


This article provides essential insights for researchers on Class 3 medical devices, underscoring their regulatory requirements, approval processes, and market access strategies. It is imperative to understand the stringent regulatory landscape and leverage strategic partnerships, such as with bioaccess®, to accelerate clinical research and ensure successful market entry for these high-risk medical products. This collaboration is crucial for enhancing patient care and outcomes, ultimately reinforcing the necessity of strategic alliances in navigating the complexities of the Medtech environment.
The landscape of class 3 medical devices is characterized by complexity and critical importance, as these high-risk instruments are essential to patient care and safety. Researchers entering this domain encounter unique challenges, ranging from navigating stringent regulatory requirements to ensuring rigorous clinical testing. This article explores vital insights that illuminate the path for innovators and researchers, examining the latest trends, regulatory frameworks, and market access strategies.
How can stakeholders effectively adapt to the evolving landscape of class 3 medical devices while maximizing their impact on healthcare outcomes?
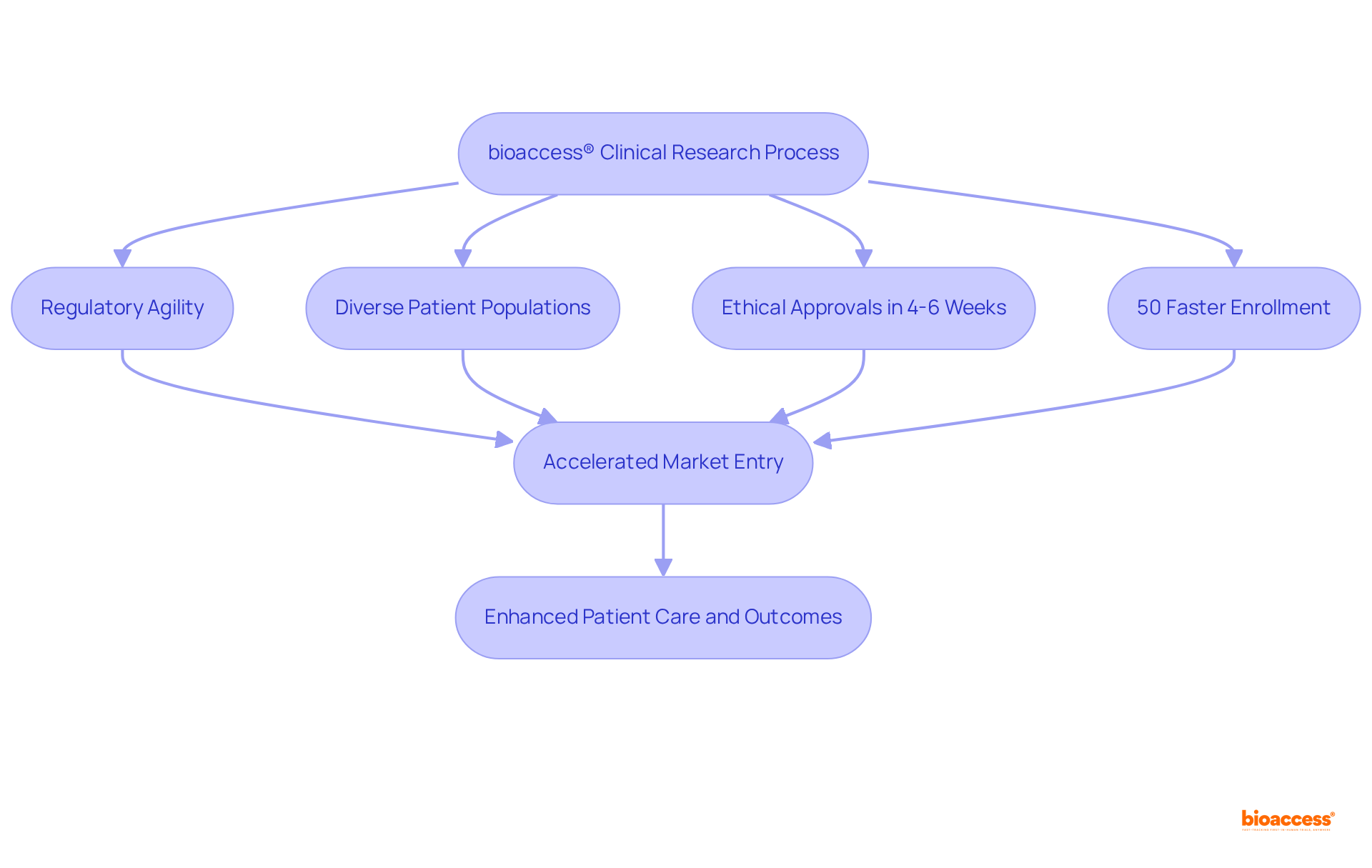
bioaccess® excels in early-phase clinical studies for Category 3 medical products, leveraging its regulatory agility and diverse patient populations across Latin America, the Balkans, and Australia. Achieving ethical approvals in an impressive 4-6 weeks—substantially quicker than the average FDA 510(k) decision time of 5 months—bioaccess® accelerates enrollment by 50%. This enables innovators to expedite their market entry, significantly enhancing patient care and outcomes.
This customized approach addresses the distinct challenges faced by medical devices class 3, which undergo more stringent regulatory scrutiny compared to lower classes. By streamlining the clinical research process, bioaccess® not only enhances efficiency but also fosters innovation in the Medtech sector, ensuring that critical advancements reach patients more swiftly. Furthermore, the healthcare market in the LAC region, estimated at approximately $29 billion, underscores the potential impact of expedited enrollment on market access for these products.
