


This article provides essential insights into Case Report Forms (CRFs) research, particularly for clinical directors, underscoring their critical role in clinical trials and the advantages of utilizing electronic formats. It highlights how well-designed CRFs significantly enhance data accuracy and compliance. Furthermore, electronic CRFs (eCRFs) showcase superior error rates and efficiency in data management, thereby improving overall research outcomes. The discussion emphasizes the necessity of integrating these advanced tools in the clinical research landscape to address key challenges effectively.
The landscape of clinical research is undergoing a rapid evolution, with the Case Report Form (CRF) positioned at the core of this transformation. This critical tool is essential for ensuring the accuracy and integrity of data collected during trials. As clinical directors navigate the complexities of research, grasping the nuances of CRF design, implementation, and management is vital for optimizing outcomes. Amidst the proliferation of electronic formats and the push for standardization, what key insights can empower clinical directors to fully harness the potential of CRFs? This article explores ten essential insights that illuminate the path forward, addressing both the opportunities and challenges inherent in CRF research.
Bioaccess® plays a pivotal role in Latin America, facilitating CRFs research by providing research directors with expedited insights into Case Report Documents while managing a diverse array of research services.
With over 20 years of experience in Medtech, bioaccess® excels in:
By leveraging local regulatory advantages and diverse patient populations, bioaccess® ensures that case report forms (CRFs) research is not only compliant but also tailored to meet the specific needs of research studies.
