


The article titled "10 Essential Insights on NDA in Pharma for Clinical Research Directors" addresses the vital components of the New Drug Application (NDA) process within the pharmaceutical industry. It underscores that a successful NDA submission necessitates:
These elements are crucial for ensuring the safety and efficacy of new medications, while also facilitating their timely entry into the market.
The New Drug Application (NDA) process serves as a pivotal gateway for pharmaceutical innovations, fundamentally shaping the landscape of medication accessibility and safety. For clinical research directors, grasping the intricacies of this process is essential, as it dictates not only the timeline for introducing new drugs to the market but also ensures compliance with stringent regulatory standards. However, navigating the complexities of NDA submissions can present significant challenges—ranging from incomplete data to regulatory hurdles.
How can organizations effectively streamline their approach to overcome these obstacles and enhance their chances of successful approvals? This article delves into ten essential insights that illuminate the NDA journey, offering strategies and best practices for clinical research directors seeking to optimize their submissions and expedite market entry.
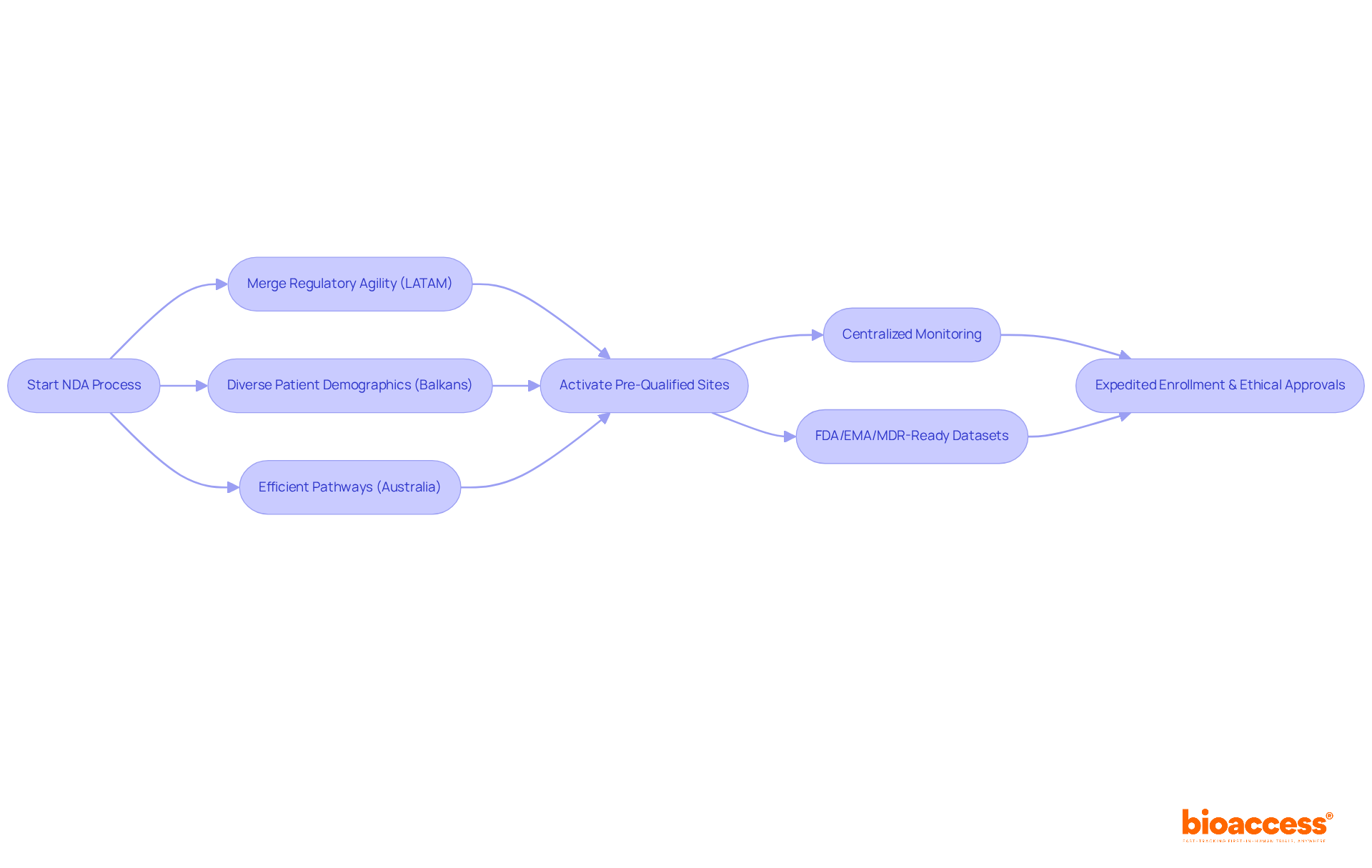
bioaccess® effectively merges Latin America's regulatory agility, the Balkans' diverse patient demographics, and Australia's efficient pathways to facilitate the process of NDA in pharma in a mere 4-6 weeks. By harnessing comprehensive acceleration for global trials, bioaccess® guarantees the activation of over 50 pre-qualified sites in under 8 weeks, alongside FDA/EMA/MDR-ready datasets and centralized monitoring. This strategic approach empowers pharmaceutical innovators to adeptly navigate intricate regulatory landscapes, ensuring expedited enrollment and ethical approvals. Additionally, by implementing parallel submissions across LATAM, the Balkans, and Australia, bioaccess® significantly accelerates the time to market for new medications.
