


This article delivers essential insights on ISO 10993-17 that clinical research directors must grasp to ensure effective compliance and safeguard patient safety. It underscores the critical nature of biocompatibility testing, the latest regulatory standards, and the necessity of ongoing risk management strategies. These elements are vital for guaranteeing that medical devices are not only safe for use but also meet the stringent regulatory requirements. Understanding these components is imperative for navigating the complexities of the Medtech landscape and addressing the challenges that arise in clinical research.
Navigating the complexities of ISO 10993-17 compliance is a critical task for clinical research directors, particularly as the landscape of medical device testing evolves rapidly. This standard governs not only the biocompatibility of devices but also directly impacts patient safety and regulatory approval processes. As research leaders strive to enhance operational efficiency and ensure adherence to stringent safety protocols, a pivotal question emerges: how can they effectively implement these standards to foster innovation while safeguarding patient health? This article delves into ten essential insights that will empower clinical research directors to master ISO 10993-17, ensuring their studies meet the highest standards of compliance and ethical responsibility.
bioaccess® is essential in assisting study leaders through the complexities of ISO 10993 17 compliance. Leveraging profound knowledge of regulatory frameworks and operational flexibility, bioaccess® guarantees compliance with essential biocompatibility standards in research studies. This strategic support accelerates the approval process and enhances patient enrollment rates, ultimately facilitating quicker market access for innovative medical devices.
With over 50 activated sites in less than 8 weeks and FDA/EMA/MDR-ready datasets, along with centralized monitoring, bioaccess® exemplifies efficiency in patient recruitment and site activation services. As updates to biocompatibility standards are anticipated in 2025, bioaccess® remains at the forefront, equipping clients with the knowledge and tools necessary to navigate these changes effectively. Clinical study leaders have observed that utilizing bioaccess®'s expertise greatly reduces risks linked to compliance, thus enhancing overall trial success rates and hastening the process from idea to market.