In conclusion, the collaboration with bioaccess® represents a strategic opportunity for innovators to navigate the complexities of clinical research effectively. As the Medtech landscape evolves, leveraging such expertise will be crucial for driving advancements that ultimately benefit patients.
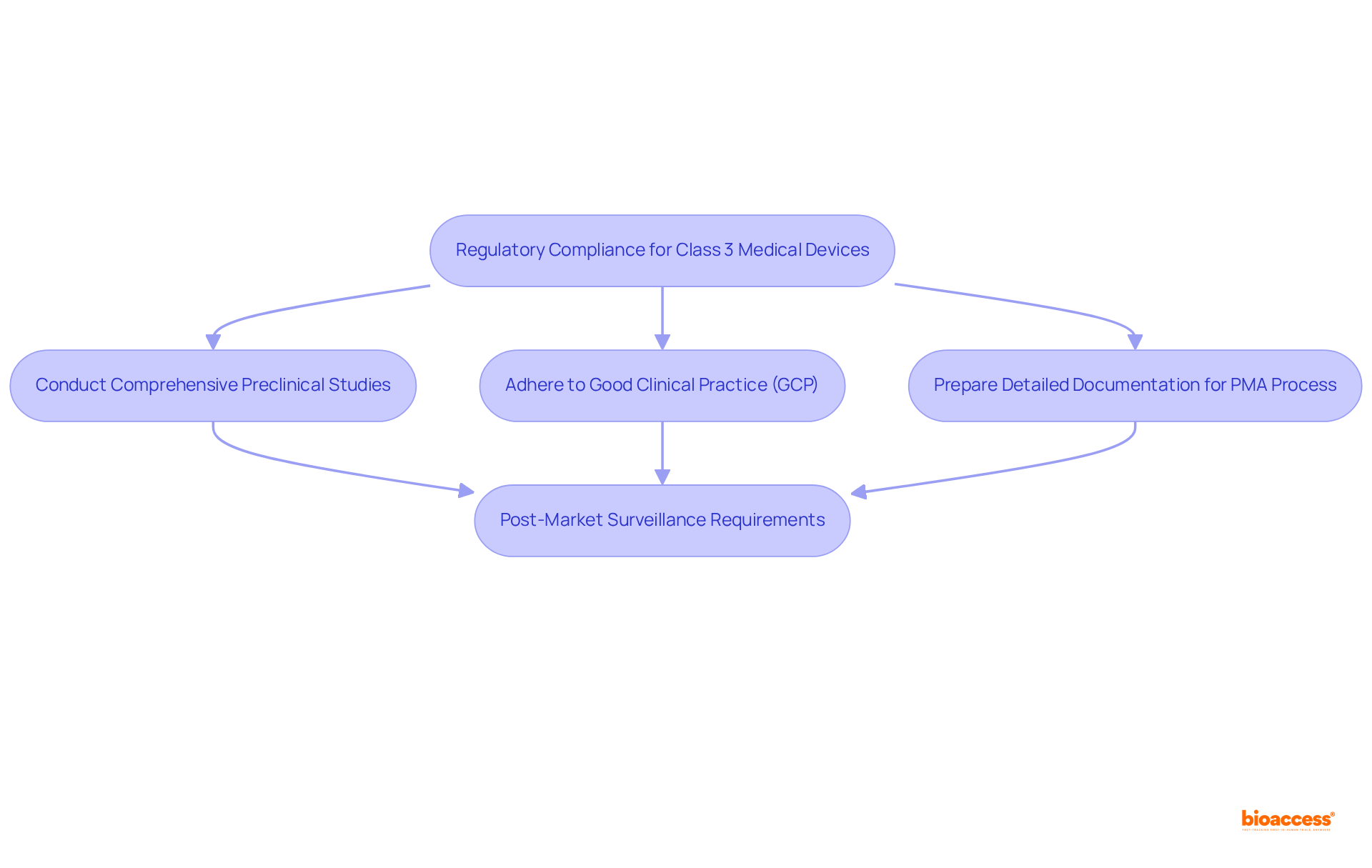
Medical devices class 3 face the most rigorous regulatory requirements due to their potential risks to patients. Researchers must ensure compliance with the guidelines established by regulatory bodies such as the FDA and EMA. Key considerations encompass:
Moreover, researchers should recognize the importance of post-market surveillance requirements to monitor product performance and safety once it is available for sale. Statistics indicate that adherence rates to FDA and EMA guidelines for medical devices class 3 products are crucial for successful market entry, with producers often required to conduct post-approval studies to confirm long-term safety and effectiveness. Understanding these regulatory frameworks and their implications is vital for researchers aiming to navigate the complexities of developing medical devices class 3 in healthcare technology.
