interactions with internal teams, oversight organizations, and external stakeholders. Transparent reporting is essential; it fosters trust and ensures that all parties are aligned on expectations and requirements. Frequent updates and proactive involvement significantly diminish misunderstandings, paving the way for more streamlined compliance processes.
For instance, embracing the electronic Common Technical Document (eCTD) format enhances organization and communication, simplifying submissions and enabling real-time updates with oversight bodies. As emphasized in various workshops, clarity in communication not only improves submission outcomes but also strengthens relationships with oversight bodies.
Ultimately, clarity in oversight communications transcends mere best practice; it stands as a fundamental principle underpinning successful pharmacy regulatory affairs.
In pharmacy regulatory affairs, regulatory submissions are paramount for compliance within the pharmacy sector. Leaders must ensure that all documentation is accurate, complete, and submitted in a timely manner. Mastery of submission formats, timelines, and the requirements of various pharmacy regulatory affairs is essential. The utilization of submission management tools significantly simplifies this process, reducing errors and enhancing adherence rates. Notably, organizations employing these tools frequently report improved visibility into their compliance posture, with 71% of users indicating better management of compliance risks.
Best practices for compliance documentation encompass:
By adopting these strategies, healthcare professionals can adeptly navigate the complexities of compliance submissions. This ensures that documentation is not only timely but also meets the stringent standards of the industry.

Integrating compliance processes with clinical research is vital for optimizing the drug development procedure. By aligning compliance strategies with clinical trial designs, industry leaders can ensure that studies adhere to guidelines from the outset. This proactive approach not only minimizes delays but also enhances the likelihood of successful submissions, ultimately accelerating the time to market for new therapies.
bioaccess® provides comprehensive clinical trial management services that empower organizations to navigate the complexities of regulatory affairs more effectively, including:
Importantly, bioaccess® offers an accelerated regulatory approval process, achieving approvals in just 6-8 weeks compared to the typical 6-12 months in the US/EU. This capability facilitates faster enrollment of treatment-naive cardiology or neurology cohorts, streamlining the approval process and significantly enhancing the efficiency of early-phase clinical trials. By leveraging these services, startups in the pharmaceutical industry can secure a competitive advantage.
Emerging trends in pharmacy regulatory affairs underscore a transformative shift toward digital technologies in submission processes, significantly enhancing efficiency and accuracy. The FDA's recent initiatives, notably the Technology and Data Modernization Action Plan, exemplify this movement, aiming to simplify submissions through advanced analytics and digital tools. The anticipated incorporation of artificial intelligence (AI) is poised to revolutionize governance frameworks, with the market for AI-driven solutions in compliance tools projected to exceed 7 billion dollars by 2030, indicating a compound annual growth rate of over 20%.
Moreover, the increasing reliance on real-world evidence (RWE) in decision-making by authorities is reshaping adherence strategies. Statistics indicate that RWE is becoming a crucial element in evaluating the safety and effectiveness of new treatments, enabling more informed assessments by regulatory bodies. As leaders in the pharmaceutical sector navigate these changes, prioritizing the adoption of digital technologies and RWE is essential for enhancing oversight strategies in pharmacy regulatory affairs and ensuring compliance with evolving requirements. This proactive approach will facilitate smoother submissions and align with the industry's transition toward patient-centric methodologies.
In Colombia, experts such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, are leading the charge in these trends. With her extensive expertise in biomedical engineering and health economics, Ana is uniquely equipped to navigate the compliance landscape, particularly in the context of cannabis legislation. Her work exemplifies the integration of digital technologies and RWE into compliance strategies, addressing industry challenges, including the 83% compliance gap in AI data security. This underscores the importance of aligning oversight practices in pharmacy regulatory affairs with emerging technological advancements.

Ethical considerations play a pivotal role in pharmaceutical compliance matters. Professionals are tasked with ensuring that all research and submissions strictly adhere to ethical guidelines, with a primary focus on patient safety and informed consent. The commitment to transparency in reporting, alongside a steadfast dedication to ethical practices, not only bolsters adherence to regulations but also cultivates public confidence in the healthcare sector. This trust is essential for sustained success in the field.
