


The article titled "10 Essential Insights on PK Sampling for Clinical Research Directors" serves as a vital resource for clinical research directors, offering essential information and strategies regarding pharmacokinetic (PK) sampling. It underscores the necessity of optimizing PK sampling methods to bolster data reliability and enhance clinical research outcomes. This is supported by insights into regulatory compliance, the integration of technology, and the critical need for collaboration with field experts.
In the ever-evolving Medtech landscape, understanding the role of PK sampling is crucial. The article delves into how effective PK sampling can address significant challenges faced by clinical research directors today. By leveraging advanced methodologies and fostering partnerships, directors can navigate the complexities of clinical trials more effectively.
Ultimately, the article emphasizes that collaboration is not just beneficial but essential for success in clinical research. It encourages directors to take actionable steps towards optimizing their PK sampling strategies, ensuring they remain at the forefront of research excellence.
The landscape of clinical research is evolving rapidly, and pharmacokinetic (PK) sampling has emerged as a pivotal element in developing new therapies. As clinical research directors navigate the complexities of drug trials, understanding the nuances of PK sampling can significantly influence the success of their studies.
This article delves into ten essential insights that highlight the importance of optimizing PK sampling methods. By revealing how strategic approaches can mitigate risks, enhance data integrity, and ultimately accelerate the path to market for innovative medical solutions, we aim to underscore the critical role of PK sampling in clinical research.
What challenges might arise in the pursuit of effective PK sampling, and how can they be overcome to ensure robust research outcomes?
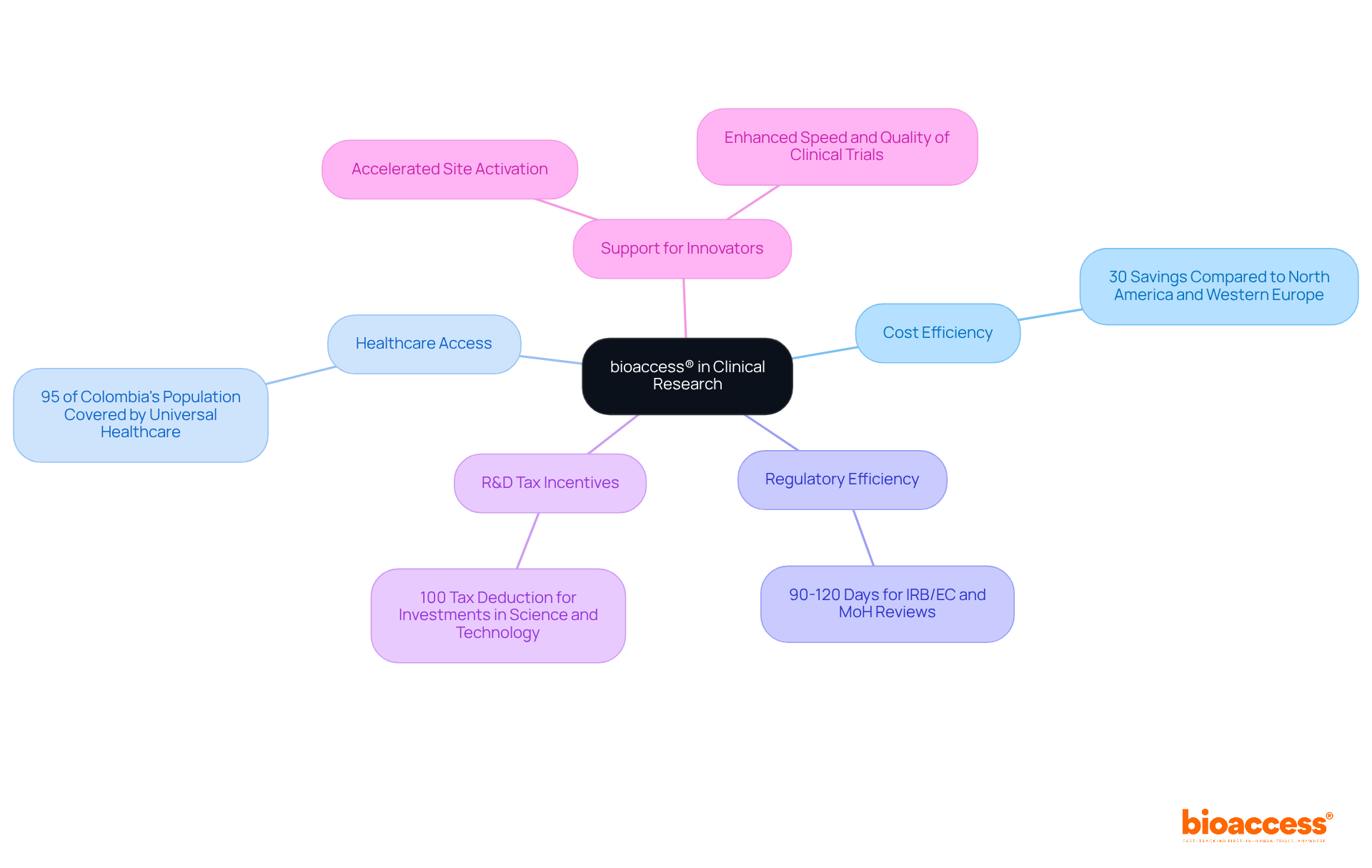
bioaccess® leverages its unique position in Latin America to provide swift and effective PK sampling insights, capitalizing on the region's cost efficiency—offering savings exceeding 30% compared to North America and Western Europe. With access to diverse patient pools, where over 95% of Colombia's population is covered by universal healthcare, and streamlined regulatory pathways that ensure IRB/EC and MoH (INVIMA) reviews are completed within 90-120 days, bioaccess® empowers clinical research directors to obtain timely and relevant information on pk sampling. This agility is crucial for Medtech, Biopharma, and Radiopharma innovators striving to expedite their product market entry, supported by Colombia's high-quality healthcare system, ranked #22 by the World Health Organization among 191 countries.