This agility empowers research directors to focus on strategic decision-making rather than administrative delays, ultimately enhancing the quality and speed of research outcomes.
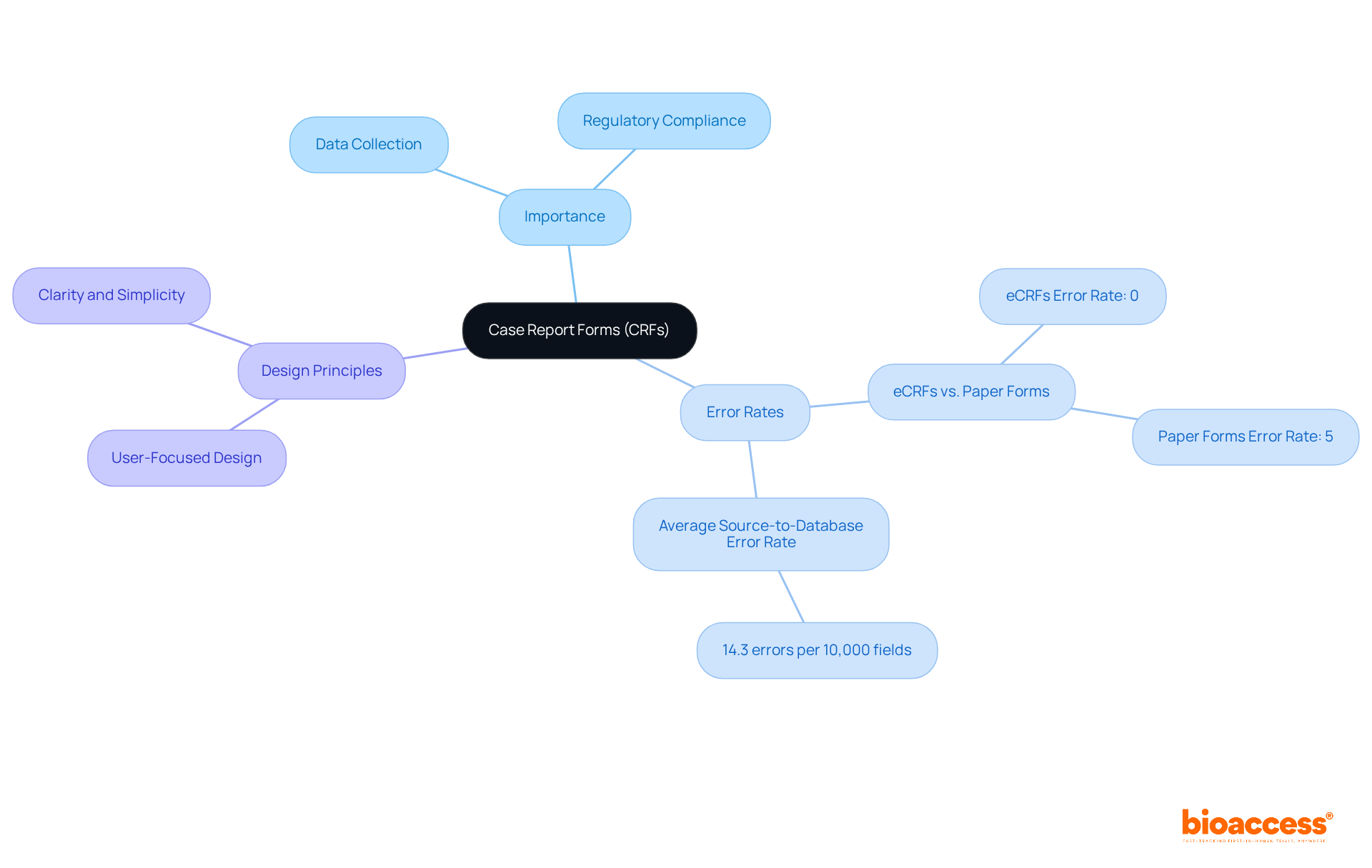
Case Report Forms (CRFs) are essential standardized documents utilized in research studies to systematically collect information from participating patients. They represent the primary mechanism for recording vital data, including patient demographics, medical history, and treatment outcomes. The significance of CRFs is underscored by their ability to ensure consistent and accurate information gathering, which is crucial for maintaining the integrity of clinical trials. Notably, research reveals that electronic case report forms (eCRFs) achieve an impressive 0% error rate, in stark contrast to a 5% error rate associated with traditional paper formats, thereby illustrating their effectiveness in enhancing information quality.
A well-structured CRF not only ensures compliance with regulatory requirements but also enhances the reliability of study results. The design of CRFs can significantly impact information accuracy; for example, a case study demonstrated that only 3.7% of over 38,000 eCRFs were queried, indicating a high level of information integrity. Furthermore, the average source-to-database error rate across various studies was found to be 14.3 errors per 10,000 fields, considerably lower than the average reported error rate of 976 errors per 10,000 fields. This emphasizes the importance of stringent information management practices in medical research.
Incorporating user-focused design principles into CRF development can significantly improve usability and compliance, ultimately leading to higher quality information. By prioritizing clarity and simplicity in CRF design, researchers can enhance data entry processes and ensure that essential information is accurately recorded, thereby supporting the overall success of clinical trials.