The NDA in pharma serves as a crucial formal request submitted to the FDA by sponsors aiming to secure marketing approval for a new pharmaceutical. This comprehensive report encompasses essential information derived from clinical trials, production methods, and proposed labeling, all directed at demonstrating the treatment's safety and effectiveness for public use. The process of NDA in pharma has undergone significant evolution since its inception, particularly after the 1962 amendment, which required manufacturers to provide evidence of both safety and effectiveness through well-controlled clinical trials prior to marketing.
Successful NDA in pharma are distinguished by their thoroughness and compliance with FDA requirements. Notably, from 2013 to 2022, an impressive 98% of new medication approvals adhered to the Prescription Drug User Fee Act (PDUFA) review target dates, underscoring the efficacy of the FDA's evaluation system. Furthermore, 87% of authorized medications during this timeframe were first-cycle approvals, reflecting robust collaboration between the FDA and developers. Additionally, 72% of medications gained approval in the US before other countries, highlighting the NDA process's significant influence on global medication accessibility.
The nda in pharma not only provides a pathway to market but also ensures that new medications fulfill stringent safety standards. For instance, 102 medications authorized in recent years included black box warnings, emphasizing the FDA's commitment to transparency regarding potential risks. Moreover, the approval landscape has witnessed a noteworthy trend, with 40% of medications classified as first-in-class, indicating a substantial contribution to innovative treatment options. Furthermore, 16% of new medications received fast track approval, showcasing the expedited pathways available for sponsors.
In conclusion, the nda in pharma represents a vital component of the pharmaceutical approval process, ensuring that new medications are both safe and effective while facilitating timely access to innovative therapies for patients.

An NDA in pharma must encompass several critical components, including:
Moreover, it is essential to include any preclinical study results and information regarding the drug's composition to substantiate the application. To facilitate a successful NDA in pharma submission, it is paramount to engage in comprehensive clinical trial management services. This includes:
Compliance evaluations and trial setup activities, such as obtaining necessary import permits and approvals from ethics committees and health ministries, constitute vital steps in the preparation phase. Effective project management, along with meticulous reporting of study status, inventory, and adverse events, further fortifies the NDA in pharma application, ensuring that all regulatory requirements are met efficiently. By prioritizing these elements, organizations can navigate the complexities of the NDA process with confidence.

The procedure for the NDA in pharma review encompasses several essential steps:
The FDA initiates the process with a 60-day period to assess whether the NDA in pharma is complete and appropriate for filing. Upon clearing this phase, a comprehensive review typically unfolds over about 10 to 12 months; however, it may extend if additional data is required. During this timeframe, the FDA meticulously evaluates the submitted data, including safety and efficacy details, production methods, and labeling information, to reach an informed decision regarding approval.
Adhering to the Common Technical Document (CTD) format is crucial, as it enhances the regulatory submission process by promoting consistency and efficiency. This structured approach not only ensures thorough scrutiny but also facilitates effective regulatory oversight, ultimately influencing the speed at which new drugs can enter the market. Understanding the potential outcomes of NDA in pharma rejection, such as incomplete data or safety concerns, is vital for managing this situation effectively.
To support this journey, bioaccess offers comprehensive clinical trial management services, including:
These services are designed to streamline the NDA in pharma workflow, ensuring that all essential documentation and compliance measures are in place, thereby enhancing the likelihood of a successful filing.