Significantly, partnerships with associates such as Avantec Vascular for first-in-human studies in Latin America further highlight bioaccess®'s dedication to promoting medical investigations in the region. This collaboration underscores the importance of strategic alliances in advancing clinical research and ensuring compliance in a rapidly evolving landscape.
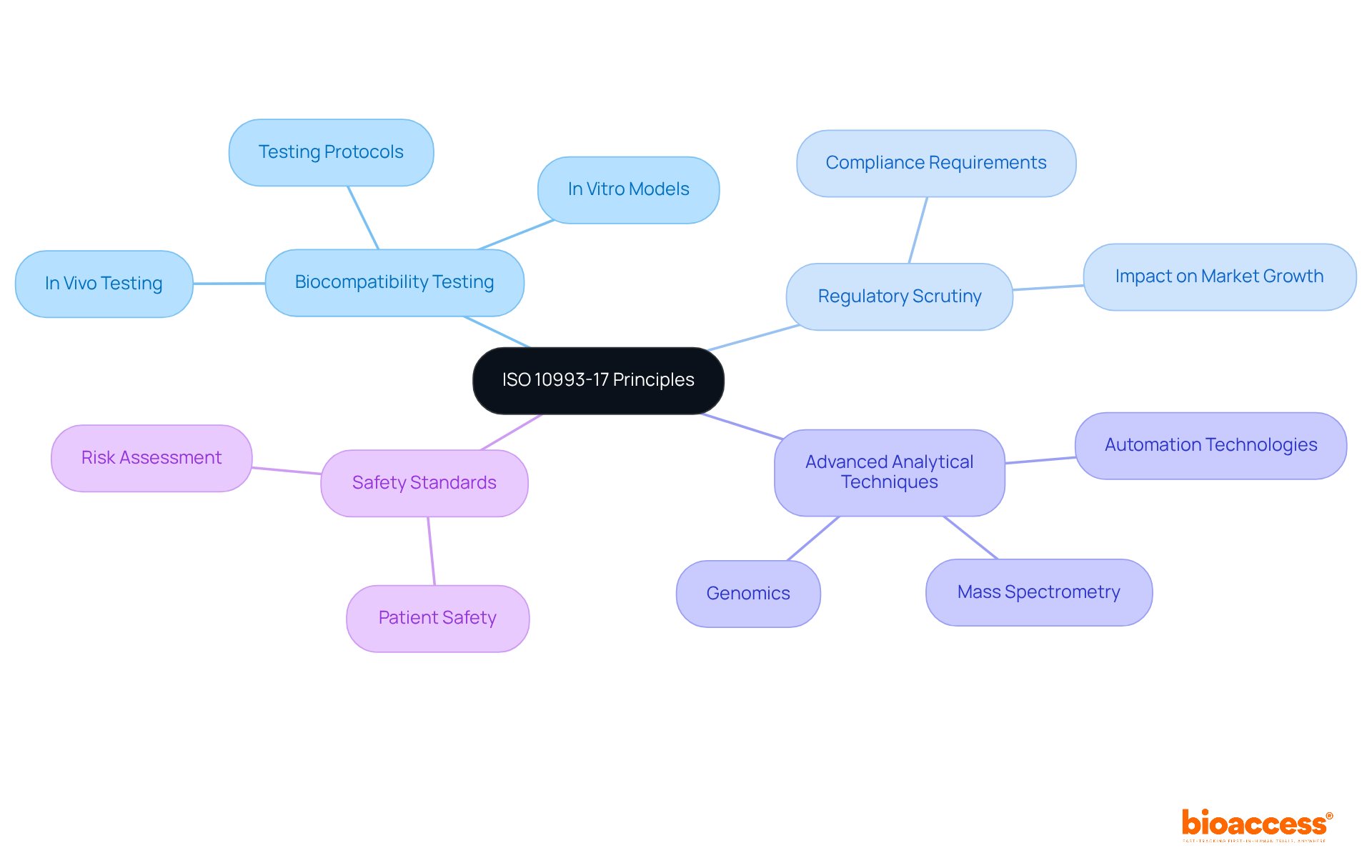
The principles established by ISO 10993 17 are essential for assessing the biological safety of medical devices, highlighting the critical role of biocompatibility testing in evaluating the interaction between devices and the human body. As we approach 2025, the significance of this testing becomes increasingly pronounced due to heightened regulatory scrutiny and the growing demand for safer medical devices.
Clinical study leaders must comprehend these principles to ensure adherence to safety standards, safeguarding patient well-being while enhancing the credibility of study results. Current trends reveal a shift towards advanced analytical techniques and in vitro testing models, which provide more reliable alternatives to traditional methods, thereby improving accuracy and efficiency in biocompatibility assessments.
Furthermore, the principles of ISO 10993 17 are applied in medical studies, exemplified by organizations adopting stringent testing protocols to meet regulatory standards, ensuring that new medical devices are both effective and safe for patient use. Understanding these dynamics is crucial for clinical leaders aiming to navigate the complexities of medical device testing and uphold the highest standards of patient safety.
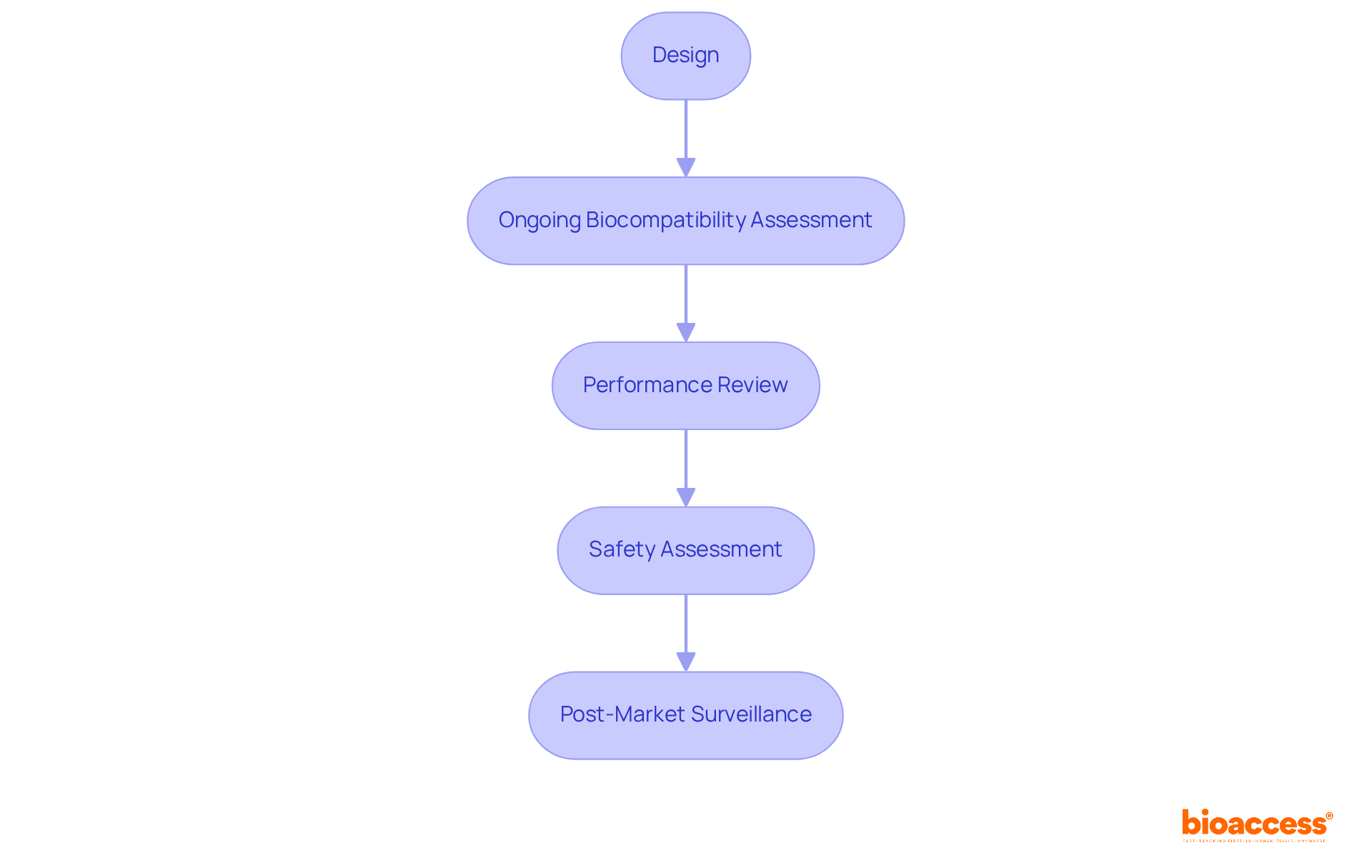