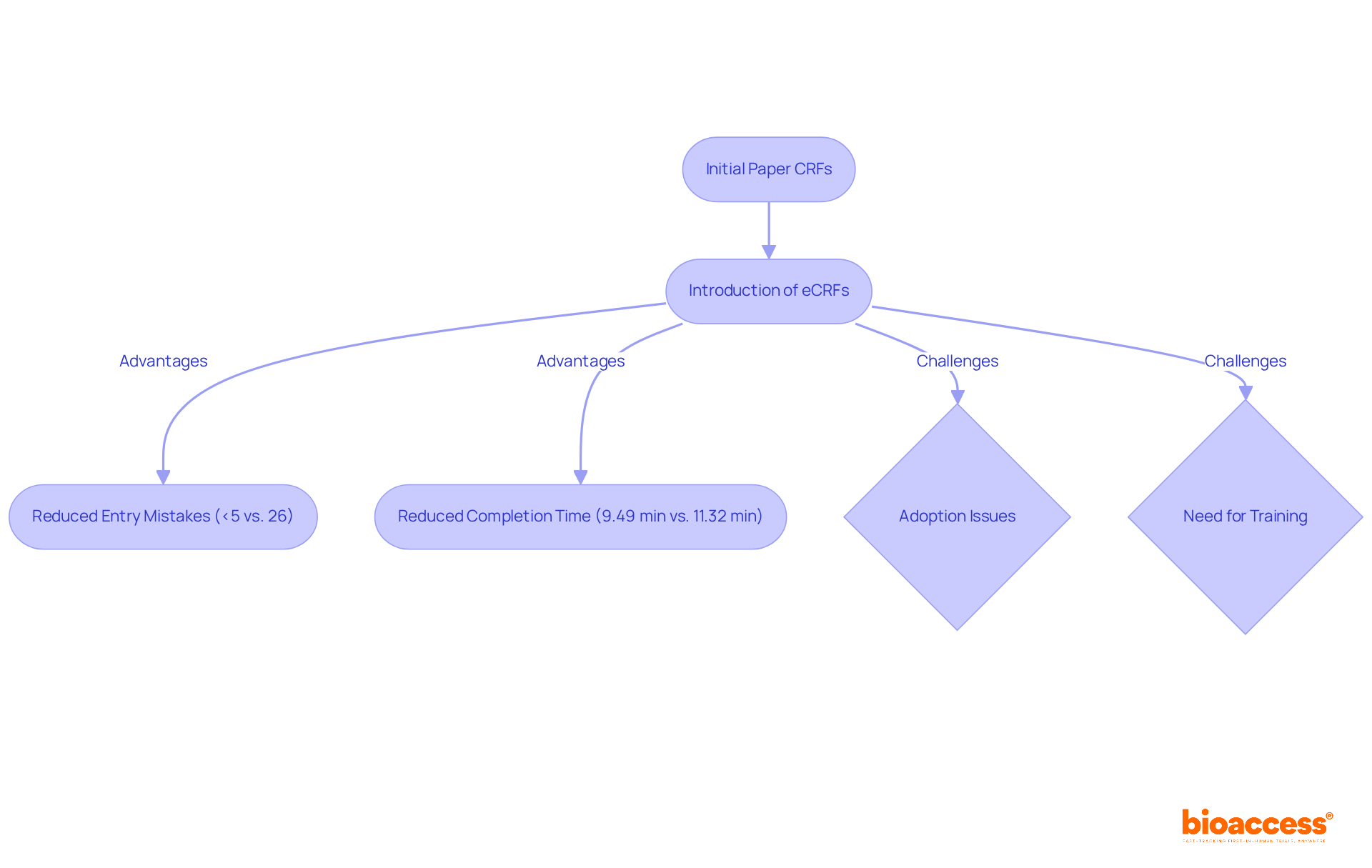
The development of Case Report Forms (CRFs) underscores the dynamic nature of CRFs research. Initially, CRFs were basic paper documents designed to gather fundamental patient information. However, as clinical trials became increasingly complex and information requirements escalated, CRFs evolved into more sophisticated documents. The introduction of electronic Case Report Forms (eCRFs) marked a pivotal advancement, facilitating real-time data entry and significantly enhancing accuracy.
Studies indicate that eCRFs can reduce entry mistakes to under 5%, compared to 26% for traditional paper forms, while also simplifying the collection process, resulting in a 16% reduction in completion time. Notably, the average completion time for eCRFs is 9.49 minutes, whereas paper forms take 11.32 minutes. This ongoing evolution is propelled by regulatory bodies and research organizations advocating for greater standardization and technological integration in trial processes, with over 80% of Phase III trials now employing eCRFs.
Nevertheless, challenges in the adoption of eCRFs remain, emphasizing the necessity for ongoing support and training. The shift from paper to electronic formats not only addresses data management issues but also enhances the overall efficiency and integrity of research in healthcare. As Katherine Ruiz emphasizes, the research on CRFs plays a vital role in maintaining high standards of medical research.
The comprehensive clinical trial management services provided by bioaccess—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential in facilitating this evolution, ensuring that the transition to eCRFs is both smooth and effective.