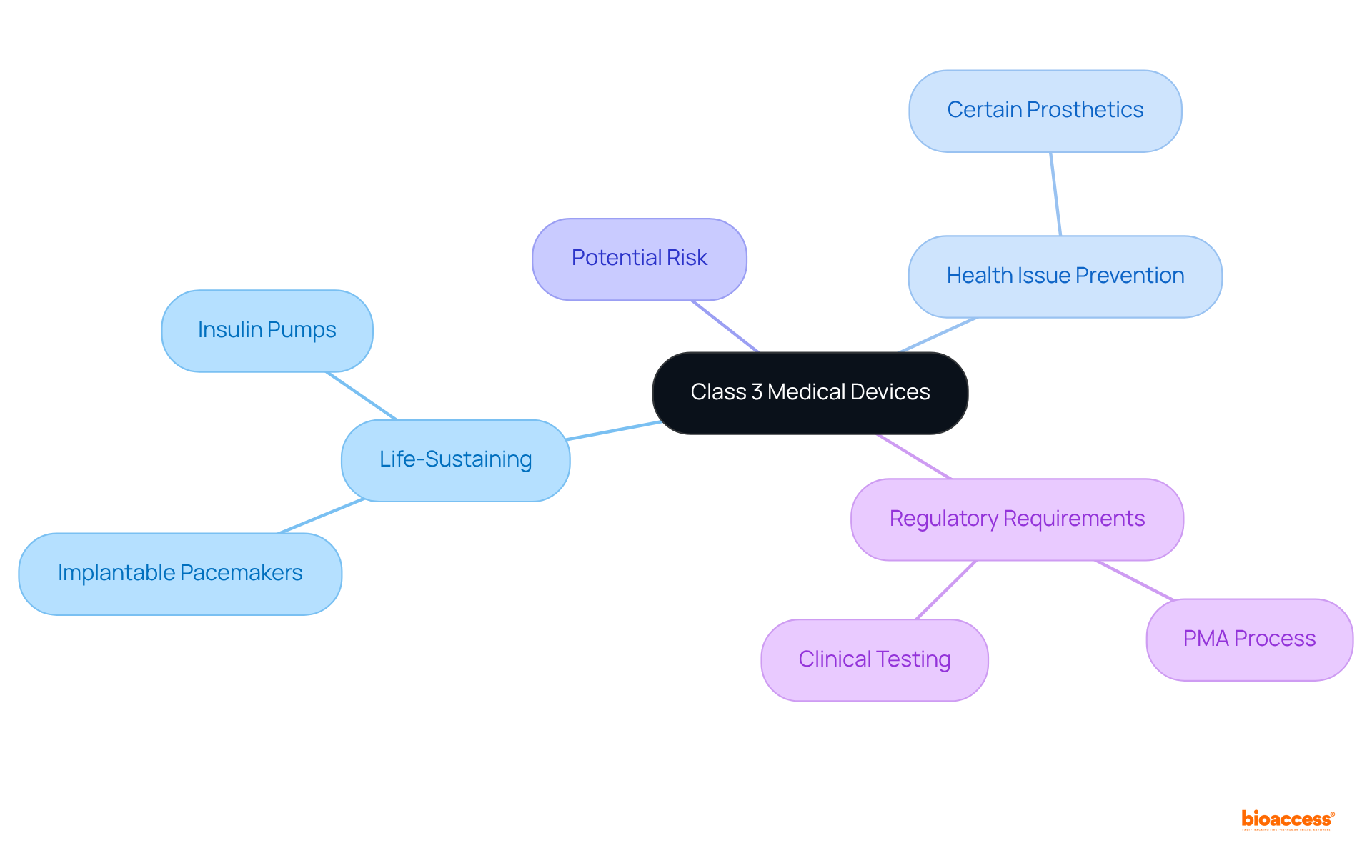
The FDA classifies medical devices class 3 into three categories based on their associated risk levels. Items classified as medical devices class 3 are particularly critical, as they either assist or sustain human life, significantly contribute to the prevention of health issues, or pose a potential unreasonable risk of illness or injury. Notable examples of medical devices class 3 include:
All of which require comprehensive clinical testing to validate their safety and efficacy. According to the FDA, approximately 9% of all regulated medical instruments fall into this category, underscoring the stringent requirements these items must fulfill. The premarket approval (PMA) process for medical devices class 3 is the most rigorous, necessitating extensive documentation and clinical studies to ensure compliance with the highest safety standards. Regulatory experts assert that the thorough evaluation of medical devices class 3 is essential for safeguarding patient health, as any malfunction could lead to severe consequences. Therefore, the classification and authorization of medical devices class 3 are vital in maintaining the integrity of healthcare and ensuring patient safety.
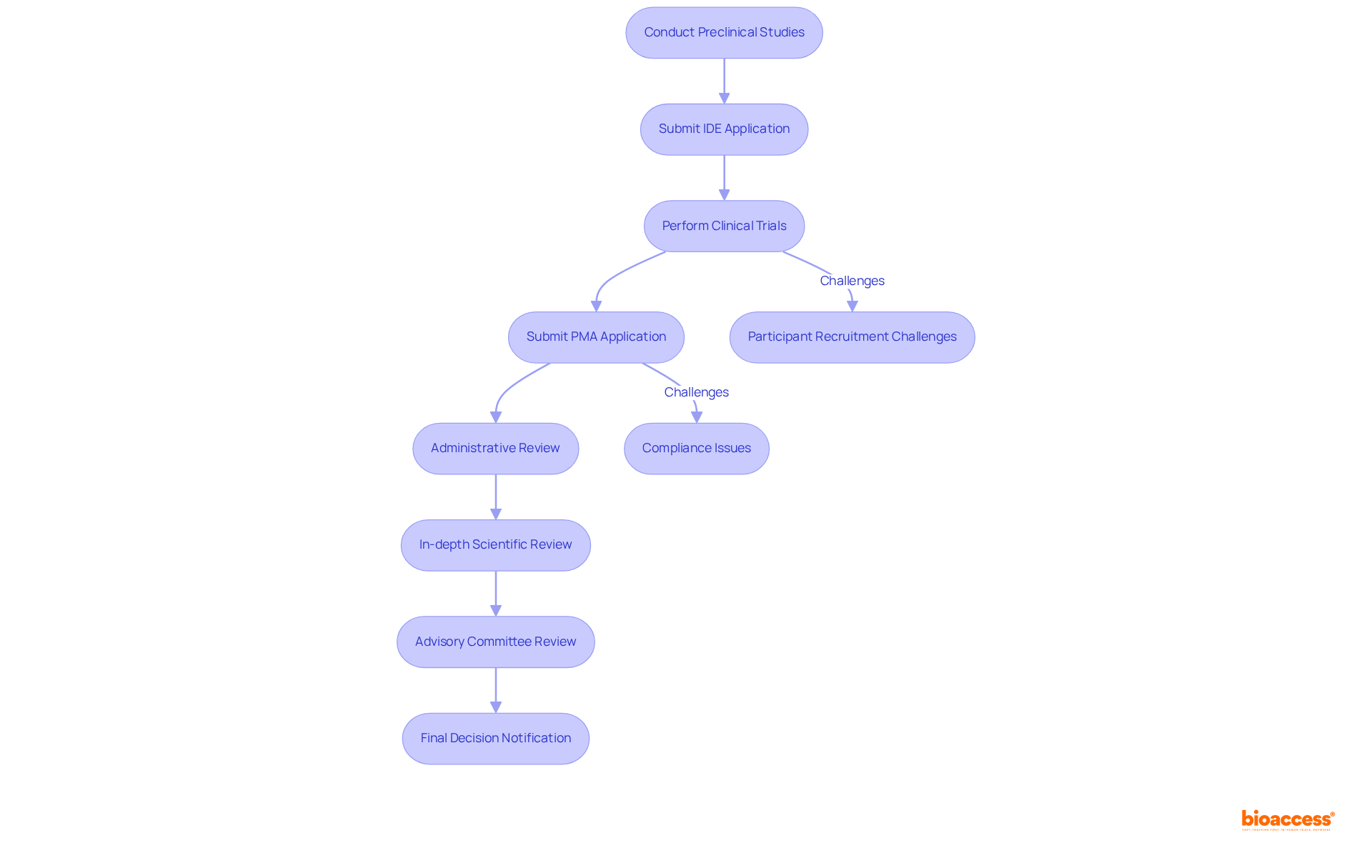
The premarket approval (PMA) procedure for medical devices class 3 encompasses several critical steps:
Each of these steps presents unique challenges, such as recruiting suitable participants for clinical trials and ensuring compliance with regulatory requirements. Researchers must also prepare for potential delays due to the detailed assessments conducted by regulatory bodies, which can significantly prolong timelines. Notably, the typical duration for rendering a decision on FDA 510(k) applications is closer to five months, highlighting the lengthy nature of the approval process.