Life cycle management according to ISO 10993 17 mandates ongoing biocompatibility assessment of medical devices from design through post-market surveillance. Clinical study leaders must implement strategies that consistently assess and review device performance and safety throughout its life cycle. This proactive approach not only ensures regulatory compliance but also significantly enhances patient safety and device efficacy. Continuous assessment is crucial, as it allows for timely identification of potential risks associated with material changes or manufacturing processes, ultimately contributing to improved safety statistics in medical device usage.
For instance, implementing a robust Biological Evaluation Plan (BEP) that aligns with ISO 10993 17 can guide the necessary evaluations and testing strategies, ensuring that biocompatibility remains a priority throughout the device's life cycle. By integrating these practices, organizations can better navigate the complexities of biocompatibility, fostering innovation while safeguarding patient health.
The 2023 updates to ISO 10993 17 introduce critical guidelines and clarifications that reshape the protocols for biocompatibility testing and risk assessment. Clinical research directors must thoroughly understand these changes to maintain compliance in their studies. Key aspects include:
These changes can substantially influence the approval process and overall study timelines. Notably, the FDA recognized the introduction of toxicological screening limits and assumed release kinetics, necessitating a reevaluation of existing testing strategies. This acknowledgment is vital for comprehending the regulatory environment and its effect on research studies.
In this context, Bioaccess provides comprehensive trial management services that include:
These services are designed to simplify the medical study process, ensuring that all biological endpoints from ISO 10993-1 are sufficiently covered. Additionally, the classification of contact duration into:
is essential for adapting risk assessments. A clear rationale for tests performed or omitted must be provided, as this is a critical aspect of the new guidelines. By proactively incorporating these updates, medical investigation teams can improve their operational efficiency and ensure that their studies align with the latest regulatory standards.