Moreover, bioaccess® accelerates site activation and regulatory compliance, further enhancing the speed and quality of clinical trials. The R&D tax incentives available in Colombia, including a 100% tax deduction for investments in science and technology, present additional financial advantages for Medtech startups. As the landscape of clinical research evolves, collaboration with bioaccess® not only addresses key challenges but also positions innovators for success in a competitive market.
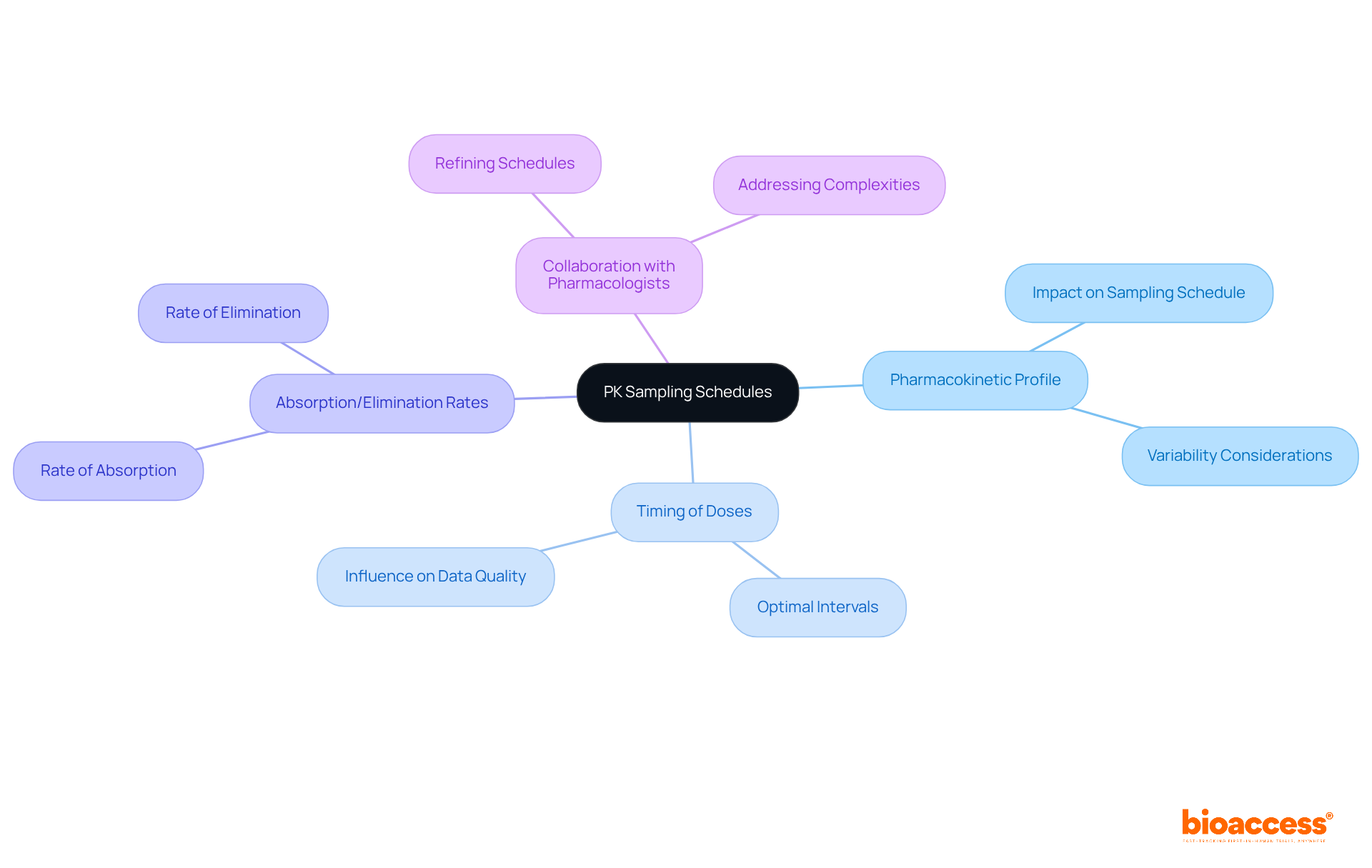
When developing PK collection schedules, clinical research directors must consider several key factors. These include:
The selection of sampling time intervals should align with the research goals, ensuring that the data collected provides significant insights into the drug's behavior within the body. Collaborating with pharmacologists can further refine these schedules, enhancing the quality of the data gathered. This collaboration is crucial for addressing the complexities of clinical research and ensuring robust outcomes.
Pharmacokinetic analyses play a crucial role in clinical research, necessitating the collection of various specimen types, including blood and urine. Blood specimens are vital for accurately measuring drug concentrations in systemic circulation, providing essential data for understanding pharmacodynamics. Meanwhile, urine specimens offer valuable insights into renal clearance and drug metabolism, further enriching the research findings. Depending on the specific objectives of the study, other specimen types, such as saliva or tissue biopsies, may also be utilized. Each specimen category comes with its own unique collection and management requirements, which must be meticulously evaluated to ensure the integrity of the information gathered.

