


The article primarily addresses the INVIMA risk classification guide for medical devices, which categorizes products into four distinct risk groups that dictate the regulatory requirements and approval processes. Understanding this classification system is essential for manufacturers, as it enables them to ensure compliance and optimize their registration efforts. Notably, lower-risk devices can benefit from expedited approval processes, while higher-risk devices encounter more stringent requirements and extended review times. This insight into the classification system is crucial for navigating the complexities of the Medtech landscape.
Understanding the intricate landscape of medical device regulation in Colombia is essential for manufacturers seeking to navigate the complexities of the INVIMA risk classification guide. This framework categorizes devices based on risk levels and dictates the regulatory scrutiny each product must undergo, significantly influencing the speed and efficiency of market entry. As the demand for innovative healthcare solutions continues to rise, the pressing challenge remains: how can manufacturers effectively align their strategies with INVIMA’s evolving requirements to ensure compliance and expedite their approval processes?
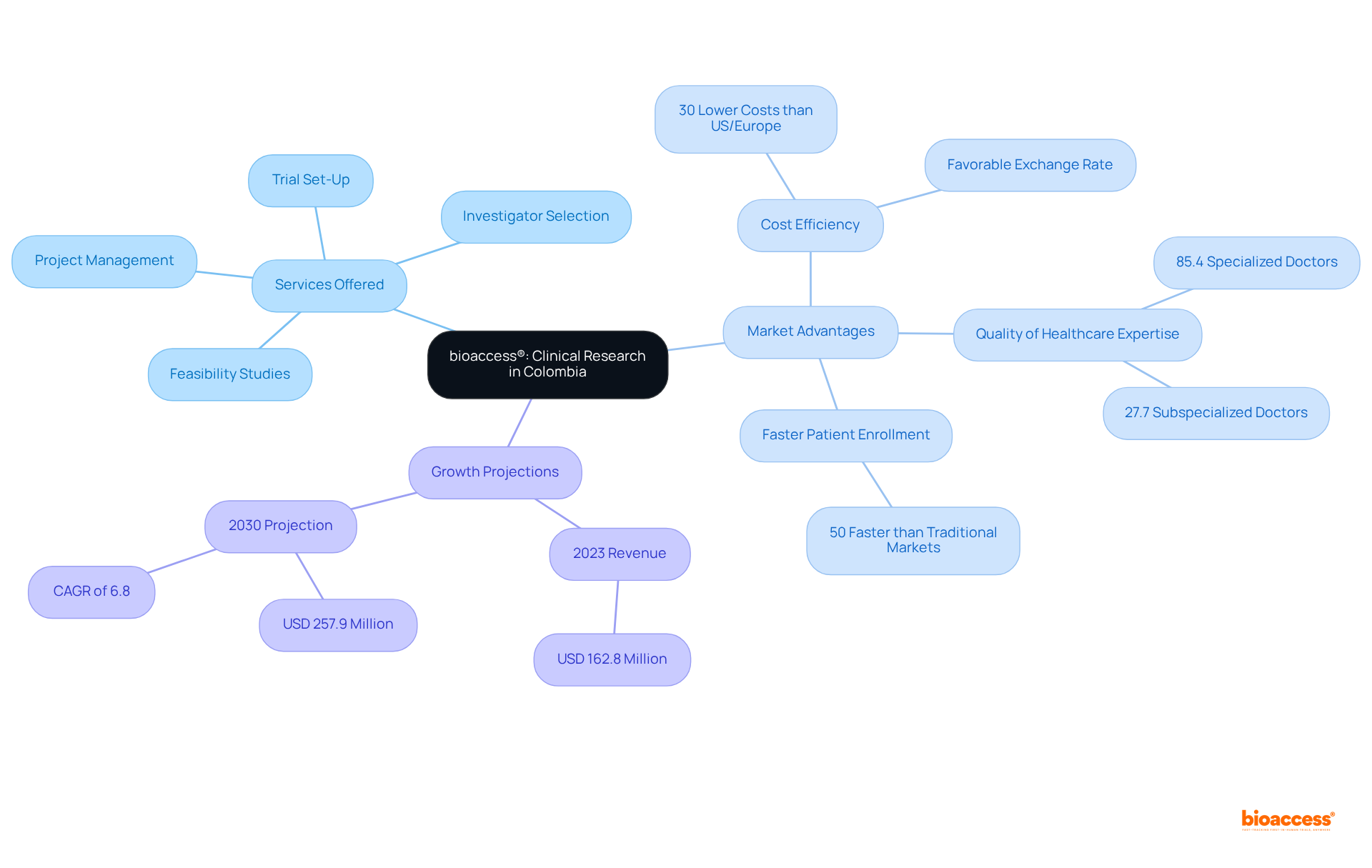
bioaccess® spearheads the advancement of clinical research for healthcare instruments in Colombia, leveraging over 15 years of industry knowledge. By expertly navigating local regulations, bioaccess® empowers Medtech innovators to efficiently manage the complexities of clinical trials. This strategic approach combines the rapid regulatory environment with access to diverse patient populations, resulting in ethical approvals delivered in just 4 to 6 weeks and patient enrollment that is 50% faster than traditional markets. Essential services encompass:
This ensures that novel healthcare devices reach those in need more swiftly.
Clinical trial costs in Colombia are 30% lower than in the United States or Europe, making it a cost-effective option for Medtech companies. Furthermore, with 85.4% of doctors in classified Colombian hospitals being specialized and 27.7% subspecialized, the quality of healthcare expertise available is exceptional. As Colombia's clinical trials market generated a revenue of USD 162.8 million in 2023 and is projected to grow significantly, reaching USD 257.9 million by 2030, bioaccess® stands out as a vital partner for Medtech companies aiming to thrive in this dynamic landscape.
Significantly, the regulatory agency has succeeded in decreasing the evaluation duration of clinical trials by over 50% in the past five years, further improving the efficiency of the regulatory process. Additionally, bioaccess® collaborates with Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America, supported by Colombia's Minister of Health. This collaboration ensures that Medtech companies benefit from accelerated patient recruitment and site activation services.