Moreover, requests for additional information during the FDA evaluation can lead to further delays and increased costs, many of which are often avoidable. In fact, nearly 67% of 510(k) submissions resulted in such requests in the year leading up to September 2022, underscoring the critical importance of thorough preparation.
As one specialist remarked, "communicating early and frequently will aid the procedure for both parties and hopefully reduce any delays in what is already a quite lengthy endeavor." This statement emphasizes the necessity for proactive engagement with regulatory bodies to ensure that submissions meet all required standards from the outset.
The evaluation of the PMA application comprises four steps:
Understanding these steps is essential for researchers aiming to effectively navigate the complexities of the PMA procedure for medical devices class 3. Furthermore, the introduction of new regulations, such as the EU's Medical Device Regulations (MDR), is anticipated to influence decision times, potentially extending approval periods. As the landscape evolves, staying informed about the latest developments in the PMA process is crucial for researchers aspiring to introduce innovative medical products to the market successfully.
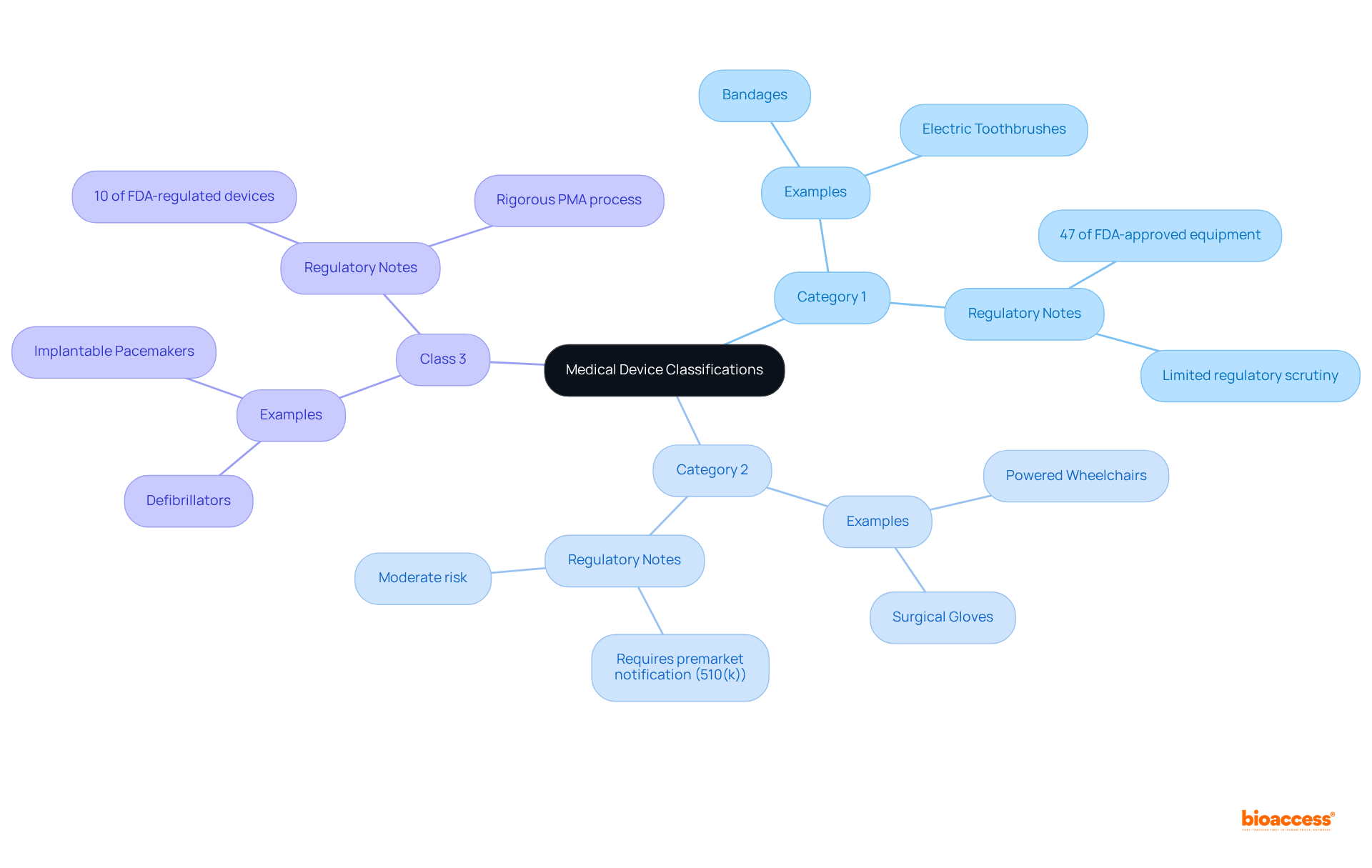
Medical instruments are classified into three categories—Category 1, Category 2, and medical devices class 3—according to their risk levels.
Category 1 items, regarded as low-risk, typically face limited regulatory scrutiny and often qualify for exceptions from premarket notification. Products such as bandages and electric toothbrushes exemplify this group, accounting for approximately 47% of FDA-approved equipment.