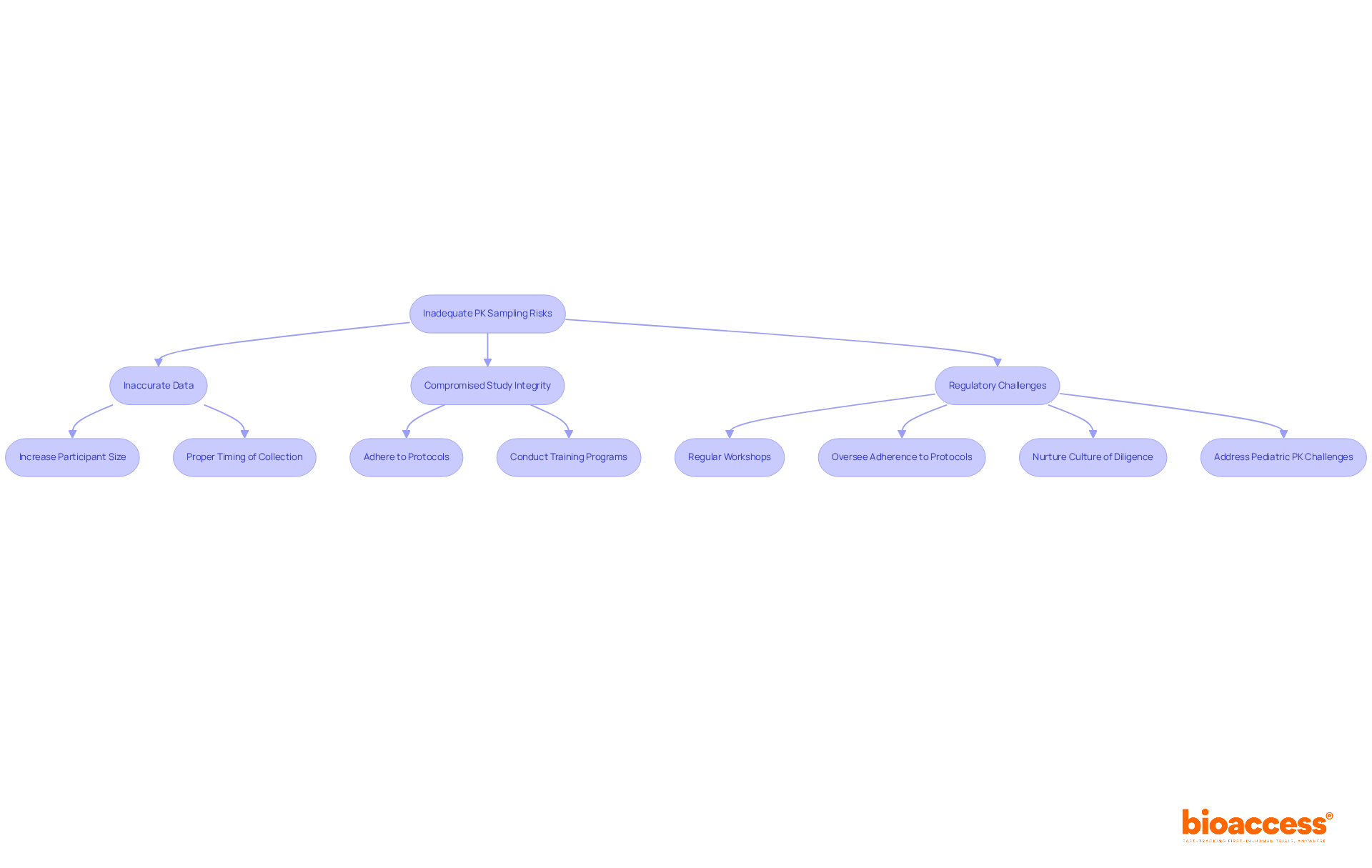
Inadequate pk sampling poses significant risks, including inaccurate data, compromised study integrity, and potential regulatory challenges. Common pitfalls include inadequate participant sizes, improper timing of collection, and deviations from established protocol specifications. For instance, the FDA recommends collecting 12 to 18 blood samples per subject, per dose, to adequately characterize the drug's pharmacokinetic phases. Additionally, data collection should continue for at least three terminal elimination half-lives to ensure comprehensive information gathering.
To mitigate these risks, clinical research directors must prioritize robust training programs that emphasize the critical nature of accurate specimen collection techniques. Conducting regular workshops on pk sampling techniques can enhance team proficiency and commitment to best practices. As Rachel Rozakis, a Senior Clinical Pharmacologist, observes, "A properly designed PK collection schedule facilitates a comprehensive understanding of a drug’s PK, which is essential for regulatory submissions."
Moreover, overseeing adherence to testing protocols is crucial to guarantee that all team members align with the project's goals. By nurturing a culture of diligence and precision in pk sampling, organizations can significantly enhance the reliability of their pharmacokinetic data and facilitate smoother regulatory submissions. Tackling distinct challenges, such as those faced in pediatric PK collection, is also essential, as these investigations frequently necessitate specialized strategies to enhance the timing and practicality of sample acquisition.