Furthermore, the impact of medtech research studies on local economies—such as job creation, economic growth, and healthcare enhancement—highlights the importance of international collaboration in this field. However, the medical device industry may face challenges in adopting these new concepts, which could impact the implementation of updated protocols. As Vaibhav Patidar, an executive toxicologist, notes, "With the recent updates to ISO 10993 17, there are significant changes that have been made to enhance clarity and relevance in the evaluation of medical devices.

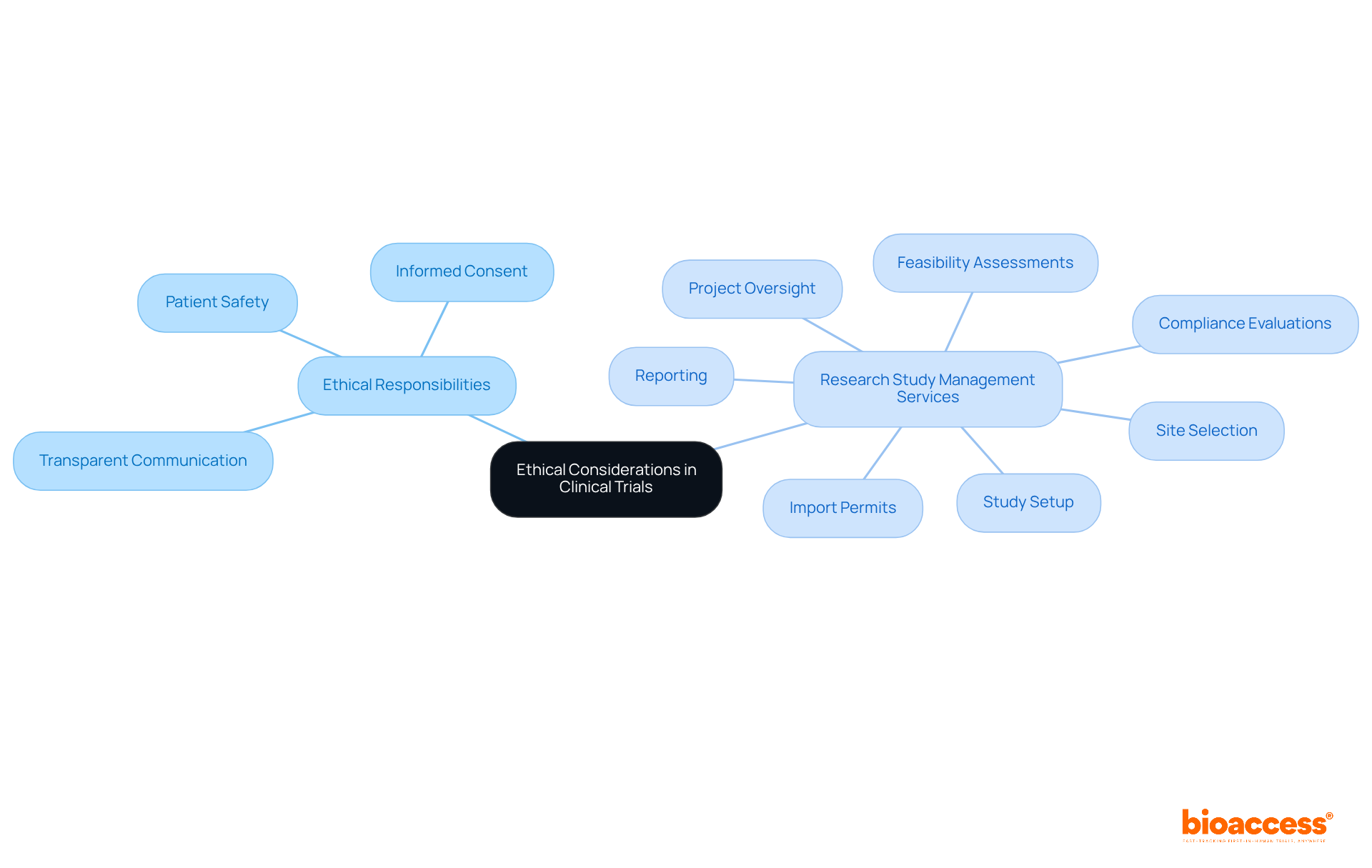
The ethical responsibilities of trial directors are underscored by ISO 10993 17, which mandates that they prioritize patient safety and informed consent. It is imperative that directors ensure all biocompatibility assessments are conducted ethically, with transparent communication to participants regarding potential risks. Upholding these ethical standards not only protects patients but also enhances the integrity of the investigative process.