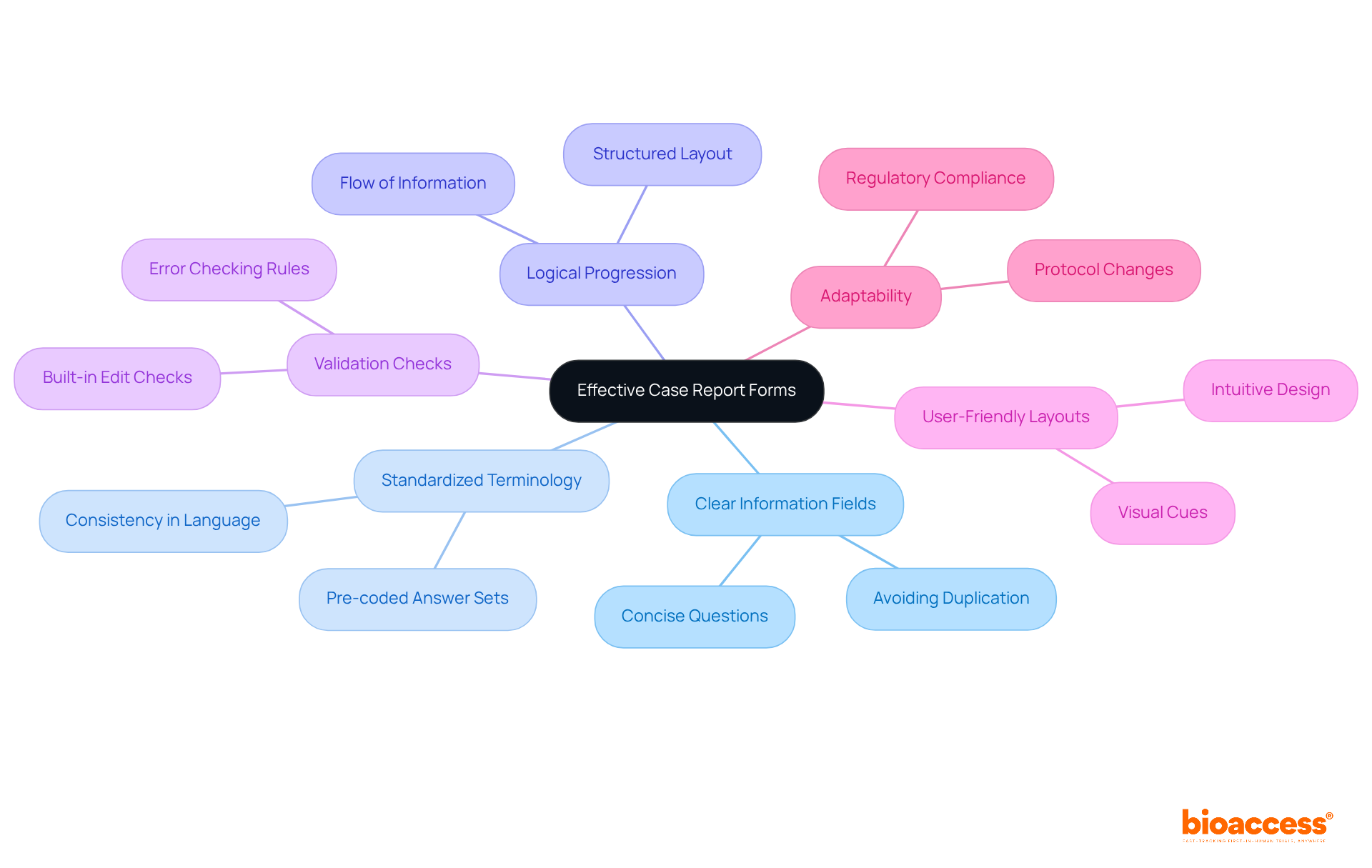
Effective Case Report Forms are critical in clinical research, necessitating the incorporation of several essential elements:
These forms must be structured to gather all necessary information while minimizing duplication. By integrating validation checks and user-friendly layouts, the accuracy of data entry and ease of use can be significantly enhanced. Moreover, it is imperative that case report forms are adaptable to accommodate changes in study protocols or regulatory requirements, thereby ensuring their continued relevance throughout the trial.