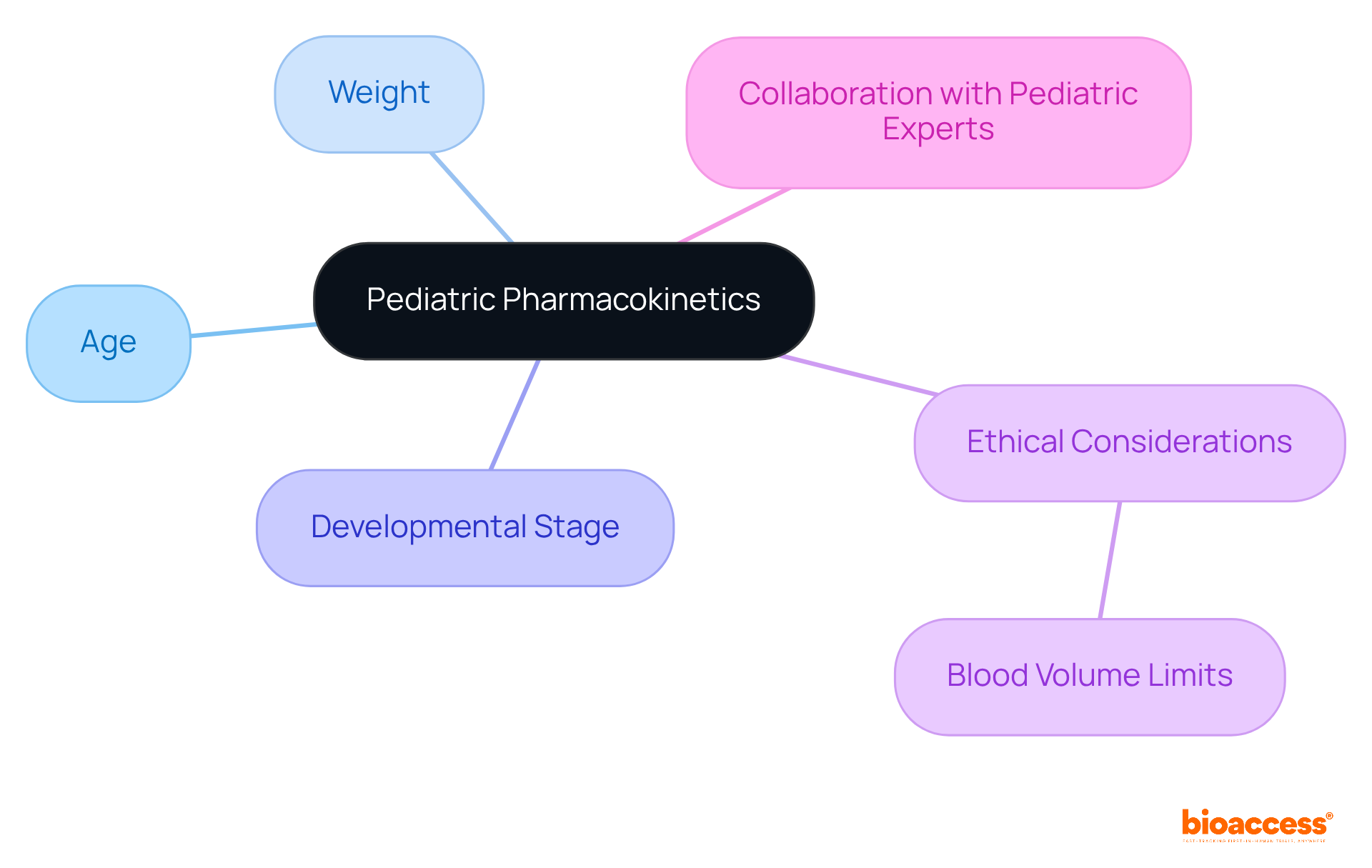
Pediatric pharmacokinetics presents unique challenges that demand tailored strategies for PK sampling. When designing pharmacokinetic (PK) sampling research for children, it’s crucial to consider factors such as age, weight, and developmental stage. Moreover, ethical considerations regarding the volume of blood that can be safely drawn from young patients are of utmost importance.
Collaborating with pediatric experts not only ensures that collection protocols are effective but also that they adhere to ethical standards. This partnership is essential for advancing clinical research in this sensitive area.
