


The article provides essential insights for Medtech innovators on compliance with the EU In Vitro Diagnostic Regulation (IVDR). It emphasizes the necessity for robust clinical evidence, effective Quality Management Systems, and proactive engagement with Notified Bodies. These elements are crucial for navigating regulatory challenges and ensuring successful market entry. Adherence to these standards is vital for maintaining product safety and efficacy, underscoring the importance of collaboration in the Medtech landscape.
Navigating the intricate landscape of EU IVDR compliance presents a formidable challenge for Medtech innovators, particularly as the 2025 deadline approaches. With stringent regulations designed to ensure the safety and effectiveness of in vitro diagnostic devices, companies must not only grasp the new standards but also strategically position themselves to meet them. This article unveils ten essential insights that will empower innovators to streamline their compliance processes, enhance product quality, and ultimately secure a competitive edge in the evolving medical technology market.
How can Medtech firms effectively adapt to these changes and thrive amidst the regulatory complexities?
bioaccess® leverages its extensive expertise in early-stage clinical research to expedite EU regulatory compliance for Medtech innovators. By harnessing the regulatory efficiency found in Latin America and the diverse patient populations of the Balkans, bioaccess® achieves ethical approvals in an impressive 4-6 weeks. This rapid turnaround is crucial for businesses striving to navigate stringent regulatory requirements while sustaining their competitive edge in the market. Given that the average time to market for medical products is significantly impacted by regulatory hurdles under the EU IVDR, bioaccess®'s strategy not only accelerates the process but also enhances the likelihood of successful market entry.
Industry leaders emphasize that a well-defined governance approach, beginning with a clear understanding of the product's intended purpose and user requirements, is vital for compliance and overall business success. As the transition period for the Medical Device Regulation (MDR) extends until 2027/2028, Medtech innovators must proactively engage in adherence efforts to prevent market delays. The recent case study of Avantec Vascular, which selected bioaccess™ for its first-in-human clinical study of an innovative vascular device in Latin America, exemplifies how bioaccess supports companies in effectively navigating regulatory submissions and site activations. This collaboration highlights the strategic advantage of partnering with bioaccess® to ensure timely compliance and successful market entry.
In this evolving landscape, bioaccess® positions itself as a strategic ally, assisting Medtech firms in adeptly maneuvering through the complexities of EU IVDR compliance with both agility and confidence.

The eu ivdr aims to ensure the safety and effectiveness of in vitro diagnostic tools for patients. Its primary objectives include enhancing the quality and reliability of IVDs, increasing transparency in the regulatory process, and requiring manufacturers to provide robust clinical evidence to substantiate their claims. The regulation covers a wide range of instruments, from simple tests to complex diagnostics, and enforces strict adherence measures designed to protect public health.
As of 2025, compliance rates for IVDs under the new regulations are anticipated to increase substantially, demonstrating the industry's adjustment to these stringent standards. Compliance specialists, such as Ana Criado, Director of Compliance Affairs at bioaccess, and Katherine Ruiz, an expert in compliance for medical devices and in vitro diagnostics, stress that the new regulation not only aligns the compliance framework across the EU but also imposes increased responsibility on producers to prove that their products satisfy rigorous safety and performance standards. Ana Criado observes, "The new regulations signify a major change in oversight expectations, urging manufacturers to emphasize clinical evidence in their product development processes." This shift is crucial for maintaining market access and ensuring that IVDs contribute positively to patient outcomes.
The execution of the eu ivdr signifies a crucial moment for the in vitro diagnostics industry, requiring that manufacturers stay informed about ongoing updates and regulatory obligations. With the evolving landscape, companies that proactively engage with these regulations will be better positioned to navigate the complexities of the market and enhance their product offerings. Katherine Ruiz states, "Keeping up with compliance changes is crucial for companies seeking to thrive in the competitive IVD market." Furthermore, producers need to recognize the transition phases for legacy devices, which permit certain products to stay available in the market under particular conditions, along with the heightened documentation and regulatory demands established by the new rules. The gradual implementation of the EUDAMED platform will also play a significant role in shaping the regulatory landscape, making it essential for companies to stay updated on its progress.