Designing and implementing Case Report Forms (CRFs) is a critical aspect of CRFs research, yet it involves navigating several challenges. Ensuring clarity and consistency across diverse sites, managing protocol changes, and effectively addressing user feedback are just a few hurdles that researchers face. Striking a balance between comprehensive information gathering and user-friendly design can be particularly challenging. To tackle these issues, research directors must prioritize early stakeholder involvement in the design process, fostering collaboration and ensuring that the design aligns with CRFs research to meet the needs of all users.
Thorough usability testing is essential; it enables researchers to identify potential issues and refine the CRF based on real-world feedback. Additionally, providing comprehensive training for users enhances their understanding and confidence, facilitating smoother implementation. Optimal methods include:
By incorporating user feedback into the CRFs research and development process, organizations can significantly enhance the quality of information and reduce errors, ultimately leading to more successful clinical trials.

Electronic Case Report Forms (eCRFs) present significant advantages over traditional paper forms, particularly in the realm of clinical research. They enhance information accuracy by minimizing transcription errors and facilitating real-time data entry, which accelerates the data collection process. Furthermore, eCRFs simplify information management and analysis through seamless integration with electronic information capture systems. Their capability for remote monitoring and information access makes them ideal for multi-site studies. Additionally, the ability to perform automated validation checks not only enhances data quality but also ensures compliance with regulatory standards.

Standardizing Case Report Forms is essential for enhancing the quality of clinical trials. By ensuring consistent information gathering across various locations and studies, standardized case report forms significantly improve the reliability of research results. For example, user-friendly case report forms can reduce entry mistakes by as much as 5%, while electronic case report forms (eCRFs) achieve an impressive 0% error rate compared to traditional paper formats. This consistency minimizes variability in information collection, making sharing and analysis more straightforward.