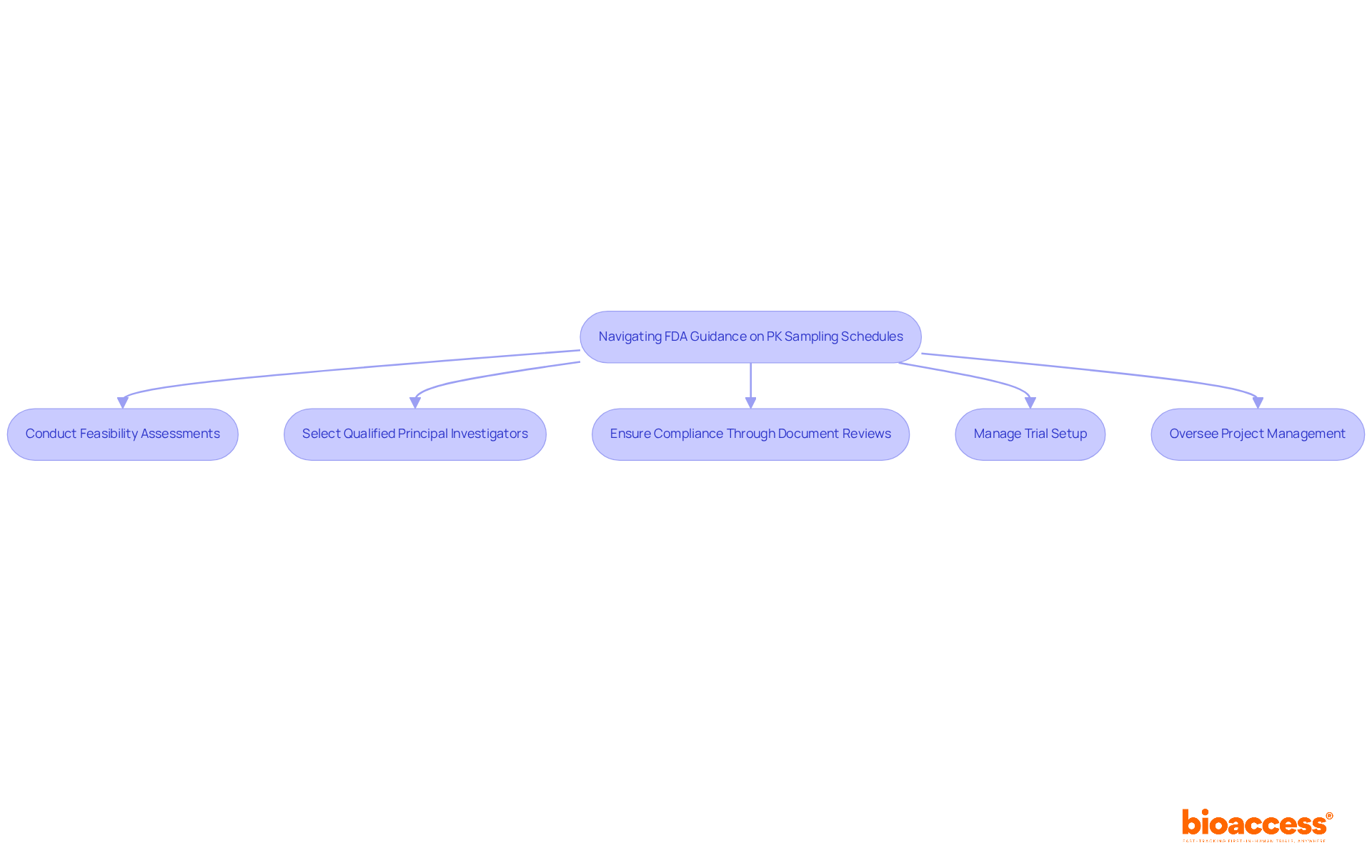
Navigating FDA guidance on pk sampling schedules is crucial for ensuring compliance and achieving successful research outcomes. The FDA provides specific guidance on the timing and frequency of pk sampling, which can vary based on the drug's pharmacokinetic characteristics and the research design.
Clinical research directors must leverage comprehensive clinical trial management services, such as those offered by bioaccess, to effectively implement these guidelines. This includes:
By incorporating these elements into study protocols, researchers not only facilitate regulatory approval but also streamline the review process, significantly enhancing the likelihood of successful outcomes.
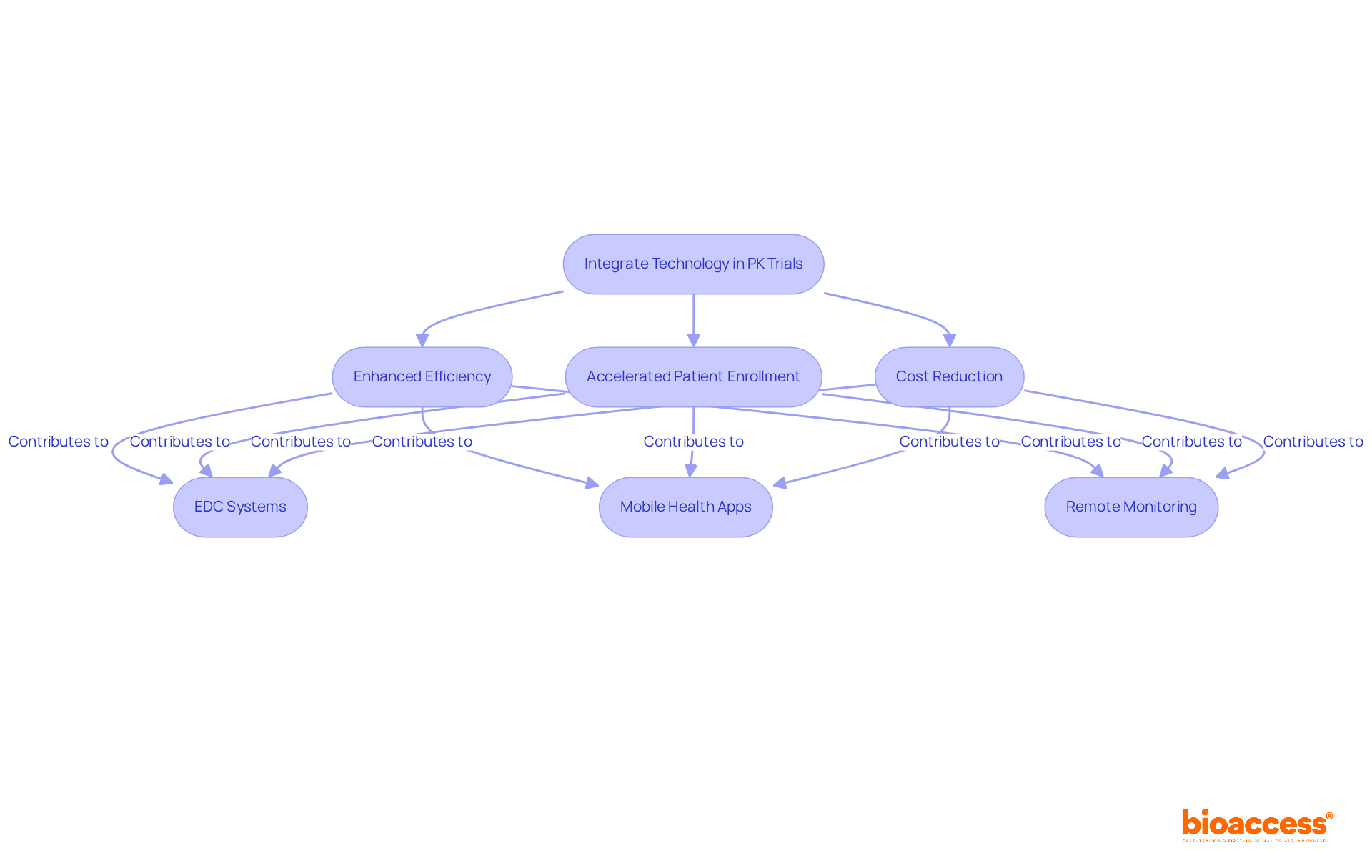
Incorporating technology into late-phase pk sampling trials significantly enhances the efficiency and accuracy of information, while also accelerating patient enrollment and reducing costs. Tools such as electronic data capture (EDC) systems, mobile health applications, and remote monitoring technologies facilitate real-time data collection and analysis.