The transition from the In Vitro Diagnostic Directive (IVDD) to the new regulations marks a pivotal moment that Medtech innovators must grasp to ensure compliance and avert potential pitfalls. The EU IVDR imposes more rigorous standards for clinical evidence, increases scrutiny from Notified Bodies, and introduces a novel risk-oriented classification system for products. Furthermore, it places a greater emphasis on post-market surveillance and continuous performance evaluation, areas that were less stringent under the EU IVDR.
With bioaccess®'s expedited clinical trial management services—including Early-Feasibility, First-In-Human, and Post-Market Clinical Follow-Up Studies—companies can adeptly navigate these compliance challenges. Katherine Ruiz, an expert in regulatory matters for medical instruments and in vitro diagnostics in Colombia, further bolsters bioaccess®'s capacity to assist innovators in achieving adherence and expediting their clinical trials.
To achieve compliance with the EU IVDR, Medtech innovators must prioritize several critical components:
Clinical Evidence: A robust body of clinical data is essential to demonstrate the safety and performance of the device. This evidence must be meticulously gathered and documented, as it plays a pivotal role in regulatory assessments.
Implementing a comprehensive Quality Management System (QMS) that aligns with EU IVDR requirements is crucial for ensuring consistent product quality. Organizations that implement effective QMS practices can anticipate enhanced operational efficiency and adherence rates, with studies suggesting that companies with developed systems achieve a 92% on-time delivery rate.
Risk Management: A thorough risk assessment and management strategy must be integrated throughout the product lifecycle. This involves recognizing possible risks related to the equipment and applying strategies to reduce them, which is essential for ensuring patient safety and adhering to regulatory standards.
Post-Market Surveillance: Ongoing observation of product performance and safety following market entry is required under the EU IVDR. This continuous alertness assists manufacturers in reacting quickly to any problems that occur, guaranteeing that the product stays safe and effective for users.
Technical Documentation: Detailed technical documentation is necessary to support adherence claims and facilitate regulatory review. This documentation should include descriptions of equipment, labeling, safety and performance data, and clinical evidence, all organized in a manner that is readily accessible for auditors.
The effective execution of these components not only guarantees adherence to the regulations but also improves the overall quality and dependability of medical products in the market. As the landscape of Medtech evolves, maintaining a strong focus on clinical evidence and robust quality management practices will be essential for innovators aiming to thrive in this competitive environment.
Post-market surveillance (PMS) is a fundamental requirement under the EU IVDR, aimed at ensuring the continued safety and effectiveness of IVDs. Manufacturers must establish a PMS plan that includes:
By adopting a strong PMS strategy, including PMCF studies supported by bioaccess®, manufacturers can proactively tackle potential problems, uphold adherence to industry standards, and guarantee the long-term success of their products.

Notified Bodies play a crucial role in the certification process under the EU IVDR, serving as independent organizations appointed by EU member states to evaluate the conformity of in vitro diagnostic devices (IVDs). Their primary responsibilities include:
Engaging with Notified Bodies early in the development process is vital. This proactive approach simplifies the certification journey and enables manufacturers to identify potential regulatory challenges at an early stage. As we approach 2025, the landscape of Notified Bodies operating under the EU IVDR is evolving, marked by a significant increase in applications and certifications. This trend underscores the growing complexity and demand for IVDs in the market. Manufacturers are strongly encouraged to initiate their compliance journeys promptly, as delays can adversely impact product timelines. Notably, 90% of Notified Bodies report that product certificates under the regulation require over 13 months for processing, with 75% of rejections attributed to incomplete applications. As emphasized by Emergo by UL, manufacturers are encouraged to commence their MDR and EU IVDR compliance journeys now.