The challenges associated with the NDA in pharma procedure include:
Incomplete entries can severely impede approval rates; the FDA may issue a Refuse-to-File (RTF) letter if critical information is absent. To mitigate these risks, clinical research directors must prioritize thorough pre-submission planning in the context of NDA in pharma, which includes conducting a validation check to identify any missing files or formatting errors.
Additionally, maintaining open lines of communication with the FDA throughout the process is crucial, as this facilitates clarification on requirements and expectations. Moreover, meticulous compilation and review of all data—covering clinical trial results, pharmacology, and toxicology summaries—are essential for a successful NDA in pharma submission. By proactively addressing these challenges, organizations can significantly enhance their chances of achieving first-cycle approval and expedite their path to market.

The distinction between an NDA in pharma and an Abbreviated New Drug Application (ANDA) is fundamental in pharmaceutical development. An NDA in pharma is submitted for new medications that require extensive clinical data to prove safety and efficacy. In contrast, an ANDA is intended for generic drugs, which must establish bioequivalence to an already approved product without the need for extensive clinical trials.
Understanding these differences is vital for strategic decision-making. The approval procedure for NDA in pharma generally encounters more stringent requirements, resulting in a lower approval rate compared to ANDAs. Recent data indicates that while the approval rate for NDA in pharma hovers around 50%, ANDAs enjoy a significantly higher success rate, often exceeding 90%. This disparity underscores the strategic implications for companies considering their development pathways, particularly in relation to the NDA in pharma.
Pharmaceutical companies now spend significantly more on R&D but produce far fewer new molecules than they did 10 years ago. This trend, coupled with pricing pressures and tighter regulatory requirements, has prompted companies to reassess their strategies. For instance, opting for an ANDA route can expedite market entry and capitalize on existing products, especially in a landscape where the traditional blockbuster model is declining. The rise of generic medicines has shifted focus towards cost-effective strategies, prompting companies to rethink their R&D investments.
Industry leaders emphasize the importance of these strategic choices. As J.P. Garnier, Chief Executive of GlaxoSmithKline, noted, "Pharma’s traditional strategy is a business model where you are guaranteed to lose your entire book of business every 10 to 12 years." This insight reflects the broader trend of pharmaceutical companies adapting to tighter regulatory requirements and pricing pressures, making informed decisions about their product development strategies more critical than ever. Additionally, the trend of R&D outsourcing among large pharmaceutical companies highlights how organizations are navigating these challenges to enhance efficiency and innovation.

Regulatory compliance is paramount in the NDA in pharma process, ensuring that all studies and documents adhere to the FDA's rigorous standards. Non-compliance can lead to significant setbacks, including delays, rejections, and potential legal issues. Therefore, clinical research leaders must prioritize compliance with Good Clinical Practice (GCP) and other regulatory guidelines to enhance the likelihood of successful applications.
The repercussions of failing to meet these standards can be severe. For instance, incomplete data entries can result in extended review periods, increasing costs and jeopardizing timelines. Conversely, organizations that maintain robust compliance structures often experience smoother filing procedures and quicker approvals.
Successful examples of regulatory compliance in the pharmaceutical industry underscore the importance of meticulous preparation and proactive engagement with regulatory bodies. Organizations that invest in educating their teams on GCP and regulatory requirements are better positioned to navigate the complexities of the NDA in pharma process.
To ensure compliance in NDA in pharma submissions, companies should collaborate with experienced Contract Research Organizations (CROs) like bioaccess, which provide comprehensive clinical trial management services, including:
By partnering with a CRO, organizations can identify potential pitfalls in the NDA submission process early and remain informed about changes in regulatory policies. Consistent communication with the FDA during the evaluation phase is also crucial for addressing inquiries and providing additional information, ultimately enhancing the chances of a successful submission. Furthermore, post-marketing surveillance information is vital for ongoing regulatory adherence, offering insights into the long-term safety and effectiveness of medications. Statements from compliance officers emphasize the importance of GCP adherence:
"Grasping and applying GCP is not merely a regulatory obligation; it is a pledge to guarantee patient safety and data integrity during the clinical trial phase."
This perspective highlights the necessity of cultivating a culture of compliance within organizations.

The FDA plays a crucial role in the NDA in pharma submission procedure, as it is responsible for ensuring that new medications are safe and effective for public use. This responsibility involves a comprehensive evaluation of clinical trial data, manufacturing processes, and proposed labeling. The agency's decisions significantly impact a medication's market entry and overall success. For clinical research directors, understanding the FDA's expectations is vital, as these evaluations can shape the trajectory of a drug's development and commercialization.