With bioaccess®'s innovative solutions, clinical trials can achieve patient enrollment 50% faster and save $25,000 per patient with FDA-ready data—effectively eliminating rework and delays. These advancements not only streamline the pk sampling process but also boost patient engagement and compliance.
Clinical research directors must explore these technologies to elevate their trials, ensuring high-quality information while reaping substantial cost savings and expedited timelines. By embracing these innovations, they can address key challenges in the Medtech landscape, ultimately leading to more successful clinical outcomes.
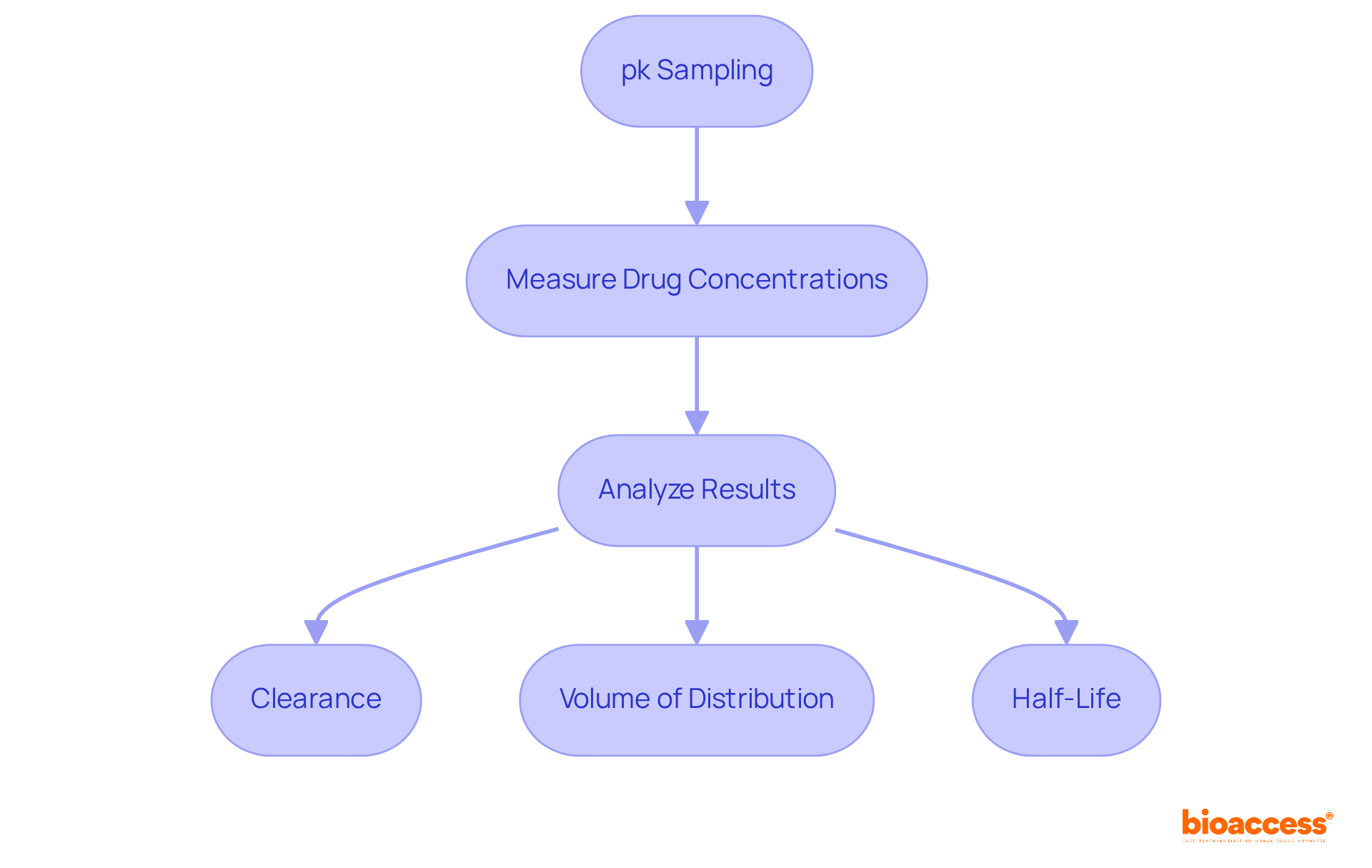
Efficient bioanalysis of specimens is essential for accurately interpreting pharmacokinetic information using pk sampling. This process includes pk sampling to measure drug concentrations in biological samples and analyze the results to determine key pharmacokinetic parameters, such as:
Clinical research directors must ensure their teams possess the necessary tools and expertise to conduct comprehensive bioanalysis. These insights are vital for shaping drug development strategies and meeting regulatory requirements.