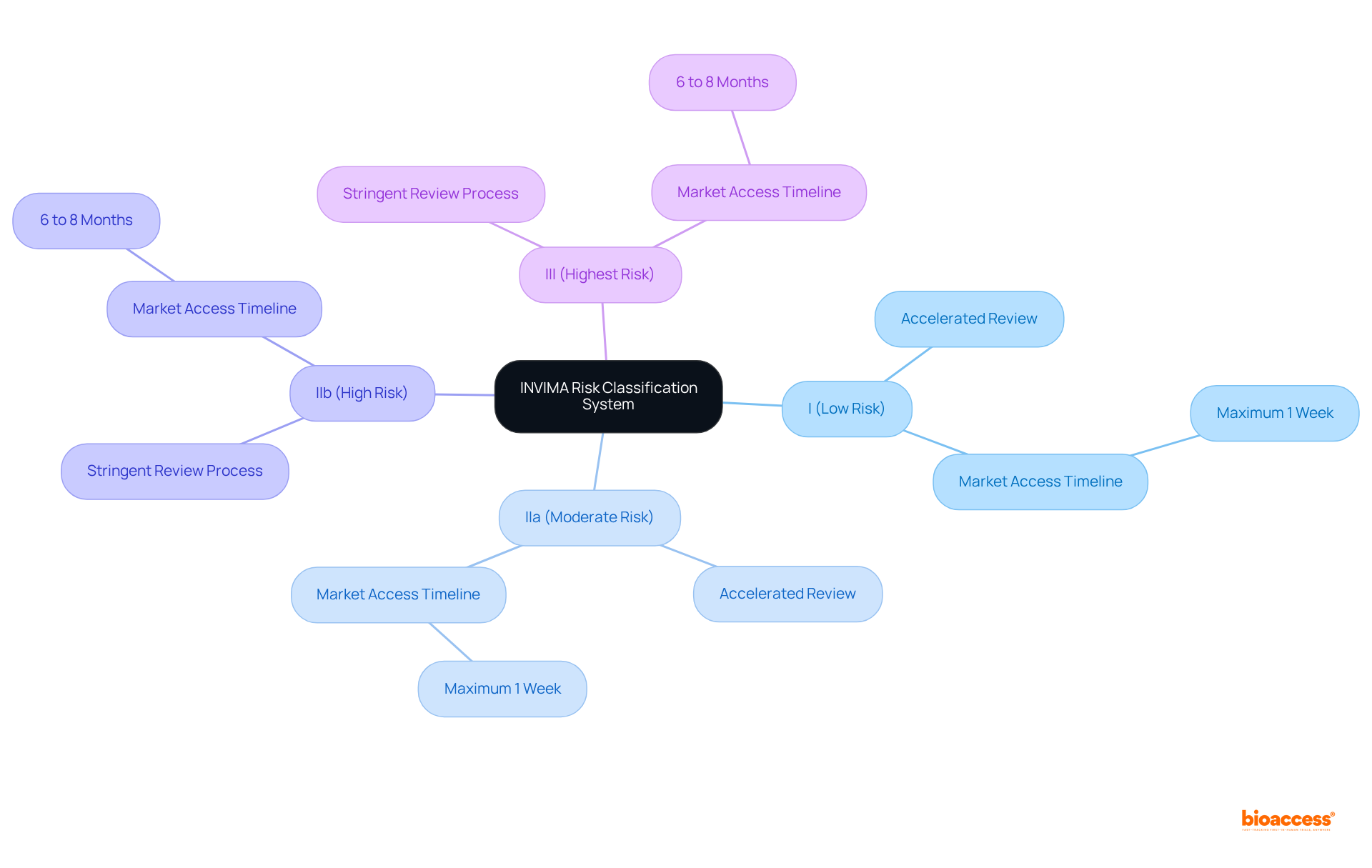
The INVIMA risk classification guide employs a risk-oriented classification system for medical instruments, dividing them into four distinct groups:
This classification framework is essential as it delineates the level of regulatory oversight and the specific criteria each item must meet during the approval process. For example, items classified as Group I or IIa may qualify for accelerated review, allowing manufacturers to introduce them upon receiving the certificate, significantly reducing time to market to a maximum of one week. Conversely, Class IIb and III instruments are subjected to a more stringent review process, which can extend from six to eight months, despite official timelines indicating shorter durations.
Understanding the INVIMA risk classification guide is essential for manufacturers aiming to ensure compliance and optimize their registration efforts. As highlighted by industry expert Julio G. Martinez-Clark, adept navigation through the classification system can facilitate quicker market access and enhance product safety, ultimately benefiting both producers and patients alike.
Additionally, it is imperative to note that healthcare product labels must be in Spanish to comply with local regulations. The Level 4 health authority recognized by PAHO/WHO plays a pivotal role in ensuring the safety, effectiveness, and quality of healthcare instruments in Colombia.
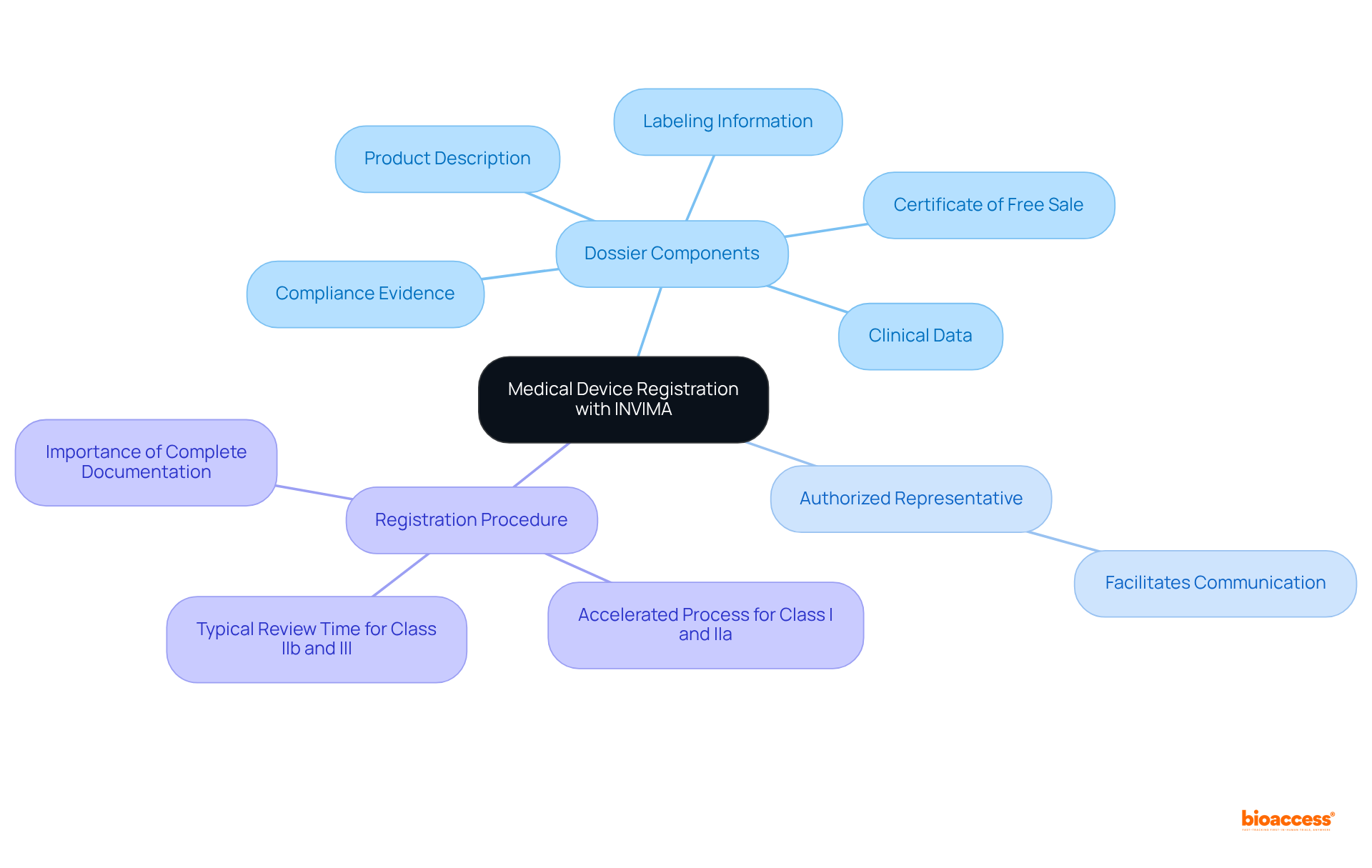
To successfully register a medical product with the regulatory authority, manufacturers must prepare a comprehensive dossier. This dossier should include:
Additionally, designating an authorized representative in Colombia is essential for facilitating communication with the regulatory authority.