Moreover, adherence to established guidelines, such as the CARE guidelines, streamlines training for site personnel, enabling them to swiftly adapt to a uniform format. This not only enhances the effectiveness of execution but also supports regulatory compliance, as standardized case report forms are crucial for meeting requirements during audits and inspections.
Additionally, mentorship is vital in improving the quality of case reports, guiding authors through the writing and submission processes. Ultimately, the adoption of standardized case report forms (CRFs) research leads to higher quality information, which is critical for the success of research studies and the advancement of medical knowledge.

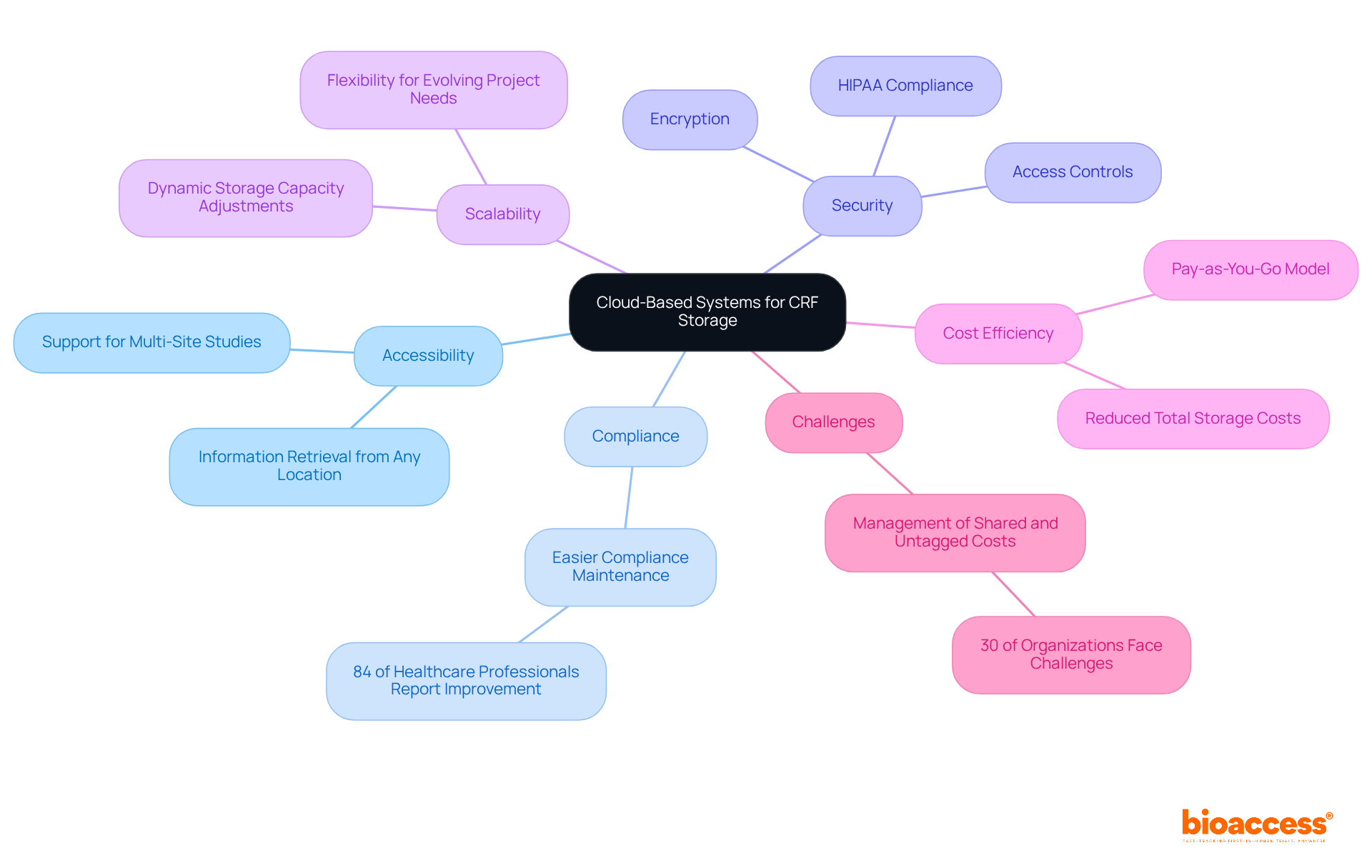
Cloud-based systems for storing Case Report Documents offer transformative advantages for CRFs research in clinical trials. These systems significantly enhance information accessibility, enabling authorized individuals to retrieve CRFs research from any location—an essential feature for multi-site studies. Notably, 84% of healthcare professionals report simpler compliance upkeep following their transition to the cloud.
However, the security of sensitive patient information remains paramount. Cloud storage solutions implement robust security measures, including:
These measures ensure that patient information is safeguarded against unauthorized access. Furthermore, the scalability of cloud systems allows for seamless adjustments in storage capacity as project needs evolve, making them a flexible choice for CRFs research organizations. This adaptability not only supports the dynamic nature of clinical trials but also aligns with the growing trend of cloud adoption in healthcare, where 80% of health services executives have embraced cloud technology for information management.
It is crucial to acknowledge that 30% of organizations face challenges in managing shared and untagged costs in the cloud, highlighting an issue that cloud storage can effectively address. Overall, cloud storage solutions prove to be more efficient than localized storage, reducing total storage costs while offering favorable service pricing.