The review process can be intricate, often requiring the FDA to seek additional information or clarification. This underscores the importance of meticulous preparation and strict compliance with regulatory standards. In this context, bioaccess offers specialized assistance for NDA in pharma applications, ensuring that the data is accurate and comprehensive, which significantly mitigates the risk of rejection or delays. Common reasons for NDA in pharma rejection include:
Furthermore, the FDA has a 60-day period to conduct an initial evaluation of NDA applications, highlighting the necessity for timely and precise data collection. As Xybion points out, "efficiencies in data collection and aggregation" are essential for successful entries. A case study illustrating the significance of precise data in NDA in pharma submissions reveals that absent or erroneous information can lead to considerable delays, emphasizing the necessity for thoroughness in the NDA in pharma procedure.

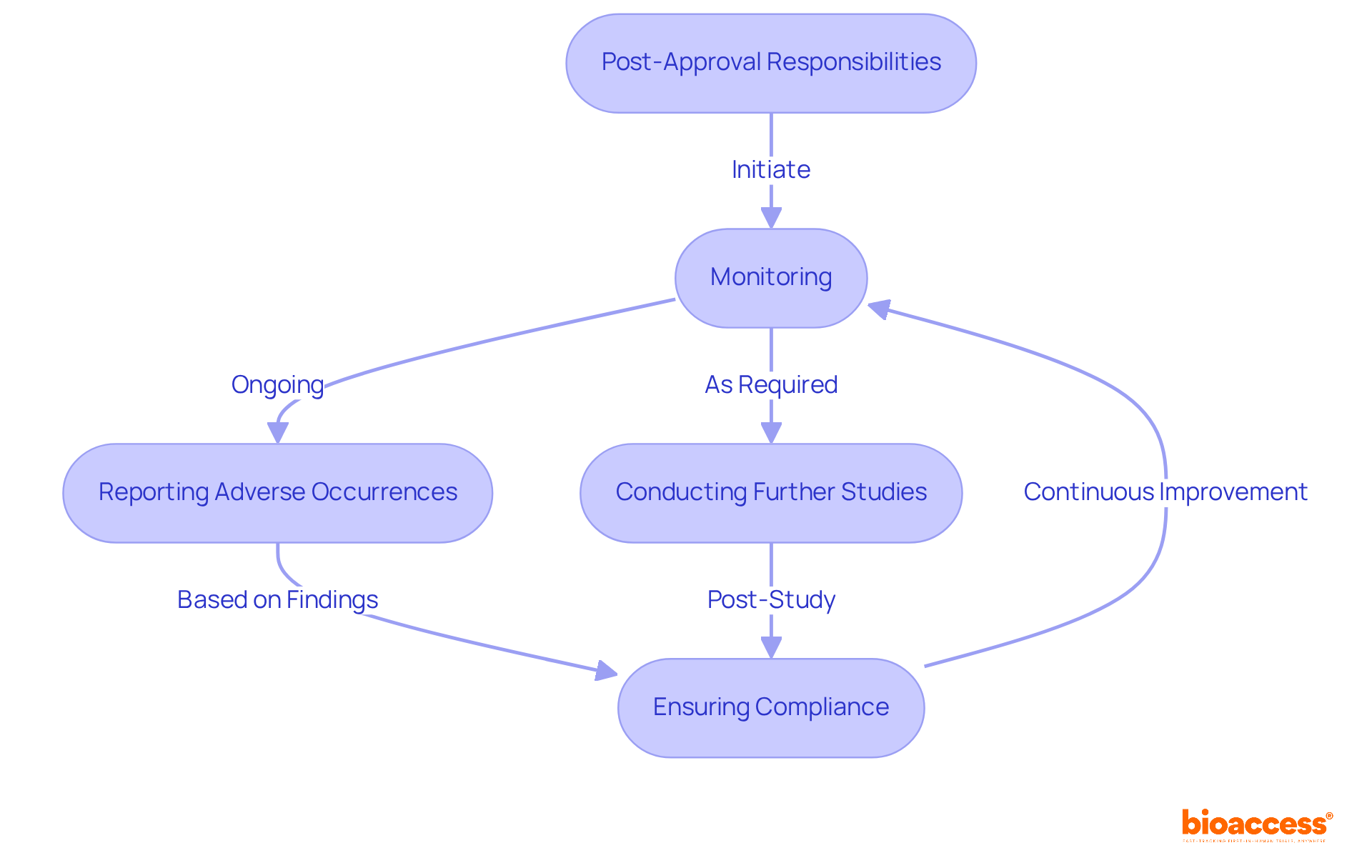
Following the NDA in pharma approval, pharmaceutical firms are subjected to rigorous post-marketing monitoring to ensure the ongoing safety and effectiveness of their medications in real-world settings. This process involves systematic reporting of adverse occurrences, which is crucial given that research indicates nearly 30% of medications experience significant adverse events post-approval.