The registration procedure can be accelerated for categories I and IIa devices, which may obtain prompt acceptance if complete technical documentation is submitted. Ensuring that all documentation is complete and accurate is crucial, as this significantly impacts the efficiency of the registration process. The regulatory agency usually requires 4-7 months for Class IIb and III submissions, making thorough preparation essential to prevent delays.
As regulatory consultants emphasize, a thoroughly prepared registration dossier is essential for navigating the complexities of the agency's requirements and obtaining timely approvals.
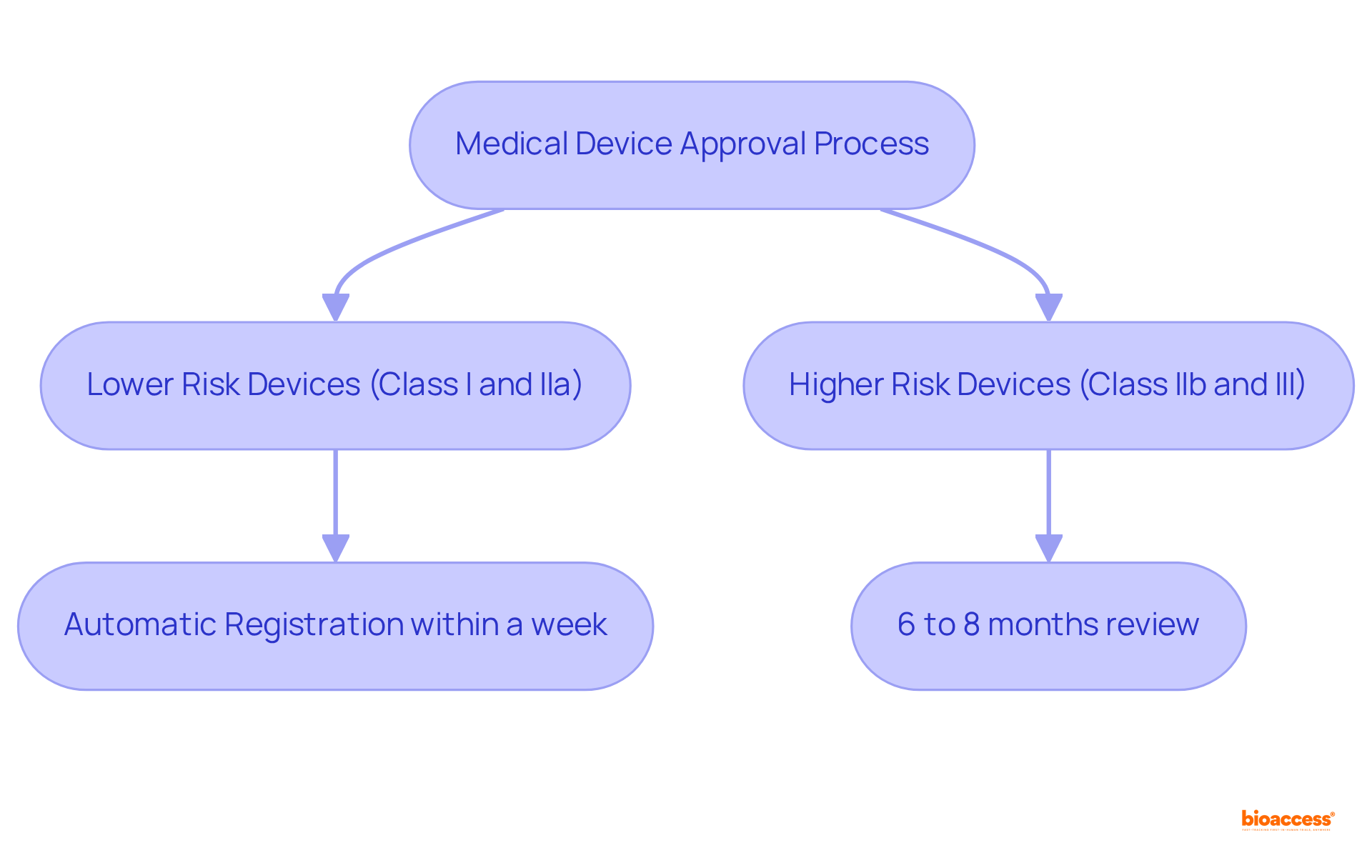
The categorization of risk assigned to a medical instrument by regulatory authorities significantly impacts its approval process. Products deemed lower risk, classified as I and IIa, enjoy a streamlined review process, often achieving market access within a week thanks to automatic registration. Conversely, higher-risk products, such as Class IIb and III, face more rigorous requirements, necessitating extensive clinical data and extended review periods, with approvals typically taking 6 to 8 months. This comprehensive legal and technical evaluation can considerably prolong the approval timeline.
Manufacturers must strategically align their development processes with the INVIMA risk classification guide to optimize their approval timelines. Notably, over 80% of health-related equipment and in vitro diagnostics (IVDs) in Colombia are supplied by international producers, which underscores the importance of the INVIMA risk classification guide for successful market entry.