In this context, the expertise of professionals like Ana Criado, Director of Compliance Affairs and a consultant with extensive experience in regulatory frameworks, is invaluable. Her background includes significant contributions at INVIMA, where she played a key role in developing compliance policies, alongside her academic positions as a professor at leading Colombian universities. Collaborating with specialists such as Ana and Katherine Ruiz, who focuses on regulatory issues for medical equipment and in vitro diagnostics in Colombia, can significantly enhance a manufacturer's understanding and implementation of compliance strategies.
Performance evaluations are paramount under the eu ivdr, emphasizing the demonstration of clinical performance and safety of in vitro diagnostics (IVDs). Manufacturers must conduct comprehensive evaluations that encompass several critical elements:
Moreover, stricter requirements apply to higher-risk IVDs, necessitating a more rigorous approach to performance evaluations. As Dr. Yupei Xiao emphasizes, "Performance assessment is not merely a pre-market necessity; producers must consistently refresh evidence to represent the most recent data, ensuring ongoing safety and effectiveness."
By adhering strictly to these requirements, manufacturers can validate their assertions, ensuring that their products not only meet regulatory expectations but also deliver real-world benefits to patients. Engaging in proactive data gathering and maintaining robust post-market monitoring further enhances adherence and safety throughout the product's lifecycle. Additionally, with the impending deadline of May 26, 2025, for the implementation of Quality Management Systems (QMS) and the submission of Performance Evaluation Reports (PER) for Class D legacy products, it is imperative for manufacturers to act swiftly to ensure compliance. The three fundamental components of in vitro diagnostic regulation performance assessment—scientific validity, analytical performance, and clinical performance—must be clearly addressed to meet compliance standards.

Under the IVDR, IVD devices are classified into four categories based on risk:
In Colombia, the oversight framework is managed by INVIMA (Colombia National Food and Drug Surveillance Institute), which plays a vital role in inspecting and supervising health products, including medical instruments. As a Level 4 health authority acknowledged by the Pan American Health Organization/World Health Organization, INVIMA ensures that items meet safety, efficacy, and quality standards. Understanding these classifications within the EU IVDR regulation, along with INVIMA's regulatory framework, is essential for manufacturers to ensure they meet the specific requirements linked to their product category.