Additionally, companies are required to conduct further studies as mandated by regulatory authorities to evaluate long-term effects and verify compliance with established labeling and manufacturing standards. Effective post-marketing surveillance not only protects public health but also strengthens regulatory adherence, thereby fostering trust among stakeholders.
Industry leaders emphasize the necessity of continuous monitoring, as it allows for timely interventions and adjustments based on real-world data, ultimately enhancing medication safety and efficacy.
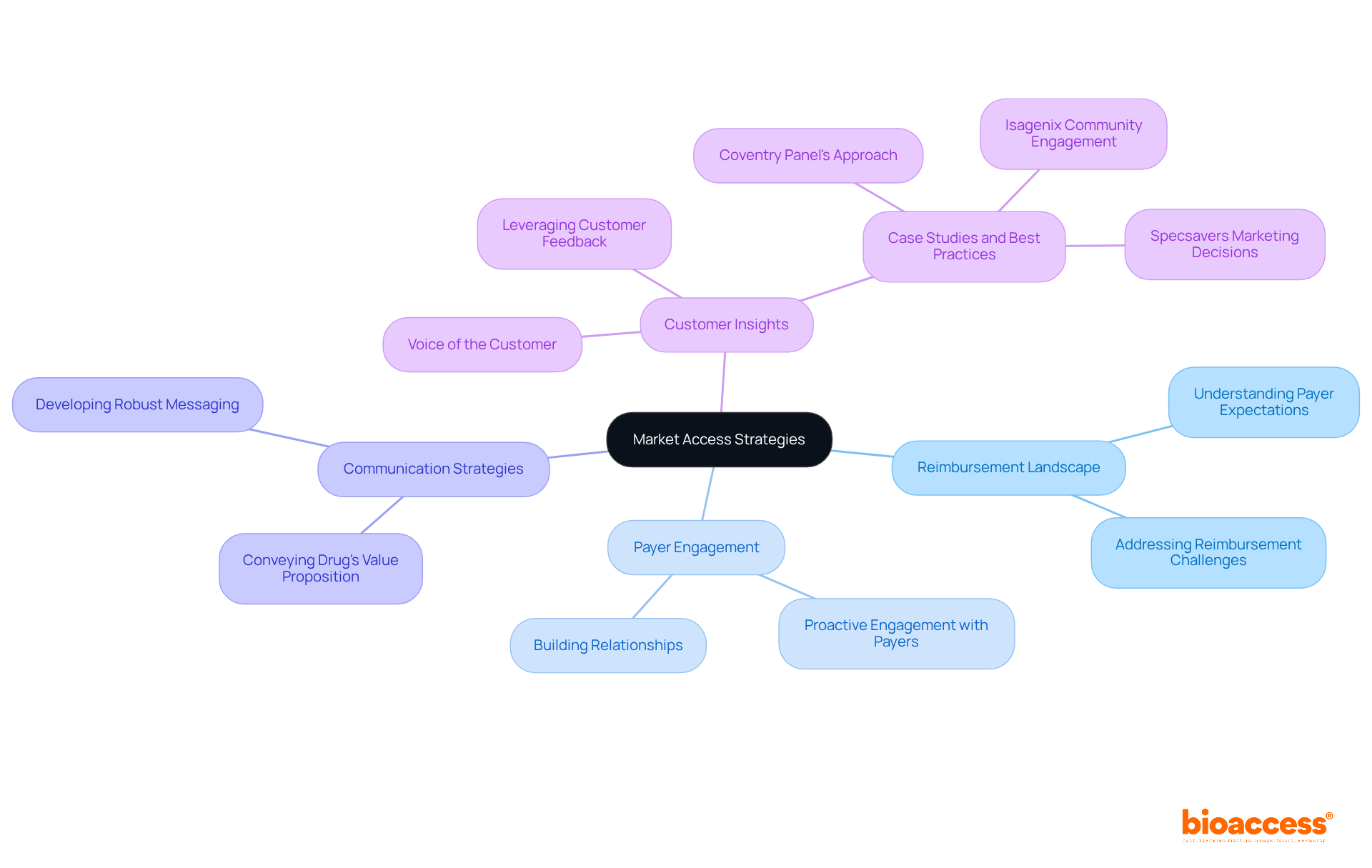
Successful commercialization following the NDA in pharma approval hinges on meticulous market access planning. This critical process demands a deep understanding of the reimbursement landscape, proactive engagement with payers, and the development of robust communication strategies that effectively convey the drug's value proposition. Clinical research directors must prioritize collaboration with market access teams from the outset, ensuring that their products are strategically positioned for successful market entry. By addressing reimbursement challenges and aligning with payer expectations, organizations can significantly enhance their chances of achieving a successful launch and sustained market presence, particularly in the context of NDA in pharma.