Industry leaders emphasize that improving approval timelines is essential for maintaining competitiveness in the rapidly evolving healthcare equipment sector. For instance, Welwaze Medical Inc. collaborated with bioaccess™ to adeptly navigate the regulatory landscape for the launch of the Celbrea® product, highlighting the critical role of expert guidance in achieving market entry.
To enhance their success prospects, manufacturers should consider consulting regulatory experts to navigate the complexities of the INVIMA risk classification guide and approval processes.
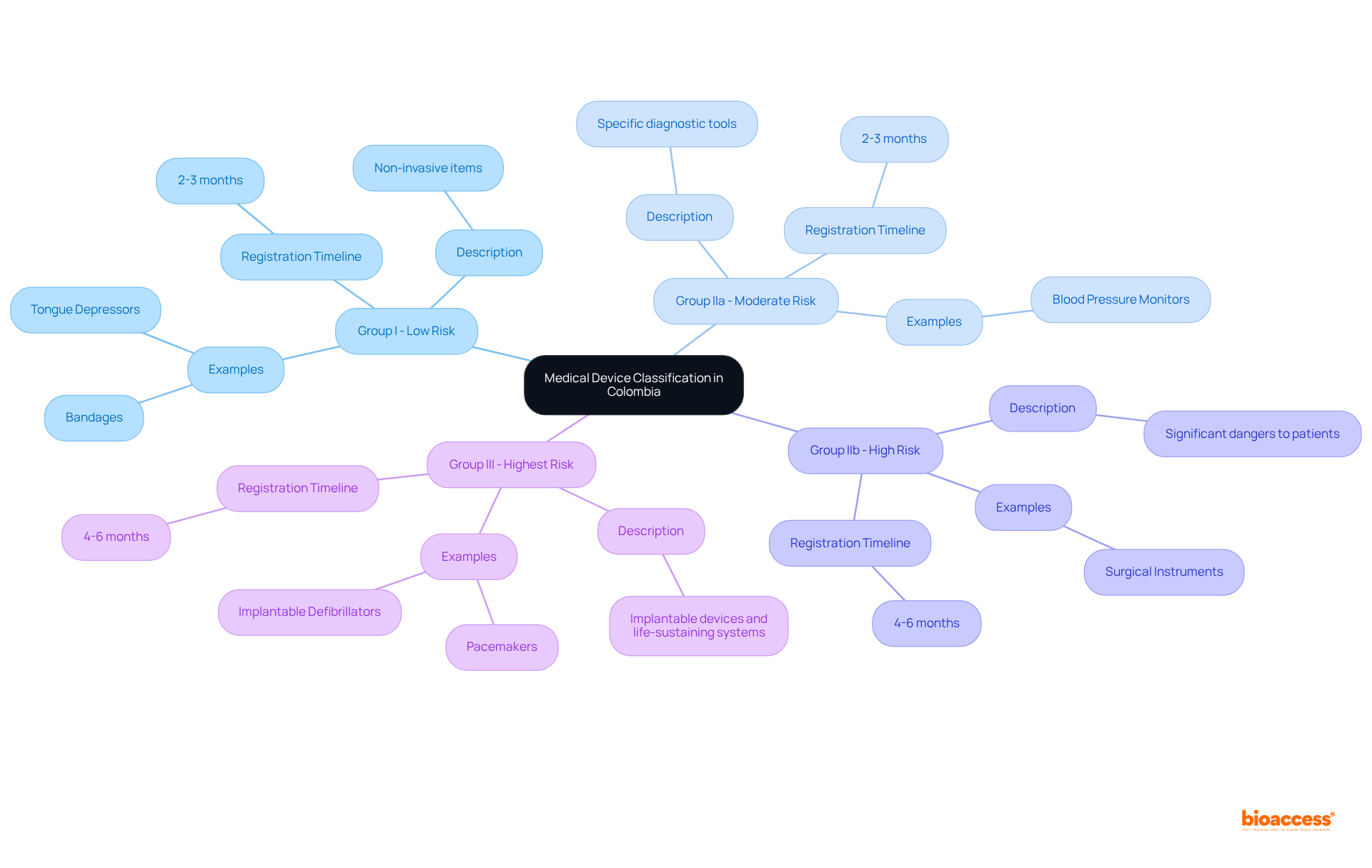
In Colombia, medical instruments are categorized into four distinct groups based on their risk levels: I, IIa, IIb, and III. Group I products, deemed low risk, include non-invasive items such as bandages and tongue depressors. Instruments classified as Group IIa present moderate risk, encompassing specific diagnostic tools like blood pressure monitors. Category IIb items are classified as high risk, including tools that pose significant dangers to patients, such as surgical instruments. Finally, Category III items represent the highest risk group, comprising implantable devices and life-sustaining systems.
Each classification entails specific regulatory requirements that manufacturers must adhere to during the registration process. For example, Class I and IIa items generally require a registration period of approximately two to three months, while Class IIb and III items may take four to six months due to their complexity and the necessity for extensive clinical data. Moreover, all relevant documents submitted for registration must be in Spanish, and producers are obligated to notify the health authority of serious adverse events within 72 hours and non-serious events within eight days to ensure patient safety.
As of 2025, the regulatory agency has approved over 150 healthcare products, highlighting the growing demand for innovative solutions in the market. Understanding the invima risk classification guide is vital for manufacturers seeking to navigate the regulatory landscape effectively. The Colombian medical equipment market is evolving, with projected revenues of US$2.51 billion in 2025, driven by increased healthcare spending and a rising demand for advanced medical technologies. Regulatory specialists emphasize the importance of adhering to the guidelines established by the agency to ensure compliance and facilitate market entry.