Category 2 items, which present moderate risk, necessitate a premarket notification (510(k)) to demonstrate safety and effectiveness, often through substantial equivalence to existing products. Examples include powered wheelchairs and surgical gloves.
Medical devices class 3 are classified as high-risk and must undergo a stringent premarket approval (PMA) process. This rigorous procedure entails extensive clinical trials and comprehensive data collection to ensure safety and efficacy. Notably, only around 10% of medical devices class 3 regulated by the FDA fall into this category, which includes critical items such as defibrillators and implantable pacemakers. The PMA process is characterized by its demanding criteria, frequently requiring significantly more time than the approval timelines for Category 1 and Category 2 items. For instance, the median initial review period for FDA drug approvals is approximately 303 days, while Category 3 products generally undergo even more thorough examination.
The ramifications of these classifications are substantial for researchers and manufacturers alike. As industry leaders assert, the classification not only directs the regulatory pathway but also impacts the speed at which innovations can penetrate the market. Understanding these distinctions is crucial for navigating the complexities of healthcare equipment development and ensuring compliance with regulatory standards.
Medical devices class 3, such as implantable defibrillators, heart valves, and neurostimulators, are pivotal in managing life-threatening conditions. These instruments undergo rigorous clinical testing to guarantee their safety and effectiveness.