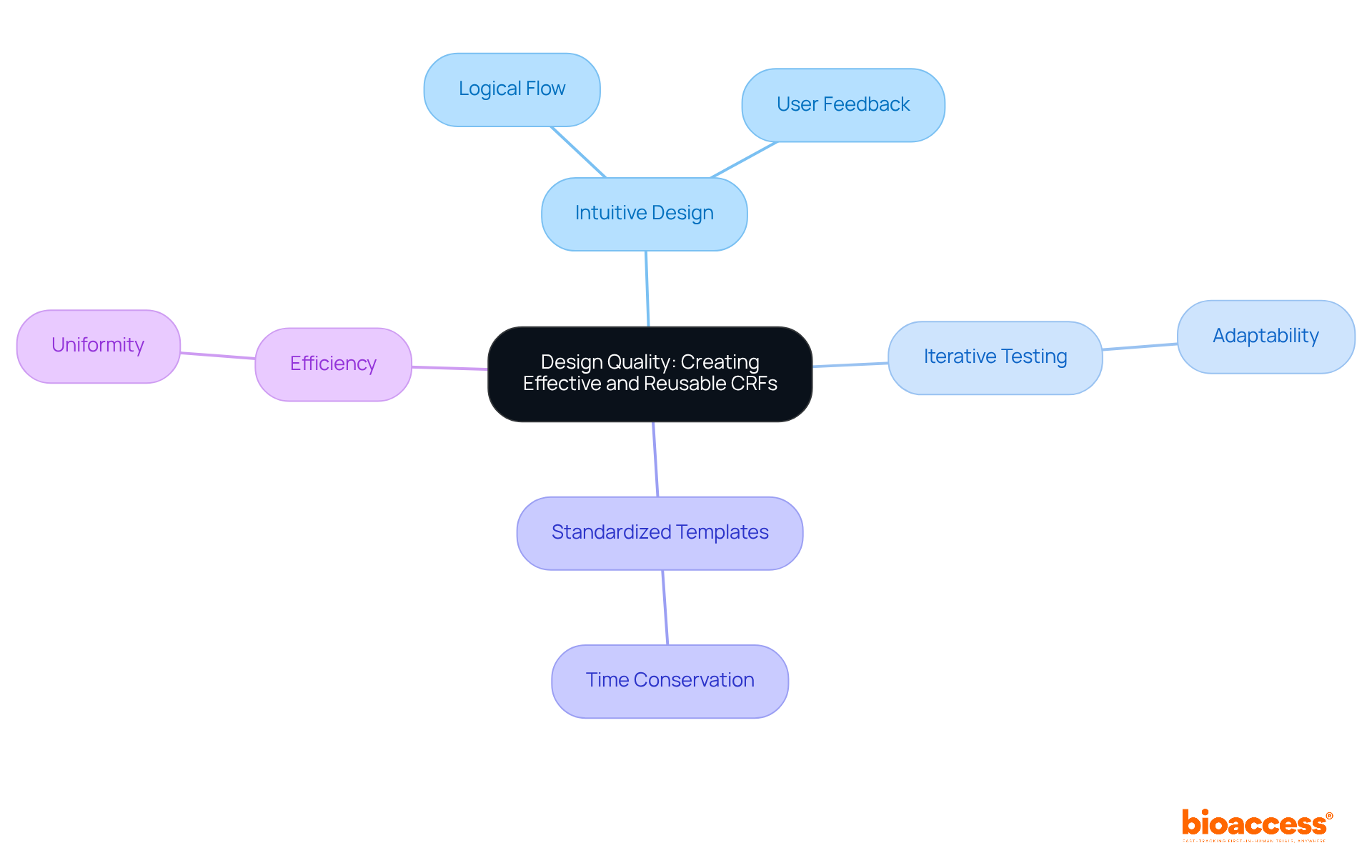
Creating effective and reusable Case Report Forms is essential in CRFs research, as it hinges on prioritizing design quality. High-quality CRFs research demonstrates that case report forms must be intuitive, featuring a logical flow that seamlessly guides users through the information entry process.
It is crucial to incorporate iterative testing and feedback from real users during the design phase of CRFs research; this approach leads to forms that are not only user-friendly but also adaptable for future studies.
Employing standardized templates significantly enhances reusability in CRFs research, allowing researchers to modify existing case report forms for new trials without starting from scratch. This strategic method not only conserves time and resources but also upholds information integrity, ensuring that the collected details remain trustworthy and valid across diverse research settings.
Moreover, well-structured CRFs enhance efficiency, saving time on information entry and corrections, while clear and concise questions ensure uniformity and clarity among raters.
What is a Case Report Form (CRF)? CRFs research indicates that a Case Report Form (CRF) serves as a standardized document utilized in clinical trials to systematically collect information from each patient participating in the study. It acts as the primary tool for recording vital patient information and treatment outcomes, ensuring that all necessary details are gathered for regulatory submissions.