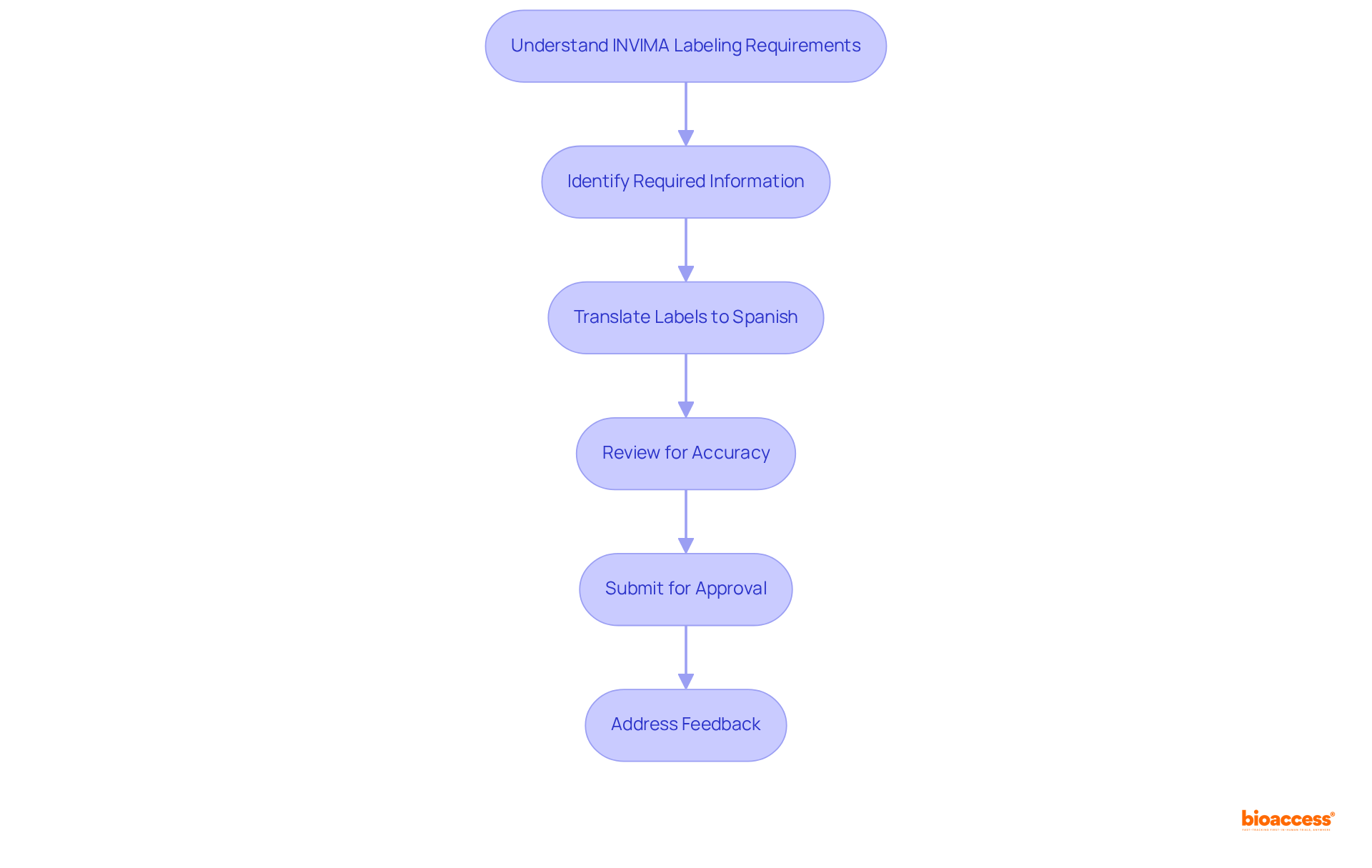
Medical devices intended for the Colombian market must adhere to the stringent labeling standards established by the regulatory authority. This requirement encompasses the display of the product's generic name or brand, the manufacturer's address, and the importer's details. Labels must be presented in Spanish and should explicitly outline instructions for use, safety information, and any relevant warnings. Compliance with the invima risk classification guide is essential for successful registration and market entry.
Manufacturers frequently face common labeling challenges, such as inadequate translations or the omission of essential information, which can result in delays during the approval process. To effectively navigate these obstacles, it is imperative to implement successful labeling strategies. This includes a thorough review of all labeling materials for accuracy and compliance with regulatory standards prior to submission.
Industry leaders underscore the significance of precise labeling in the registration process, highlighting that well-structured labels can considerably expedite approval timelines. Recent updates in labeling regulations further stress the necessity for manufacturers to stay informed about compliance requirements as outlined in the invima risk classification guide, since the regulatory authority continuously refines its guidelines to enhance product safety and efficacy in the market.
By prioritizing adherence to labeling standards, manufacturers of healthcare products can facilitate more seamless registration procedures and build trust with providers and patients within Colombia's evolving healthcare landscape.
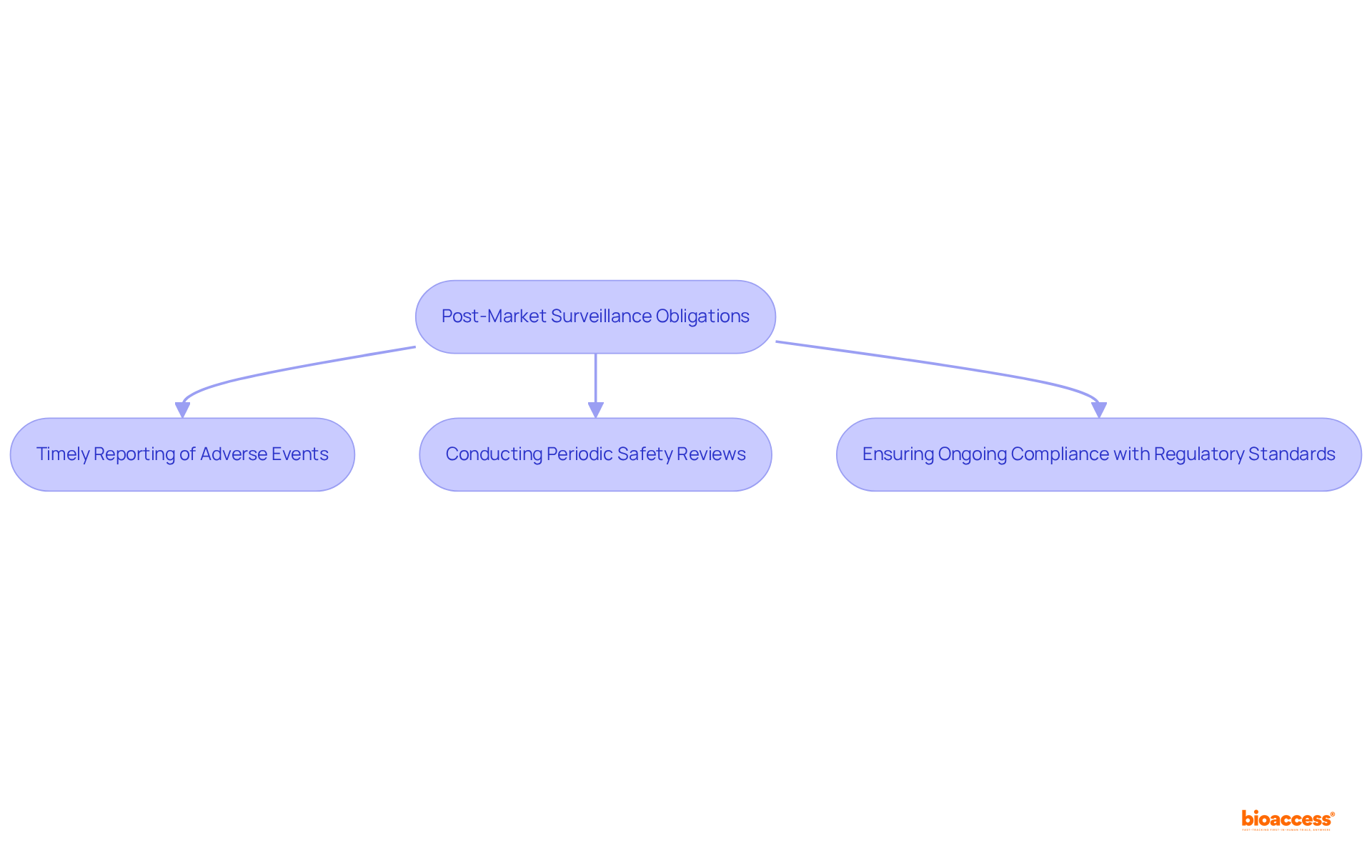
Once a medical device is on the market, manufacturers must implement a robust post-market surveillance plan to monitor its performance and safety. This plan includes:
Regulatory authorities require manufacturers to create such plans as part of their compliance duties, which are crucial for ensuring product integrity and protecting public health. The organization is classified as a Level 4 health authority by PAHO/WHO, underscoring its competence in health regulation.