For instance, implantable defibrillators continuously monitor heart rhythms and can deliver life-saving shocks upon detecting arrhythmias. This capability significantly enhances survival rates, especially in patients with a history of cardiac issues.
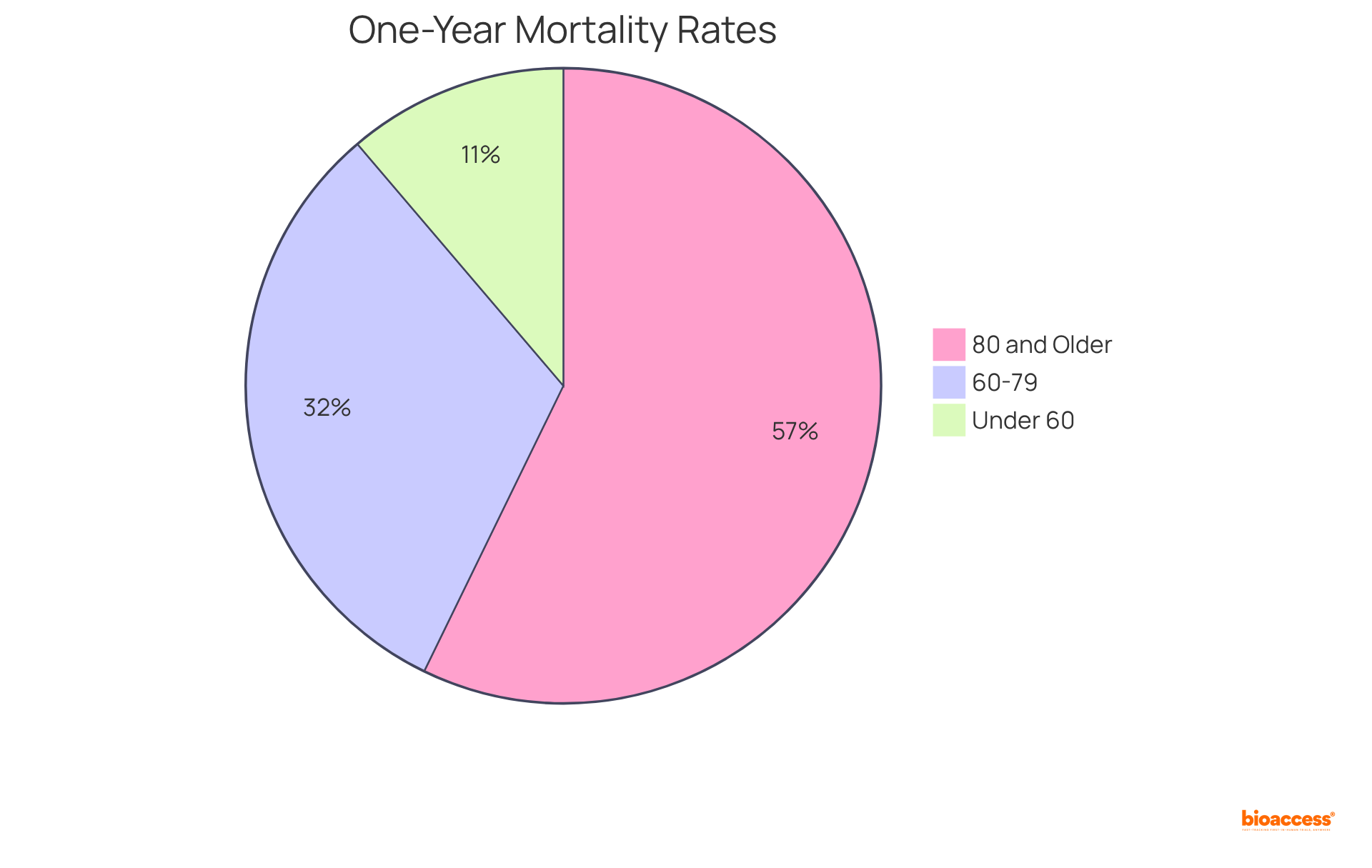
Studies reveal that the one-year mortality rate for patients receiving implantable cardioverter-defibrillators (ICDs) is markedly lower than for those who do not receive such interventions, with rates of:
Furthermore, the hazard ratio for one-year mortality is:
Heart valves, which fall under medical devices class 3, are essential for addressing conditions like aortic stenosis, where they can greatly enhance quality of life and reduce hospitalization rates. For example, the median duration of hospital stay for individuals aged 80 years and above is just 3 days, showcasing the efficiency of these tools in minimizing hospitalizations.
Understanding these applications not only underscores the importance of thorough clinical research but also highlights the substantial impact these tools have on patient care and outcomes. As emphasized by healthcare experts, integrating these instruments into treatment strategies is crucial for enhancing patient survival and quality of life.
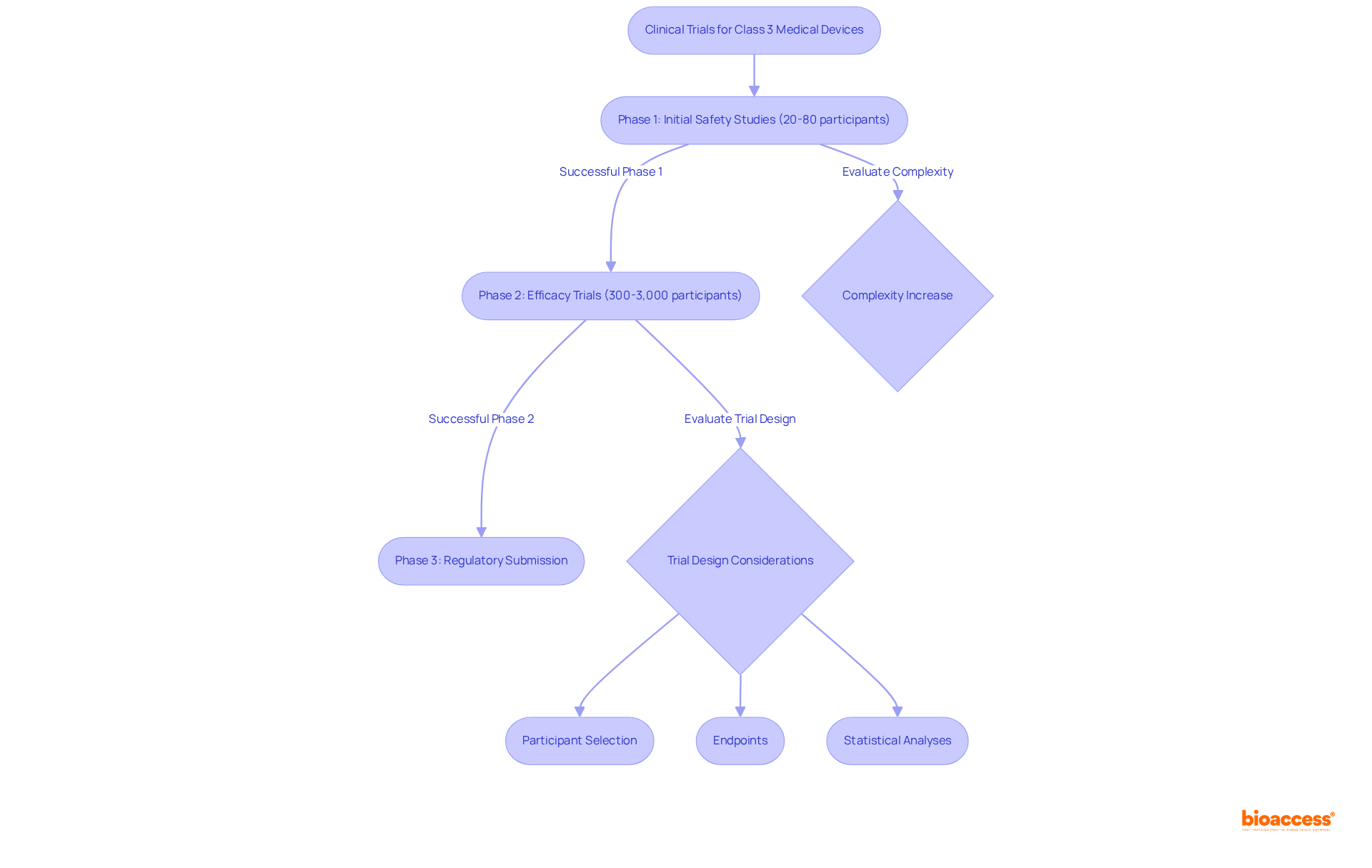
Clinical trials are indispensable for the approval of medical devices class 3, as they provide critical information to demonstrate safety and efficacy. These trials are methodically structured into multiple phases, commencing with small-scale studies that evaluate initial safety, typically involving 20 to 80 participants. As the process advances, larger trials are conducted to assess efficacy, often involving between 300 to 3,000 participants to ensure diverse population representation.