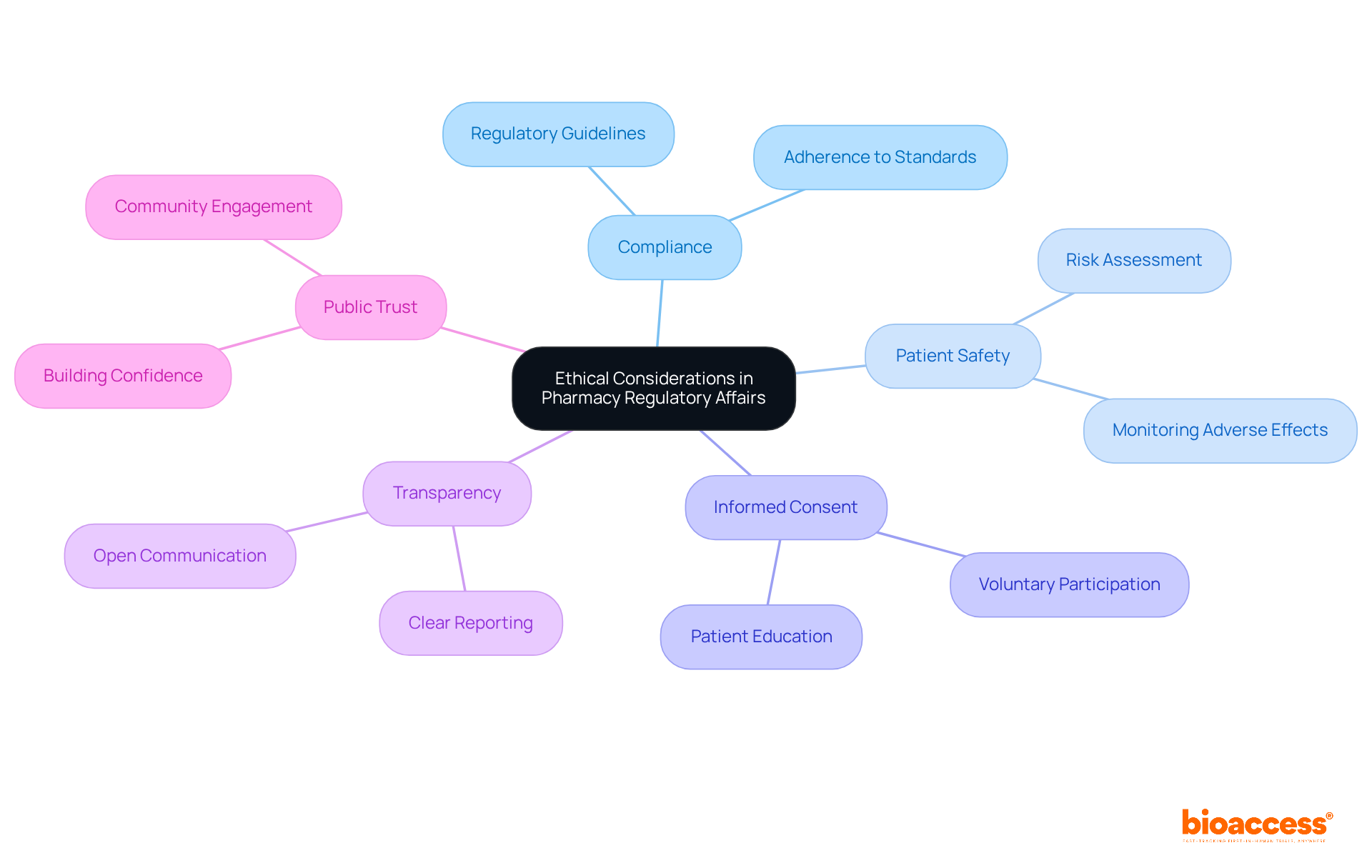
In summary, pharmacy leaders must navigate the complex legal environment of pharmacy regulatory affairs, which is characterized by evolving frameworks, adherence challenges, and ethical considerations. These challenges include disconnected systems and diverse workflows that complicate adherence efforts.