Effective post-market surveillance strategies involve proactive monitoring of product performance and prompt reporting of any adverse events. In Colombia, the number of adverse events reported has significantly increased, reflecting improved compliance practices and the importance of patient involvement in incident reporting. For instance, the Colombian National Food and Drug Surveillance Institute (INVIMA) has enhanced its National Pharmacovigilance Program, leading to a dramatic rise in reported adverse events from 5,447 in 2013 to 95,658 in 2017. This increase underscores the critical role of structured training programs, which have been shown to elevate compliance rates to over 85% among companies that prioritize education.
Recent advancements in post-market monitoring emphasize the incorporation of sophisticated data analytics, which can boost early signal detection and enhance the overall safety of products. Companies that leverage these technologies not only meet regulatory requirements but also achieve significant returns on investment, with some reporting a 430% ROI from advanced patient protection analytics. Regulatory experts emphasize that maintaining product integrity post-market is not merely a compliance task but an ongoing commitment to patient safety and product quality. By implementing thorough post-market monitoring strategies, manufacturers can manage the intricacies of regulatory adherence while ensuring the safety and effectiveness of their health products.
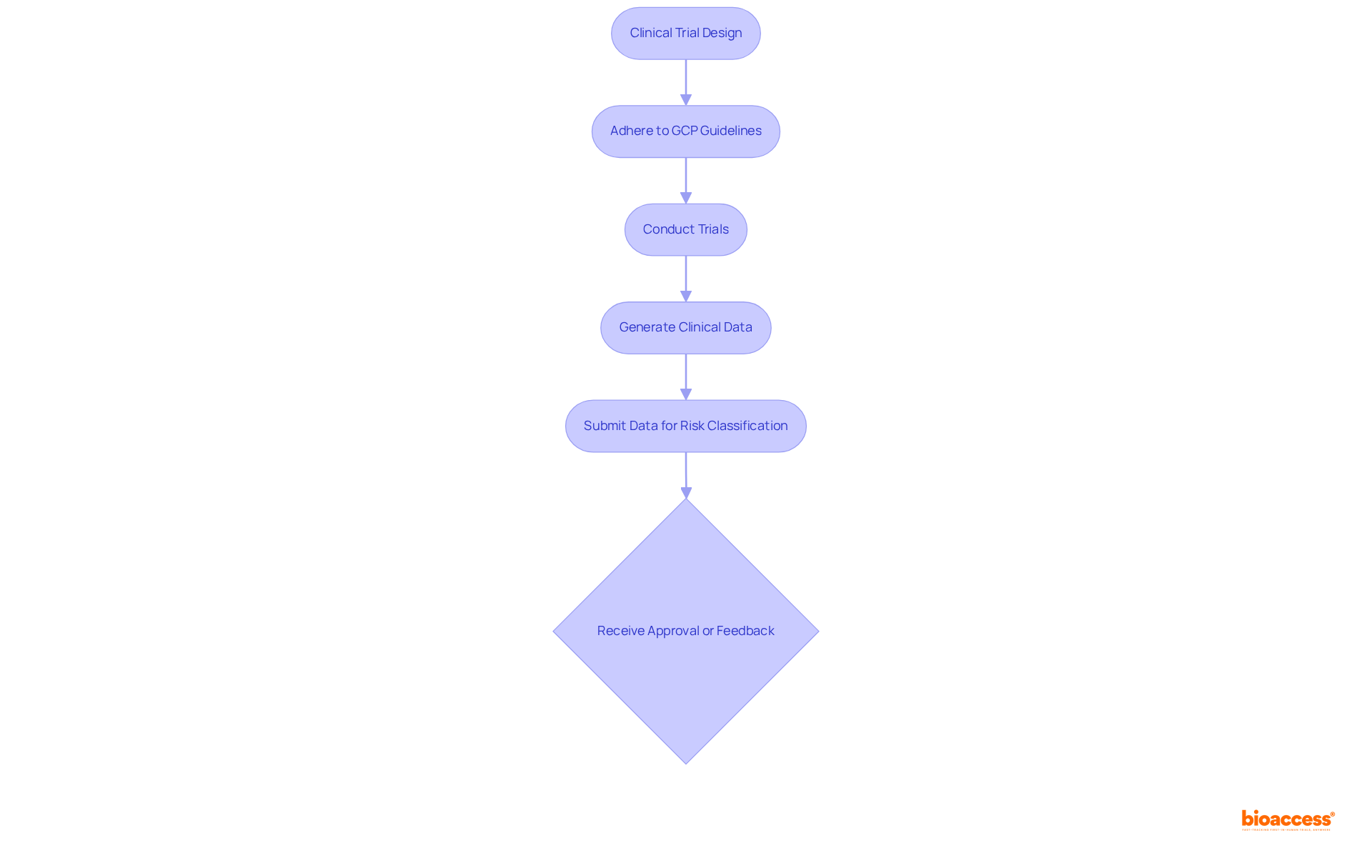
Clinical trials are pivotal in assessing the safety and effectiveness of medical instruments, especially those classified as Class IIb and III. Regulatory bodies mandate comprehensive clinical data as outlined in the invima risk classification guide to substantiate risk classification and the approval process. Manufacturers must design and conduct trials that adhere to Good Clinical Practice (GCP) guidelines, ensuring the reliability of the data generated and its capacity to withstand regulatory scrutiny. The outcomes of these trials are critical, as they directly influence the invima risk classification guide and the subsequent approval of the device.