Furthermore, a comprehensive suite of research study management services—including:
is crucial in fostering these ethical practices. By effectively managing these components, clinical study leaders can ensure that assessments comply with regulatory requirements, ultimately benefiting both participants and the broader healthcare community.
Implementing robust risk management strategies is essential for compliance with ISO 10993 17 standards. Clinical research directors must conduct comprehensive risk assessments to identify potential biocompatibility issues early in the study process. This proactive strategy not only alleviates difficulties that may emerge during tests but also guarantees compliance with regulatory standards.
Current best practices highlight the importance of incorporating risk evaluations throughout the research process, enabling timely modifications based on new information. For instance, utilizing methodologies such as Failure Mode and Effects Analysis (FMEA) can help pinpoint critical failure modes and their impacts on device safety. Moreover, participating in ongoing risk evaluation allows teams to adjust to new information, improving study integrity and participant safety. Expert insights suggest that fostering a culture of risk awareness among stakeholders is vital for effective communication and decision-making.
By emphasizing risk management, research leaders can maneuver through the intricacies of biocompatibility evaluations and enhance their processes, ultimately resulting in successful study outcomes. Utilizing extensive research study management services—such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—can greatly improve the effectiveness of these risk management strategies.
As a practical tip, leaders should regularly review and update their risk management plans to reflect new insights and data throughout the trial process.

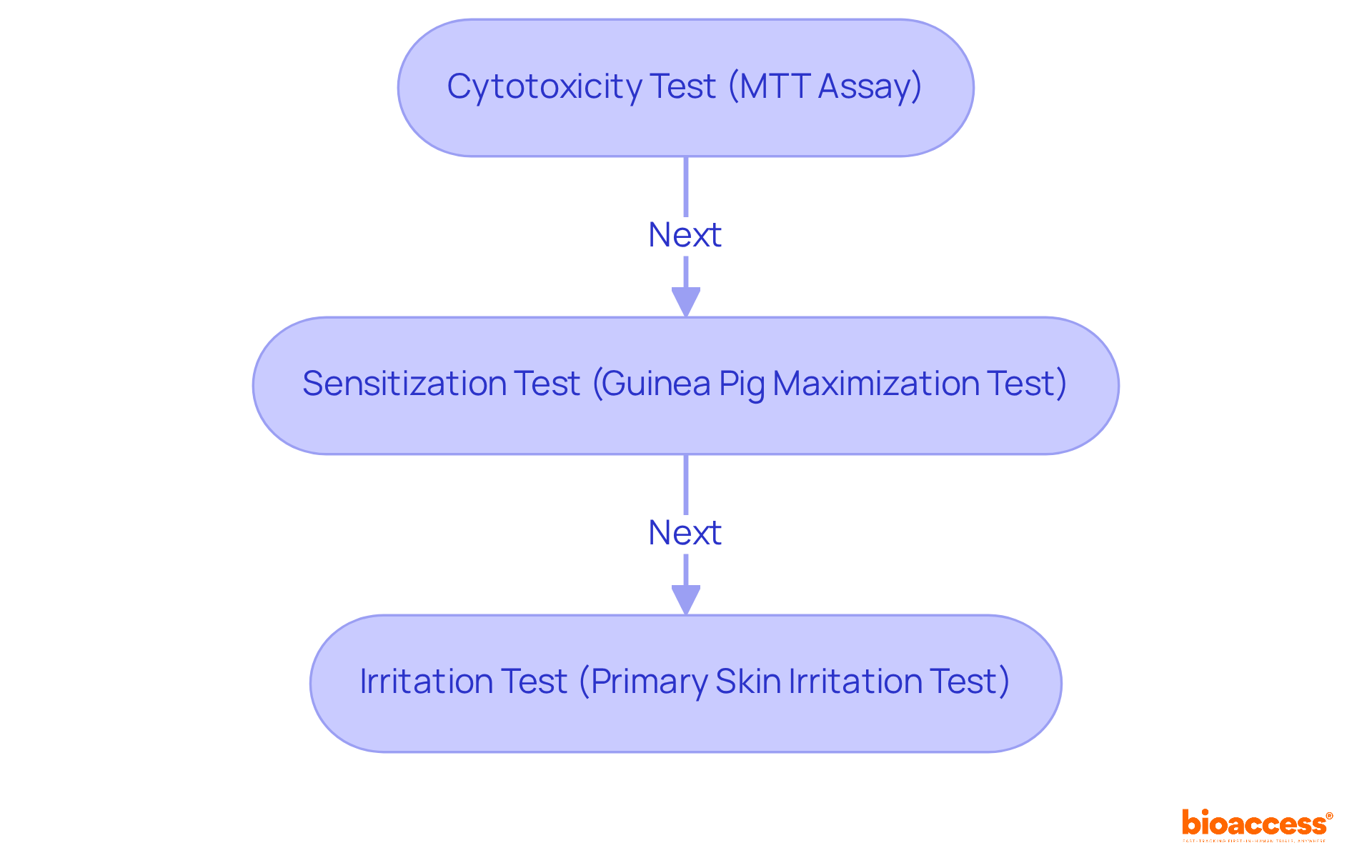
The critical biocompatibility testing requirements outlined in ISO 10993 17, such as cytotoxicity, sensitization, and irritation tests, must be prioritized by study leaders. These assessments are paramount for evaluating the safety of medical devices and ensuring compliance with regulatory standards, particularly under the oversight of INVIMA, Colombia's National Food and Drug Surveillance Institute. INVIMA plays a vital role in inspecting and supervising the marketing and manufacturing of health products, guaranteeing that medical devices meet established safety and efficacy standards.