Notably, more than 50% of innovation stems from the voice of the customer, underscoring the importance of understanding payer expectations and customer feedback in the commercialization process. Case studies, such as the Coventry Panel's approach to maintaining an award-winning customer experience, illustrate the profound impact of customer insights on decision-making. Additionally, quotes from market access professionals provide authoritative perspectives on successful commercialization strategies, further reinforcing this argument. Organizations must also recognize the challenges they face in transitioning from discussion to action regarding customer focus, as this is crucial for effective market access strategies.
The NDA process in the pharmaceutical industry is integral to ensuring that new medications are safe, effective, and accessible. This formal request to the FDA not only facilitates the entry of innovative therapies into the market but also upholds public health standards through rigorous assessments and compliance with regulatory requirements. Understanding the significance of the NDA process empowers clinical research directors to navigate its complexities effectively and contribute to the successful commercialization of new drugs.
Throughout the article, key insights have been shared regarding the NDA's definition, its critical components, and the steps involved in the review process. Emphasis has been placed on the importance of regulatory compliance, the role of the FDA, and the strategic differences between NDA and ANDA submissions. Moreover, the article highlights the challenges faced during the NDA process and offers actionable solutions to overcome these hurdles, ensuring a smoother pathway to approval.
As the pharmaceutical landscape continues to evolve, staying informed about the intricacies of the NDA process is essential for industry leaders. By prioritizing thorough preparation, regulatory adherence, and effective market access strategies, organizations can significantly enhance their chances of successful drug approvals and ultimately improve patient outcomes. Embracing these insights will not only streamline the NDA submission process but also foster innovation and collaboration within the pharmaceutical sector, paving the way for future advancements in healthcare.
What is bioaccess® and how does it facilitate the NDA process for pharmaceutical innovators?
bioaccess® merges regulatory agility from Latin America, diverse patient demographics from the Balkans, and efficient pathways from Australia to expedite the New Drug Application (NDA) process in pharmaceuticals, achieving this in 4-6 weeks. It activates over 50 pre-qualified sites in under 8 weeks and provides FDA/EMA/MDR-ready datasets and centralized monitoring, aiding innovators in navigating complex regulatory landscapes.
What is a New Drug Application (NDA) in the pharmaceutical industry?
An NDA is a formal request submitted to the FDA by sponsors seeking marketing approval for a new pharmaceutical. It includes essential information from clinical trials, production methods, and proposed labeling to demonstrate the treatment's safety and effectiveness for public use.
How has the NDA process evolved over time?
The NDA process has evolved significantly, particularly after the 1962 amendment, which mandated manufacturers to provide evidence of safety and effectiveness through well-controlled clinical trials before marketing. This has led to improved thoroughness and compliance with FDA requirements.
What are the success rates of NDA submissions from 2013 to 2022?
From 2013 to 2022, 98% of new medication approvals met the Prescription Drug User Fee Act (PDUFA) review target dates, indicating an effective FDA evaluation system. Additionally, 87% of authorized medications were first-cycle approvals, showing strong collaboration between the FDA and developers.
What are the key components required in an NDA submission?
An NDA must include clinical data demonstrating safety and effectiveness, comprehensive manufacturing details, proposed labeling, and a summary of the medication's pharmacology. It should also contain preclinical study results and information about the drug's composition.
What steps are involved in preparing for a successful NDA submission?
Preparing for an NDA involves conducting feasibility studies, selecting suitable research sites, compliance evaluations, and trial setup activities, such as obtaining necessary permits and ethics committee approvals. Effective project management and meticulous reporting of study status and adverse events are also critical.
How does the NDA process ensure medication safety?
The NDA process ensures safety by requiring thorough documentation and evidence of a medication's safety and effectiveness. For instance, recent approvals included black box warnings, reflecting the FDA's commitment to transparency regarding potential risks associated with new medications.