In Colombia, the approval process has been streamlined, with recent initiatives reducing the typical evaluation timeframe from 135 days to just 60 days, significantly enhancing the efficiency of clinical trials. This accelerated process not only facilitates quicker market entry but also underscores the importance of meticulous trial design and execution in meeting regulatory standards. Notably, Colombia ranks sixth in research studies within Latin America, accounting for 5.9% of examinations in the region, illustrating its growing significance in the clinical research landscape. Conducting clinical trials in Colombia can yield cost savings exceeding 30% compared to experiments in the U.S. and Europe, making it an appealing option for manufacturers.
Moreover, manufacturers must establish a quality management system (QMS) that complies with international standards such as ISO 13485 to ensure regulatory compliance. As highlighted by a clinical research professional, adherence to GCP guidelines is essential for generating reliable data that meets regulatory expectations. To enhance clinical trial design, manufacturers should prioritize careful preparation of their registration applications and maintain open lines of communication with the regulatory authority. Leveraging the expertise of bioaccess®, with over 20 years of experience in Medtech, in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies can further bolster the success of clinical trials in this region.
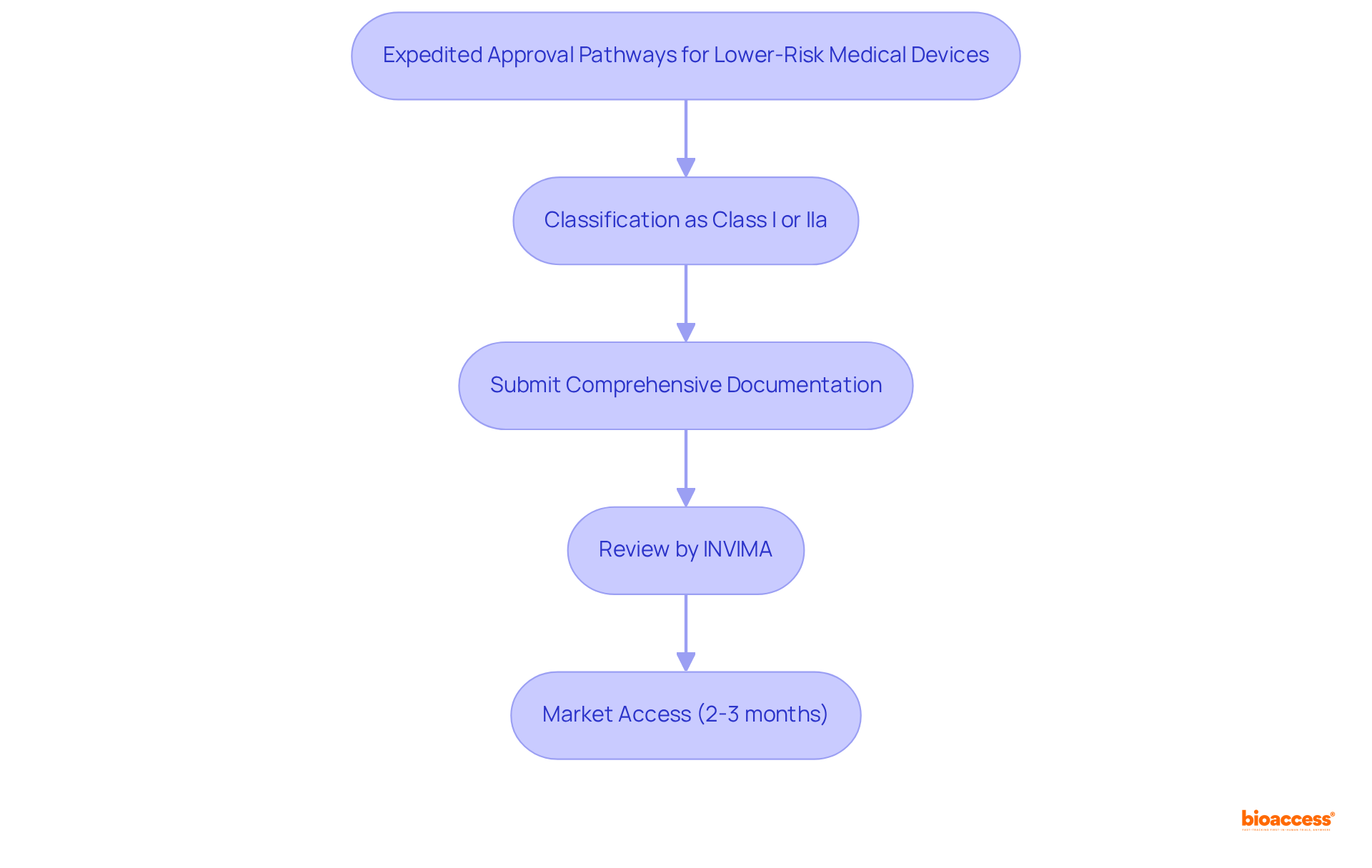
According to the invima risk classification guide, INVIMA has established expedited approval routes for lower-risk products, specifically those classified as Class I and IIa. This streamlined submission process allows manufacturers to achieve market access significantly faster, with registration durations averaging merely 2 to 3 months for these product categories. To fully capitalize on these expedited pathways, manufacturers must ensure their documentation is comprehensive and compliant with the invima risk classification guide as well as all regulatory requirements.
The success of expedited submissions underscores the effectiveness of this approach, facilitating quicker patient access to innovative health technologies. Industry leaders have observed that these pathways not only enable swifter market entry but also enhance the overall efficiency of the regulatory process in Colombia. As the healthcare equipment market continues to expand, the ability to expedite lower-risk products will be crucial in meeting the growing demand for innovative health solutions.