In the evolving Medtech landscape, the role of bioanalysis cannot be overstated. It addresses critical challenges faced in clinical research, providing the data needed to make informed decisions. By prioritizing bioanalysis, organizations can enhance their research outcomes and streamline the path to market.
Collaboration among teams is crucial in this endeavor. By fostering an environment where expertise is shared and tools are readily available, clinical research can thrive. The next steps involve investing in training and resources to ensure that bioanalysis is conducted with the utmost precision and reliability.
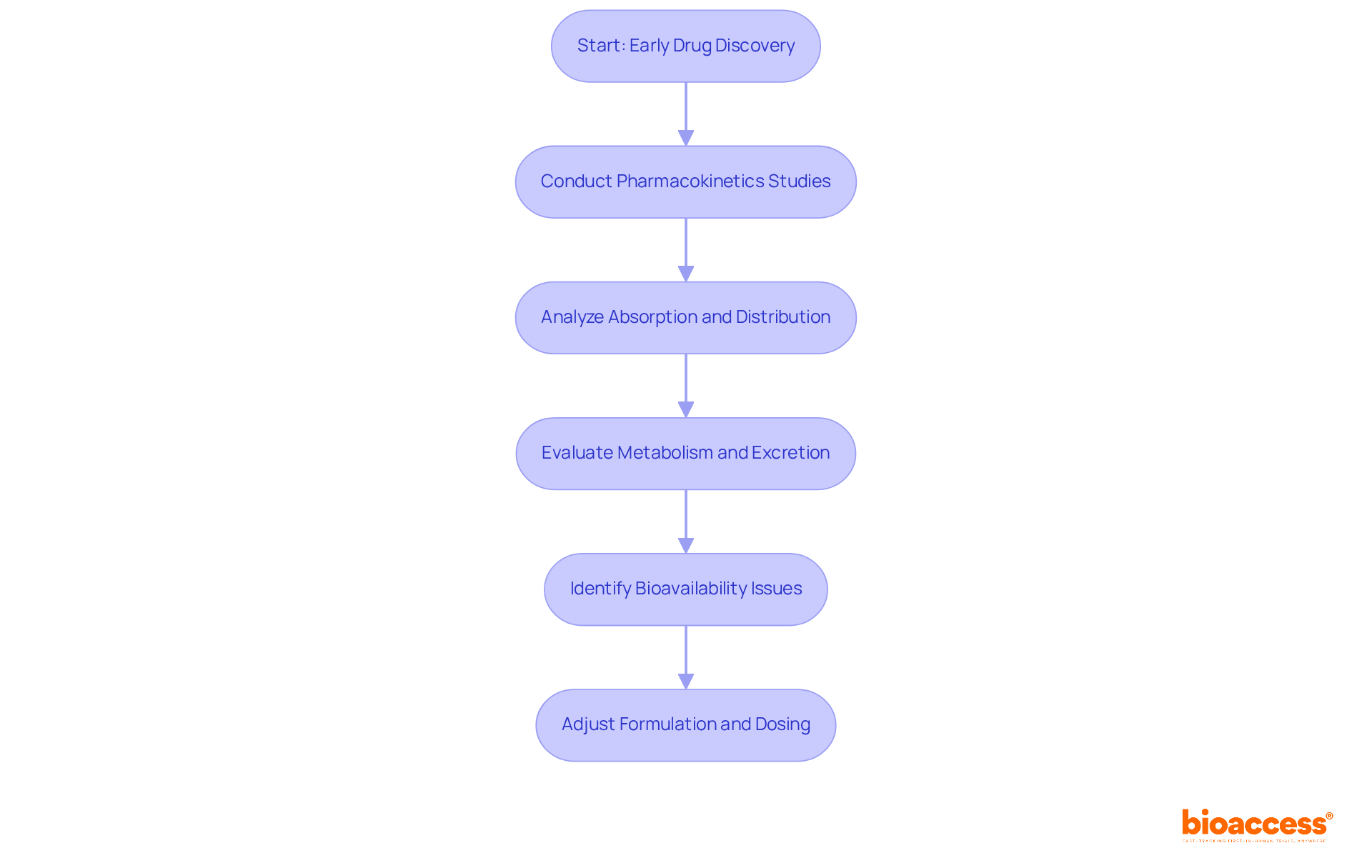
Pharmacokinetics is essential in early drug discovery, significantly influencing formulation and dosing strategies. Understanding how a drug is absorbed, distributed, metabolized, and excreted allows researchers to make informed decisions about its development. Early pk sampling information is invaluable; it helps identify potential issues related to bioavailability and toxicity, enabling necessary adjustments before advancing to later phases of clinical trials. Therefore, clinical research directors must prioritize pk sampling in the early phases to enhance the likelihood of successful drug development.