By employing effective communication strategies—such as building connections with oversight organizations like INVIMA, which monitors the marketing and production of health products in Colombia—and involving multidisciplinary teams, professionals can enhance their adherence efforts and accelerate the development of innovative therapies.
Thorough clinical trial management services, encompassing feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting, are essential for effectively navigating this landscape. Maintaining precise documentation of drug management is critical for adherence and safety, underscoring the necessity for coordinated communication in compliance matters.
Staying informed about emerging trends and compliance changes is vital for healthcare leaders to proactively address challenges and seize opportunities. As noted by Sai Prathyusha Bhamidipati, an experienced compliance professional, 'Navigating intricate legal frameworks necessitates a dedication to improving practices in governance.'
The insights provided in this article serve as a foundation for leaders striving to excel in their roles within pharmacy regulatory affairs.

Pharmacy regulatory affairs occupy a pivotal position at the intersection of compliance, innovation, and patient safety, underscoring the essential role that leaders play in navigating this intricate landscape. The insights articulated throughout this article highlight the imperative for professionals to stay abreast of evolving global regulations, cultivate critical skills, and implement effective communication strategies. By doing so, leaders can enhance adherence efforts, streamline compliance processes, and ultimately facilitate the successful introduction of new therapies into the market.
Key arguments presented emphasize the significance of leveraging technological advancements and real-world evidence to refine regulatory submissions, alongside the necessity for robust project management and collaboration within multidisciplinary teams. These components are vital for overcoming the challenges tied to compliance and ensuring that pharmaceutical innovations meet rigorous industry standards. Moreover, ethical considerations must remain paramount in regulatory practices to cultivate public trust and confidence in the healthcare sector.
In light of these insights, pharmacy leaders are urged to actively engage with emerging trends and adopt best practices that resonate with the dynamic nature of regulatory affairs. By prioritizing continuous improvement in governance and compliance strategies, professionals can adeptly navigate the complexities of their roles while contributing to the advancement of patient care and safety within the pharmaceutical industry. As the landscape continues to evolve, embracing these principles will be crucial for achieving sustained success and fostering innovation in pharmacy regulatory affairs.
What is bioaccess® and how does it impact clinical research in pharmacy regulatory affairs?
bioaccess® utilizes its expertise in early-phase clinical research to enhance compliance for healthcare professionals. By leveraging Colombia's advantages, such as significant cost savings and fast review speeds, bioaccess® improves the efficiency of clinical trials, reduces time to market, and ultimately enhances patient care.
What are the competitive advantages of conducting clinical trials in Colombia through bioaccess®?
The competitive advantages include cost savings exceeding 30% compared to North America and Western Europe, review speeds of 90-120 days, and a high-quality healthcare system recognized globally.
How does compliance speed affect clinical trial outcomes?
Companies that leverage compliance speed can achieve enrollment rates 50% faster than traditional markets, which increases the likelihood of successful trial outcomes.
What role do strategic partnerships play in bioaccess®'s approach to clinical trials?
Strategic partnerships, such as with the Caribbean Health Group, help position Barranquilla as a premier location for clinical trials in Latin America, enhancing compliance efficiency and resource management.
What global regulatory frameworks must pharmacy professionals understand?
Pharmacy professionals should understand the FDA in the United States, EMA in Europe, and TGA in Australia, as each has distinct requirements for drug approval, clinical trials, and post-market surveillance.
What essential skills are required for regulatory affairs specialists in pharmacy?
Essential skills include a profound understanding of pharmacy regulatory affairs, robust analytical abilities, exceptional communication skills, and proficiency in computer skills for submissions and adherence.
Why are soft skills important for compliance experts in pharmacy regulatory affairs?
Soft skills facilitate efficient collaboration and negotiation, which are crucial for interacting with oversight organizations and ensuring compliance, ultimately contributing to successful project management.
What is the significance of expertise in pharmacy regulatory affairs?
Expertise is crucial for navigating the complexities of compliance submissions and enhancing adherence, which contributes to the overall success of pharmaceutical innovations.
What is the average annual salary for a Regulatory Pharmacist in the United States?
The average annual salary for a Regulatory Pharmacist in the United States is $99,793.