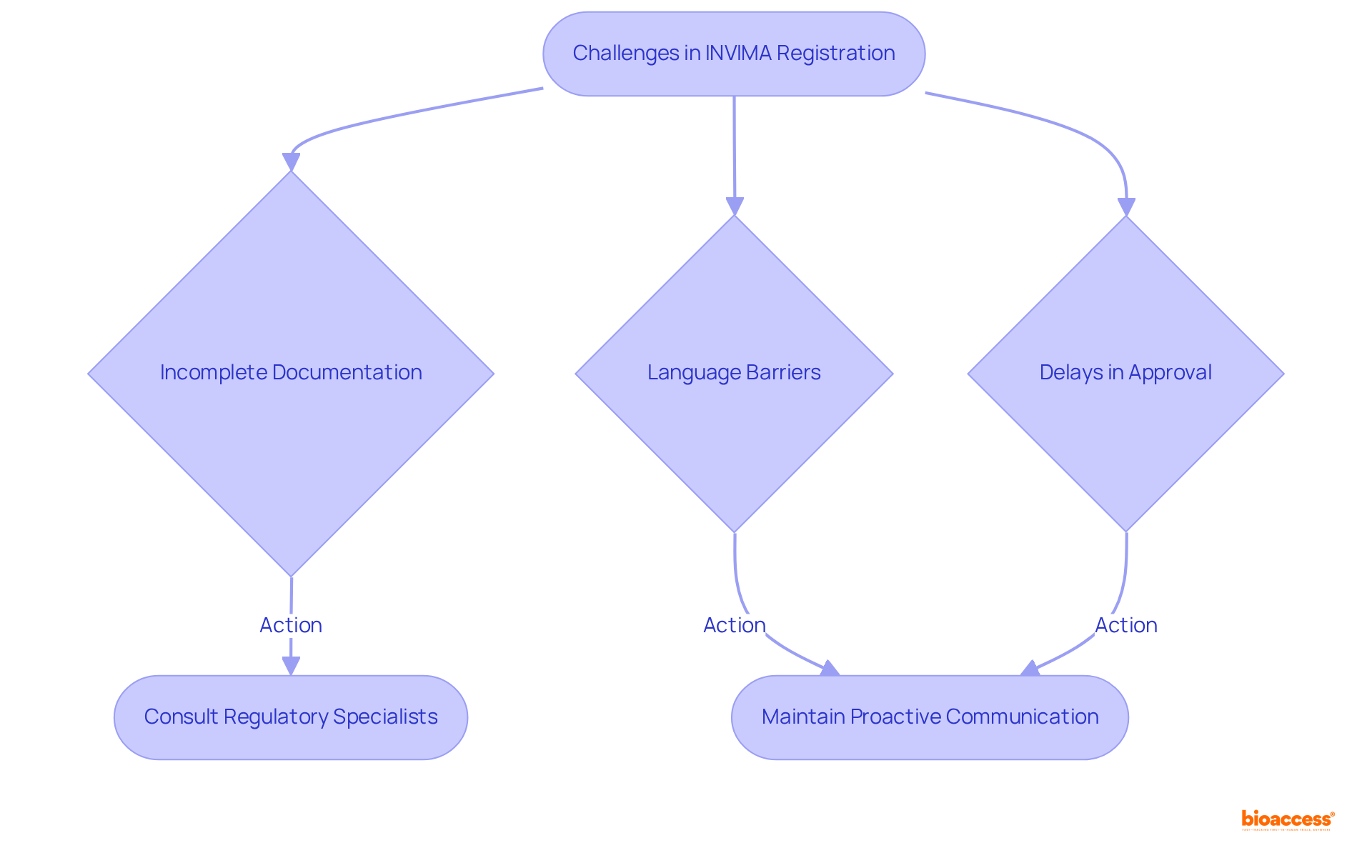
Producers frequently face significant challenges during the registration process for healthcare instruments. The Colombia National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in inspecting and supervising the marketing and manufacturing of health products, including medical devices. Notably, incomplete documentation affects up to 30% of applications, while language barriers further complicate communication. Additionally, delays often arise due to requests for supplementary information or clarification, extending the approval timeline.
As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA's stringent standards outlined in the invima risk classification guide require manufacturers to strictly adhere to regulatory requirements. To effectively navigate these challenges, it is essential for manufacturers to collaborate with experienced regulatory consultants who can offer strategic guidance in accordance with the invima risk classification guide. Engaging with these specialists ensures that all documentation is meticulously prepared and submitted according to relevant standards, ultimately facilitating a more efficient registration process.
Furthermore, proactive communication with INVIMA has been demonstrated to significantly reduce approval times, highlighting the importance of maintaining regular contact with regulatory authorities.
Understanding the INVIMA risk classification guide is crucial for manufacturers aiming to navigate the complexities of medical device registration in Colombia. This guide categorizes devices based on their risk levels and outlines the regulatory requirements that dictate the approval process. By aligning their strategies with this framework, manufacturers can ensure compliance and optimize their market entry timelines.
Key insights from the article highlight:
The emphasis on compliance with labeling requirements underscores the need for manufacturers to stay informed and proactive in their approaches. As the Colombian healthcare market continues to grow, understanding these elements will be vital for success.
Manufacturers are encouraged to leverage the insights provided by the INVIMA risk classification guide and seek expert guidance to overcome common challenges in the registration process. By doing so, they can enhance their competitiveness and contribute to the advancement of healthcare solutions in Colombia. The evolving landscape of medical device regulation presents both challenges and opportunities; staying informed and adaptable will be key to thriving in this dynamic environment.
What is bioaccess® and what role does it play in clinical research in Colombia?
bioaccess® is an organization that accelerates clinical research for medical devices in Colombia, utilizing over 15 years of industry experience to navigate local regulations. It helps Medtech innovators manage clinical trials more efficiently, resulting in ethical approvals in 4 to 6 weeks and faster patient enrollment compared to traditional markets.
What services does bioaccess® provide to support clinical trials?
bioaccess® offers essential services including feasibility studies, investigator selection, trial set-up, and thorough project management to ensure that new healthcare devices reach patients more quickly.
How do clinical trial costs in Colombia compare to those in the United States or Europe?
Clinical trial costs in Colombia are approximately 30% lower than in the United States or Europe, making it a cost-effective option for Medtech companies.
What is the significance of the INVIMA risk classification system for medical devices?
The INVIMA risk classification system categorizes medical instruments into four groups based on risk: I (low risk), IIa (moderate risk), IIb (high risk), and III (highest risk). This classification determines the level of regulatory oversight and the criteria that each device must meet during the approval process.
What are the timelines for the approval process based on the INVIMA classification?
Items classified as Group I or IIa may qualify for accelerated review, allowing for market introduction within a week. In contrast, Class IIb and III devices undergo a more rigorous review process that can take six to eight months.
What are the key requirements for registering a medical device with INVIMA?
Manufacturers must prepare a comprehensive dossier that includes a detailed product description, evidence of compliance with standards, clinical data (if required), labeling information, and a Certificate of Free Sale from the country of origin. Additionally, appointing an authorized representative in Colombia is necessary for communication with the regulatory authority.
How long does the registration process typically take for different device classes?
The registration process usually takes 4-7 months for Class IIb and III devices, while categories I and IIa can receive prompt acceptance if complete technical documentation is submitted.
Why is it important to have a thoroughly prepared registration dossier?
A well-prepared registration dossier is crucial for navigating the complexities of regulatory requirements and obtaining timely approvals, as incomplete or inaccurate documentation can lead to delays in the registration process.