A Quality Management System (QMS) is essential for adherence to the eu ivdr, which ensures that manufacturers consistently provide safe and effective devices. Essential components of an effective QMS include:
By creating a thorough QMS, manufacturers can not only enhance product quality but also simplify their regulatory efforts, ultimately enabling easier market access under the eu ivdr regulation.
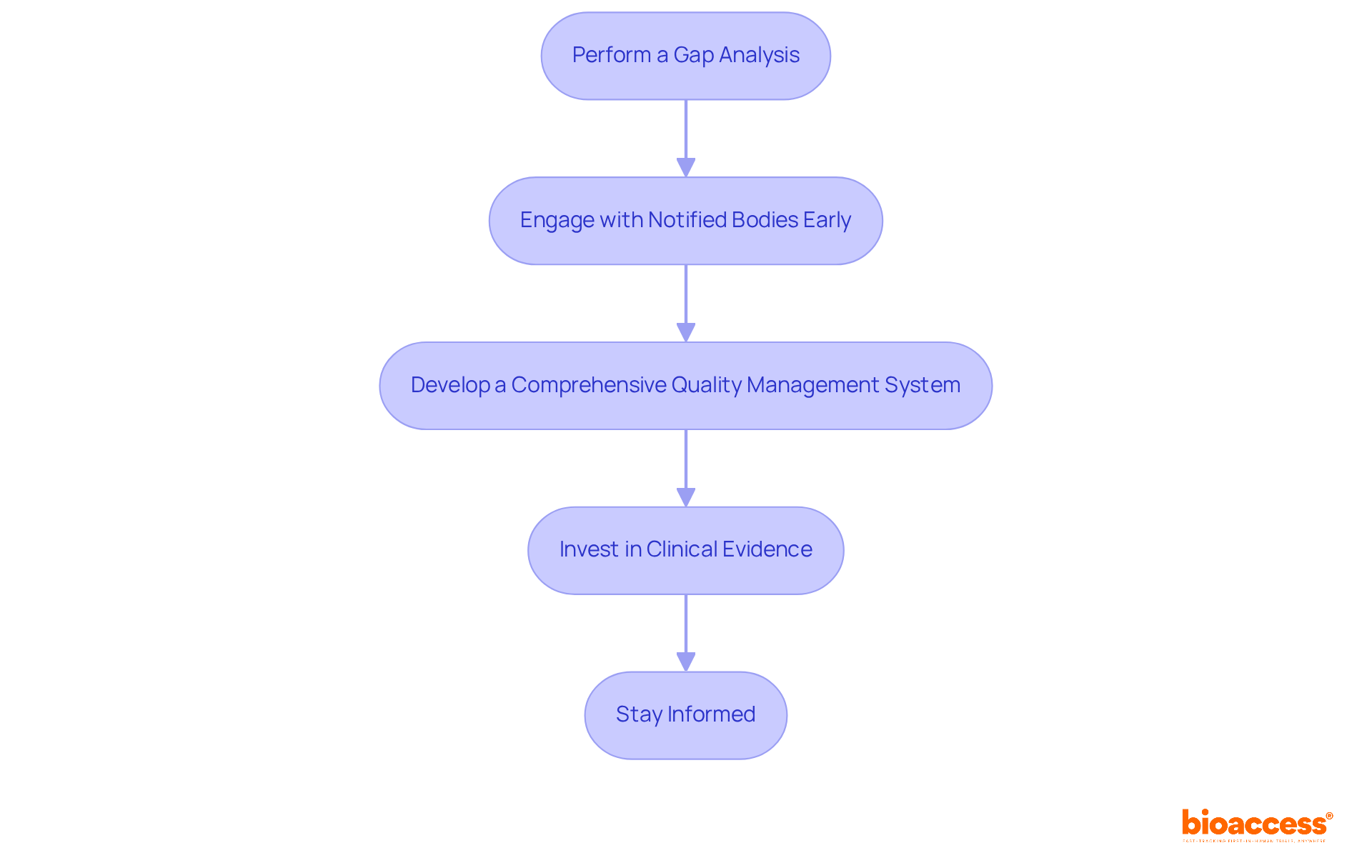
Shifting to the new regulations necessitates meticulous planning and execution. Medtech innovators can adopt the following strategies to ensure compliance and bolster their market position:
Perform a Gap Analysis: Assess your current adherence status against In Vitro Diagnostic Regulation requirements to pinpoint areas for improvement. This analysis is critical, as successful examples have demonstrated that early identification of gaps can streamline the transition process and mitigate risks. Incorporating a Performance Evaluation Plan (PEP) within this analysis offers a structured approach to gathering essential clinical data.
Engage with Notified Bodies Early: Initiate dialogue with Notified Bodies to clarify their expectations and requirements. Early engagement is linked to smoother compliance processes, allowing for proactive adjustments to meet regulatory standards. Under the new regulations, it is anticipated that 80-90% of IVD products will require the involvement of notified bodies, making this step indispensable.
Develop a Comprehensive Quality Management System (QMS) that aligns with the eu ivdr standards, focusing on documentation, risk management, and staff training. A robust QMS is vital for maintaining compliance and ensuring product safety throughout the lifecycle.
Invest in Clinical Evidence: Prioritize the accumulation of substantial clinical data to substantiate device efficacy and safety claims. As the eu ivdr mandates rigorous performance evaluations, having strong clinical evidence is essential for successful market entry. bioaccess® offers comprehensive clinical trial management services, including feasibility studies, trial setup, project management, review processes, and reporting, to support this critical phase.