The design of these trials is paramount; researchers must meticulously consider:
The complexity of these trials has escalated significantly, with eligibility criteria increasing from an average of 31 between 2001 and 2005 to 50 between 2011 and 2015, reflecting a 61 percent increase in complexity. Furthermore, 56 percent of clinical trial sites reported that trials are more complex than three years ago, underscoring the challenges encountered in trial design and execution.
This diligent approach is crucial, as successful completion of each phase does not guarantee overall success; for instance, over 58 percent of drugs fail in Phase III trials. Additionally, the FDA's approval process for Category 3 products is intricate and time-consuming, with the overall cost of developing a new medication in the U.S. estimated at about $2.6 billion as of 2023. Therefore, a well-organized trial framework is essential for navigating the complex landscape of approval for medical devices class 3, including compliance with the Unique Identification (UDI) system, which is vital for regulatory adherence.
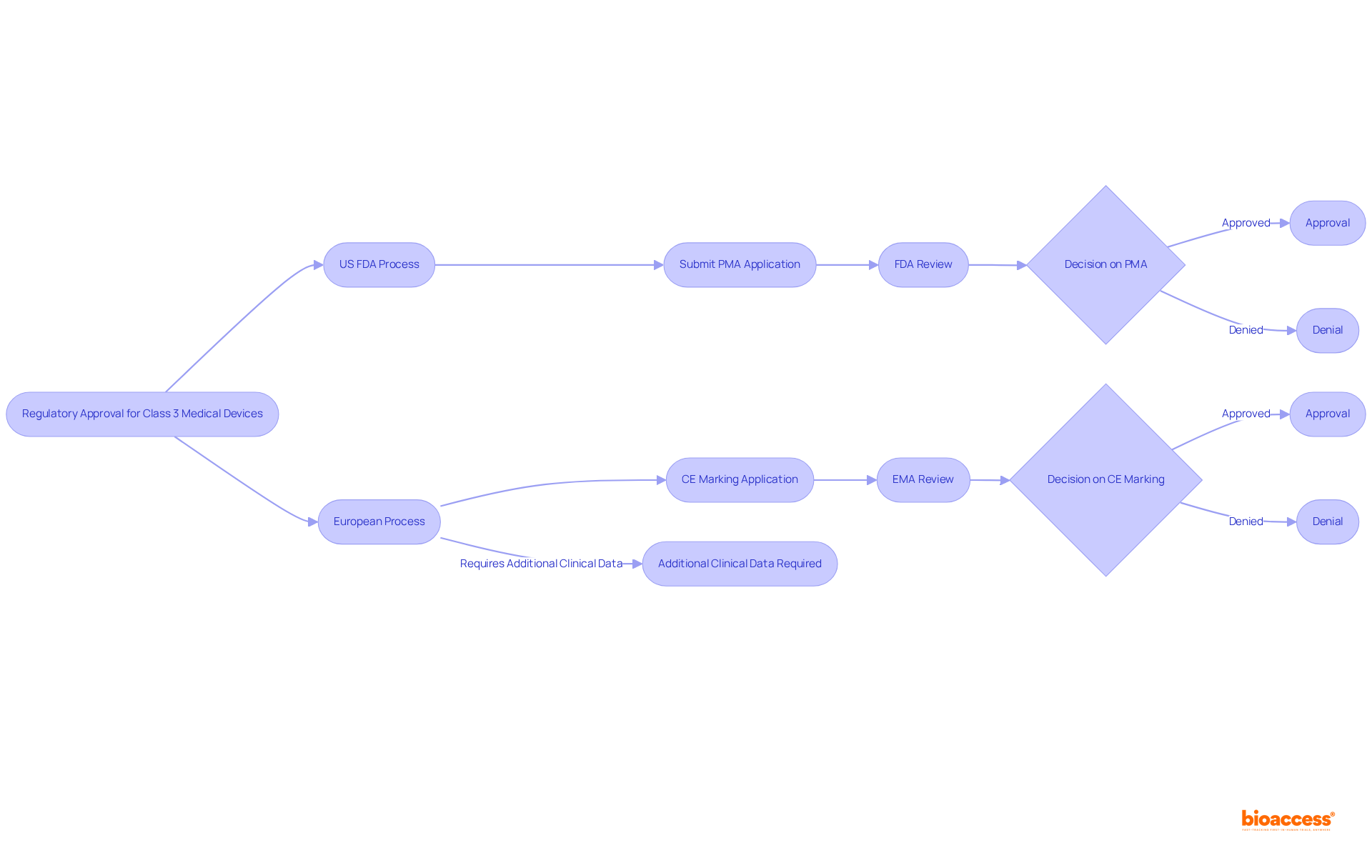
The regulatory landscape for medical devices class 3 is crucial for clinical research, as it varies significantly across countries. In the United States, the FDA oversees the approval procedure, while Europe has its own regulatory organizations, such as the European Medicines Agency (EMA). Researchers must be acutely aware of these differences, as they can profoundly impact timelines, requirements, and market access.