For instance, cytotoxicity tests, such as the MTT assay, quantitatively measure the viability of cells exposed to materials, providing insights into potential toxic effects. Sensitization tests, like the Guinea Pig Maximization Test and the Murine Local Lymph Node Assay, are essential for determining allergic reactions that may arise from prolonged exposure to device materials. Furthermore, irritation tests, including the Primary Skin Irritation Test and Intracutaneous Test, assess the local irritation potential of materials, ensuring that devices do not provoke adverse reactions upon contact with body tissues.
Failure to conduct adequate biocompatibility testing can lead to significant risks; studies indicate that up to 30% of medical devices fail due to insufficient evaluation of their biocompatibility. By adhering to ISO 10993 17 standards and understanding the regulatory environment shaped by INVIMA, medical professionals can enhance patient safety and facilitate more efficient regulatory approvals, ultimately advancing the development of safe and effective medical technologies.
The documentation and reporting standards under ISO 10993 17 are rigorous, demanding a high level of precision and thoroughness. Clinical investigation leaders must maintain comprehensive documentation of all biocompatibility evaluations, risk analyses, and ethical factors. This meticulous documentation serves not merely as a compliance requirement; it is a vital asset during regulatory reviews, ensuring that every aspect of the study remains transparent and verifiable.
Effective reporting practices significantly influence regulatory approval rates, as comprehensive documentation demonstrates adherence to safety and efficacy standards. Research indicates that thoroughly documented medical trials encounter greater approval rates due to the clarity and dependability of the data provided. Expert insights emphasize that aligning reporting practices with ISO 10993 17 not only enhances compliance but also fosters trust with regulatory bodies.
As the landscape of biocompatibility assessment evolves in 2025, it is essential for project leaders to stay abreast of the latest reporting obligations. This knowledge will be vital for navigating the complexities of regulatory submissions effectively.
Implementing ISO 10993 17 presents significant challenges, including resource constraints, regulatory complexities, and the necessity for specialized knowledge. Clinical research leaders must proactively identify these challenges and develop effective strategies to address them. This may involve:
Moreover, utilizing comprehensive research management services—including:
can substantially enhance the efficiency of clinical studies. By effectively overcoming these obstacles, leaders can ensure adherence to ISO 10993 17 standards while simultaneously enhancing the overall efficiency of their trials.