Why are case report forms significant? Case report forms are essential in maintaining the integrity of clinical trials by ensuring that information is collected consistently and accurately. This consistency is crucial for the validity of CRFs research findings, as inconsistencies in data collection can lead to significant challenges in analysis and regulatory compliance.
What are the benefits of using eCRFs? Electronic Case Report Forms (eCRFs) offer numerous advantages over traditional paper forms, including enhanced accuracy, efficient data management, and the ability for immediate data entry. Research indicates that eCRFs can decrease data entry time by approximately 20%, markedly improving the efficiency of clinical trials.
How can clinical research forms be standardized? CRFs research entails standardizing by developing uniform templates and adhering to established guidelines, such as those from the Clinical Data Interchange Standards Consortium (CDISC). This approach ensures uniformity across studies, facilitating easier comparison and analysis, which is vital for regulatory approval.
What challenges are associated with CRF design? Common challenges in CRF design include ensuring clarity in data entry fields, managing modifications in study protocols, and effectively incorporating user feedback. Proactively addressing these challenges—such as through user-centered design workshops—can result in more effective and user-friendly CRFs research, ultimately enhancing the quality of data and the integrity of trials.

The insights provided on Case Report Forms (CRFs) research underscore their critical role in enhancing the quality and efficiency of clinical trials. Understanding the evolution, design, and implementation of CRFs enables clinical directors to leverage these tools to improve data accuracy and streamline research processes. The transition towards electronic Case Report Forms (eCRFs) exemplifies the ongoing advancements in this field, offering significant benefits over traditional paper formats.
Key arguments presented throughout the article emphasize the importance of:
These elements not only enhance the reliability and integrity of data collection but also facilitate compliance with regulatory standards. Furthermore, addressing challenges in CRF design through stakeholder involvement and iterative testing is essential for optimizing usability and effectiveness.
In conclusion, the significance of CRFs in clinical research is paramount. As the landscape of clinical trials continues to evolve, embracing innovative technologies and best practices in CRF development will be crucial. Clinical directors are encouraged to prioritize these insights and implement strategies that ensure the successful execution of trials, ultimately advancing medical knowledge and improving patient outcomes.
What is bioaccess and what role does it play in clinical research?
Bioaccess® is a key player in Latin America that facilitates Case Report Forms (CRFs) research by providing clinical research directors with expedited insights into Case Report Documents while managing various research services.
What types of studies does bioaccess specialize in?
Bioaccess specializes in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
How does bioaccess enhance the quality and speed of research outcomes?
By leveraging local regulatory advantages and diverse patient populations, bioaccess ensures that CRFs research is compliant and tailored to specific study needs, allowing research directors to focus on strategic decision-making rather than administrative delays.
What are Case Report Forms (CRFs) and why are they important?
CRFs are standardized documents used in research studies to systematically collect information from participating patients. They are crucial for maintaining the integrity of clinical trials by ensuring consistent and accurate information gathering.
How do electronic Case Report Forms (eCRFs) compare to traditional paper forms?
eCRFs achieve a 0% error rate, while traditional paper forms have a 5% error rate. eCRFs also reduce entry mistakes to under 5% compared to 26% for paper forms and simplify the collection process, leading to a 16% reduction in completion time.
What impact does the design of CRFs have on information accuracy?
A well-structured CRF enhances compliance with regulatory requirements and improves the reliability of study results. User-focused design principles can significantly improve usability and compliance, ensuring accurate data entry.
How have CRFs evolved in clinical trials?
CRFs have evolved from basic paper documents to sophisticated electronic formats (eCRFs) that facilitate real-time data entry and enhance accuracy. The shift to eCRFs has been driven by the need for greater standardization and technological integration in trial processes.
What challenges exist in the adoption of eCRFs?
Challenges in adopting eCRFs include the need for ongoing support and training to address the transition from paper to electronic formats effectively.
What comprehensive services does bioaccess provide to facilitate the evolution of CRFs?
Bioaccess provides clinical trial management services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting to ensure a smooth transition to eCRFs.