For example, the CE marking process in Europe for medical devices class 3 may necessitate additional clinical data compared to the FDA's PMA process. This disparity underscores the need for careful planning when conducting international studies.
To effectively navigate market entry for Category 3 health products, researchers must develop comprehensive strategies that encompass an understanding of reimbursement pathways, engagement with key opinion leaders, and the execution of health economic assessments.
Collaborating with local partners significantly facilitates smoother entry into diverse markets, particularly in regions such as Latin America, where bioaccess® offers invaluable support in navigating regulatory complexities and reimbursement dynamics.
Given that the healthcare equipment market in LATAM is projected to reach USD 37.23 billion by 2025, it is imperative for researchers to stay informed about evolving regulations and market trends to adapt their strategies accordingly.
Early engagement with stakeholders, especially payers, is crucial for enhancing the likelihood of successful reimbursement outcomes.
The future of medical devices class 3 development is poised for significant transformation due to advancements in technology, particularly artificial intelligence (AI) and machine learning. These innovations not only enhance equipment functionality but also improve patient outcomes, paving the way for personalized healthcare solutions that cater to individual patient needs. As the industry transitions towards personalized medicine, researchers must anticipate increased regulatory oversight and the necessity for robust post-market monitoring, especially as devices grow more sophisticated. Staying informed about these trends is essential for fostering successful innovation within the medical devices class 3 sector.
The integration of AI represents more than just a passing trend; it is revolutionizing healthcare innovation. For example, AI-driven algorithms are improving diagnostic accuracy and efficiency, facilitating earlier disease detection and more precise surgical interventions. This technological evolution is supported by a growing body of evidence indicating that AI can accelerate clinical research by analyzing vast amounts of biomedical data, thus identifying promising pathways for product development.
Furthermore, industry thought leaders underscore the significance of these advancements. As the healthcare technology sector continues to evolve, the focus on personalized medicine will drive the development of medical devices class 3 that are not only effective but also tailored to the specific needs of patients. This transition highlights the imperative for researchers to engage in continuous learning and adapt to the swiftly changing technological landscape, ensuring they remain at the forefront of innovation in medical devices class 3.
The intricate world of Class 3 medical devices is pivotal to enhancing patient care and safety, demanding a comprehensive understanding of regulatory frameworks and clinical research methodologies. Navigating this complex landscape is essential for researchers and innovators who aim to bring life-saving technologies to market efficiently and effectively.
Key insights from the article reveal the critical importance of:
By leveraging platforms like bioaccess® that streamline these processes, researchers can significantly expedite market entry and improve patient outcomes. Additionally, recognizing the differences between Class 3 and other medical device classifications is vital for developing effective strategies that address the unique challenges associated with high-risk devices.
Looking ahead, the integration of advanced technologies such as artificial intelligence and machine learning will undoubtedly shape the future of Class 3 medical devices. Staying informed about these trends and engaging in continuous learning will empower stakeholders to adapt and thrive in this dynamic environment. As the healthcare landscape evolves, the commitment to innovation and patient-centered solutions remains paramount, underscoring the significant role that Class 3 medical devices play in advancing healthcare outcomes.
What is bioaccess® and what role does it play in clinical research for Class 3 medical devices?
bioaccess® specializes in early-phase clinical studies for Class 3 medical devices, utilizing its regulatory agility and diverse patient populations in regions such as Latin America, the Balkans, and Australia. It significantly speeds up the ethical approval process and enrollment, allowing innovators to bring products to market more quickly.
How quickly can bioaccess® achieve ethical approvals compared to the FDA?
bioaccess® can achieve ethical approvals in approximately 4-6 weeks, which is much faster than the average FDA 510(k) decision time of about 5 months.
What are the benefits of using bioaccess® for clinical research?
By accelerating enrollment by 50% and streamlining the clinical research process, bioaccess® helps innovators expedite market entry for Class 3 medical devices, enhancing patient care and outcomes.
What are the key regulatory requirements for Class 3 medical devices?
Key regulatory requirements for Class 3 medical devices include conducting comprehensive preclinical studies, adhering to Good Clinical Practice (GCP), and preparing detailed documentation for the premarket approval (PMA) process.
Why are post-market surveillance requirements important for Class 3 medical devices?
Post-market surveillance is essential to monitor the performance and safety of medical devices once they are available for sale, ensuring long-term safety and effectiveness.
How does the FDA classify Class 3 medical devices?
The FDA classifies Class 3 medical devices based on their associated risk levels, identifying them as critical devices that either assist or sustain human life, prevent health issues, or pose a significant risk of illness or injury.
Can you provide examples of Class 3 medical devices?
Examples of Class 3 medical devices include implantable pacemakers, insulin pumps, and certain types of prosthetics.
What is the significance of the premarket approval (PMA) process for Class 3 medical devices?
The PMA process is the most rigorous regulatory pathway, requiring extensive documentation and clinical studies to ensure compliance with the highest safety standards, which is crucial for protecting patient health.