Stay Informed: Consistently update your knowledge of regulatory changes and industry best practices to ensure continuous compliance. This proactive strategy not only aids in fulfilling regulatory requirements but also positions your organization as a leader in the Medtech sector. Collaborating with bioaccess, a vetted CRO and consulting partner for U.S. medical device companies in Colombia, can provide invaluable insights and support throughout this process.
By implementing these strategies, Medtech innovators can adeptly navigate the transition to eu ivdr, ensuring compliance while maintaining a competitive edge in the evolving regulatory landscape.
bioaccess® plays a pivotal role in guiding Medtech innovators through the intricate landscape of EU IVDR compliance. By emphasizing the importance of robust clinical evidence, effective quality management systems, and proactive engagement with Notified Bodies, the article underscores the necessity for manufacturers to adapt swiftly to the evolving regulatory environment. Understanding the nuances between the IVDR and its predecessor, the IVDD, is crucial for ensuring successful market entry and maintaining competitive advantage.
Key arguments presented include:
As the deadline for full compliance approaches, Medtech innovators are called to action to prioritize their transition strategies. By leveraging resources like bioaccess® and staying informed about regulatory changes, companies can not only meet compliance requirements but also position themselves as leaders in the Medtech sector. Embracing these insights will pave the way for successful navigation of the EU IVDR landscape, ensuring that innovations contribute positively to patient outcomes while adhering to the highest standards of safety and effectiveness.
What is bioaccess® and how does it assist Medtech innovators?
bioaccess® is a company that leverages its expertise in early-stage clinical research to expedite EU regulatory compliance for Medtech innovators. It helps businesses achieve ethical approvals in 4-6 weeks, facilitating quicker market entry while navigating stringent regulatory requirements.
What is the significance of the EU IVDR for in vitro diagnostic tools?
The EU IVDR aims to ensure the safety and effectiveness of in vitro diagnostic (IVD) tools. Its primary objectives include enhancing quality and reliability, increasing transparency in the regulatory process, and requiring manufacturers to provide robust clinical evidence for their products.
How does bioaccess® improve the time to market for medical products?
By utilizing regulatory efficiencies found in Latin America and diverse patient populations in the Balkans, bioaccess® accelerates the approval process, which is crucial for Medtech companies facing regulatory hurdles under the EU IVDR.
What are the critical differences between the EU IVDR and the previous IVDD?
The EU IVDR imposes more rigorous standards for clinical evidence, increases scrutiny from Notified Bodies, introduces a risk-oriented classification system, and emphasizes post-market surveillance and continuous performance evaluation compared to the IVDD.
What role does governance play in compliance with EU IVDR?
A well-defined governance approach is vital for compliance and business success. It begins with a clear understanding of the product's intended purpose and user requirements, which is essential for navigating regulatory processes effectively.
What is the anticipated impact of the EU IVDR on compliance rates by 2025?
Compliance rates for IVDs under the new regulations are expected to increase substantially by 2025, as the industry adjusts to the stringent standards set by the EU IVDR.
How does bioaccess® support companies during clinical trials?
bioaccess® offers expedited clinical trial management services, including Early-Feasibility, First-In-Human, and Post-Market Clinical Follow-Up Studies, helping companies navigate compliance challenges effectively.
What should manufacturers know about the transition phases for legacy devices under the EU IVDR?
Manufacturers need to recognize that transition phases allow certain legacy devices to remain on the market under specific conditions, while also adhering to heightened documentation and regulatory demands established by the new regulations.
Why is it important for companies to stay informed about compliance changes in the IVD market?
Keeping up with compliance changes is crucial for companies to maintain market access, enhance their product offerings, and thrive in the competitive IVD market.
What is the role of the EUDAMED platform in the regulatory landscape?
The gradual implementation of the EUDAMED platform will significantly shape the regulatory landscape, making it essential for companies to stay updated on its progress and understand their obligations under the new regulations.