Adhering to ISO 10993 17 offers considerable strategic benefits for research leaders, especially in enhancing patient safety and strengthening trust with regulatory bodies. This standard emphasizes a systematic approach to toxicological risk assessment, enabling directors to implement rigorous evaluations that directly contribute to improved patient outcomes. For instance, the introduction of the Toxicological Screening Limit (TSL) facilitates effective screening of constituents, ensuring that only those with a low toxicological risk are deemed safe for medical use.
Bioaccess's comprehensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—directly support adherence to ISO 10993 17 by ensuring that each phase of the trial process complies with regulatory standards. This proactive compliance simplifies the risk evaluation process and accelerates market entry for medical devices, positioning organizations as leaders in medical studies. Furthermore, adherence to ISO 10993 17 fosters trust among stakeholders and reinforces the regulatory credibility that is essential for successful product commercialization.
Ultimately, by prioritizing compliance, clinical research directors can significantly advance medical knowledge and enhance patient safety, reflecting a commitment to ethical and effective healthcare solutions.
Adhering to ISO 10993-17 is crucial for clinical research directors, as it lays the foundation for ensuring the biocompatibility and safety of medical devices. This standard not only facilitates compliance with regulatory requirements but also enhances patient safety and strengthens trust with stakeholders. By understanding and implementing the principles of ISO 10993-17, clinical leaders can navigate the complexities of medical device testing and significantly improve trial outcomes.
Key insights throughout the article highlight the importance of:
The emphasis on ethical considerations and proactive compliance strategies illustrates the multifaceted approach required to meet the evolving demands of regulatory frameworks. Bioaccess® emerges as a pivotal partner, providing essential services that streamline the compliance process, thus accelerating the path from concept to market.
In conclusion, embracing the strategic advantages of ISO 10993-17 compliance fosters innovation and enhances patient safety, positioning clinical research organizations as leaders in the medical field. As the landscape continues to evolve, staying informed and adaptable will be vital for success. Engaging with resources and partnerships that support ISO 10993-17 compliance is imperative for advancing medical research and ensuring the delivery of safe and effective healthcare solutions.
What is bioaccess® and how does it assist with ISO 10993-17 compliance?
bioaccess® helps study leaders navigate the complexities of ISO 10993-17 compliance by leveraging regulatory knowledge and operational flexibility to ensure adherence to biocompatibility standards, accelerating the approval process and enhancing patient enrollment rates for medical devices.
How quickly can bioaccess® activate study sites?
bioaccess® has successfully activated over 50 sites in less than 8 weeks.
What advantages does bioaccess® offer for clinical research?
bioaccess® provides FDA/EMA/MDR-ready datasets, centralized monitoring, and expertise that significantly reduces compliance risks, enhances trial success rates, and speeds up the process from idea to market.
Why is understanding ISO 10993-17 important for clinical study leaders?
Understanding ISO 10993-17 is crucial for clinical study leaders to ensure adherence to safety standards, which safeguards patient well-being and enhances the credibility of study results.
What are the current trends in biocompatibility testing according to the article?
Current trends indicate a shift towards advanced analytical techniques and in vitro testing models, which offer more reliable alternatives to traditional methods, improving the accuracy and efficiency of biocompatibility assessments.
What does life cycle management of medical devices under ISO 10993-17 entail?
Life cycle management requires ongoing biocompatibility assessment from the design phase through post-market surveillance, ensuring regulatory compliance and enhancing patient safety and device efficacy.
How can organizations ensure biocompatibility throughout a device's life cycle?
Organizations can implement a robust Biological Evaluation Plan (BEP) that aligns with ISO 10993-17 to guide necessary evaluations and testing strategies, prioritizing biocompatibility throughout the device's life cycle.
What is the significance of strategic partnerships in clinical research as mentioned in the article?
Strategic partnerships, such as with Avantec Vascular for first-in-human studies in Latin America, are essential for advancing medical investigations and ensuring compliance in a rapidly evolving regulatory landscape.