In the ever-evolving Medtech landscape, the role of pharmacokinetics cannot be overstated. By addressing key challenges through robust pk sampling, researchers can navigate the complexities of drug development more effectively. This proactive approach not only mitigates risks but also fosters collaboration among stakeholders, paving the way for innovative solutions.
In summary, the importance of pharmacokinetics in drug discovery is clear. By prioritizing early pk sampling studies, clinical research directors can significantly enhance the chances of successful outcomes in drug development. The next steps involve integrating these insights into strategic planning and fostering collaboration across the research spectrum.
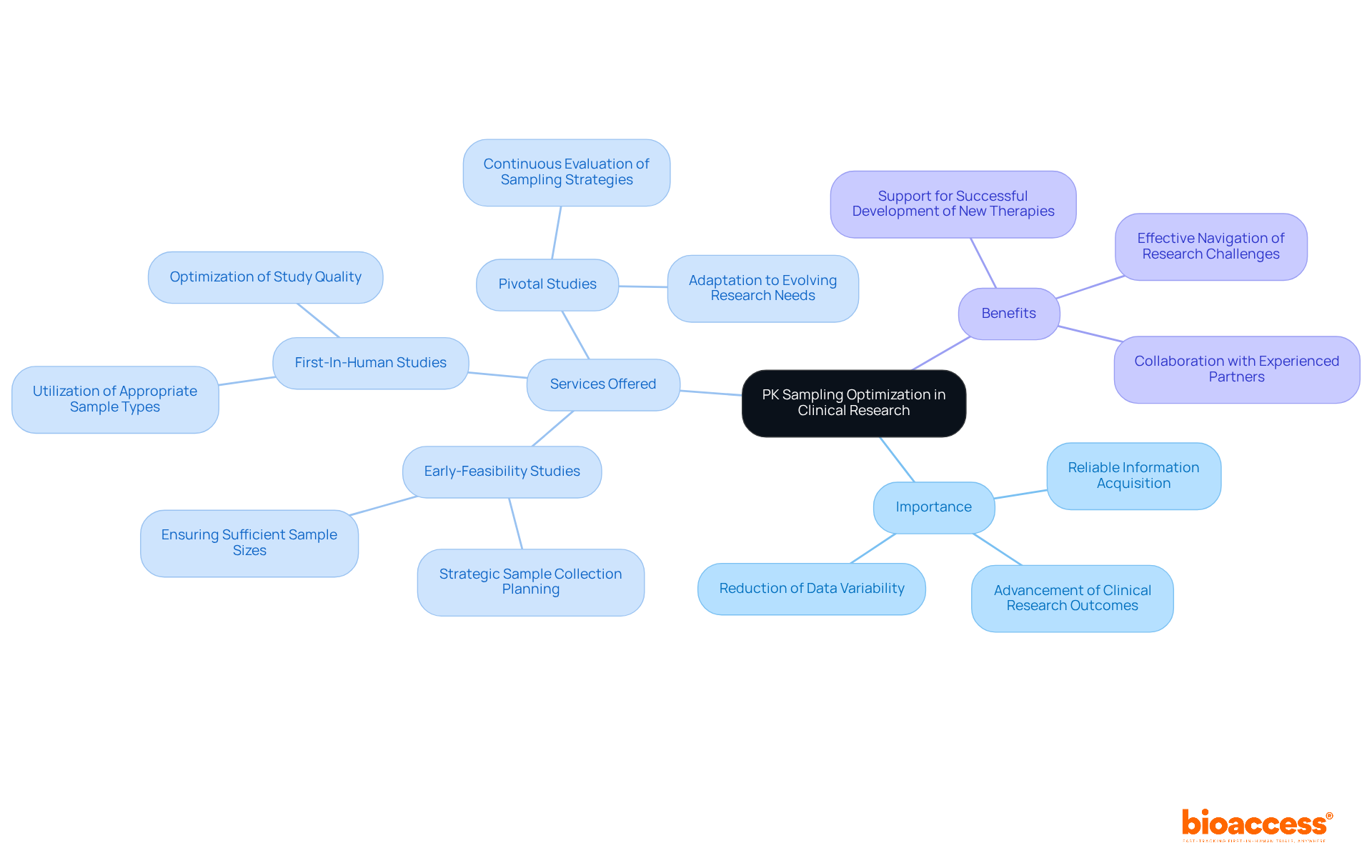
Enhancing pk sampling methods is essential for acquiring reliable information and advancing clinical research outcomes, particularly in medical device studies. With over 20 years of experience in Medtech, bioaccess stands out by offering comprehensive clinical trial management services, including:
This expertise allows clinical research directors to strategically plan sample collection times, ensure sufficient sample sizes, and utilize appropriate sample types. Such optimization not only enhances study quality but also reduces data variability, ultimately supporting the successful development of new therapies.
Continuous evaluation and refinement of pk sampling strategies, combined with bioaccess's specialized knowledge, are vital for adapting to the evolving landscape of research needs and regulatory requirements. As the Medtech field grows increasingly complex, collaboration with experienced partners like bioaccess can make a significant difference. Are you ready to elevate your clinical research efforts? By leveraging our expertise, you can navigate challenges more effectively and achieve your research goals.
The insights on pharmacokinetic (PK) sampling highlight its vital role in advancing clinical research, especially for directors navigating the complexities of drug development. By optimizing PK sampling strategies, organizations can significantly enhance data reliability, reduce variability, and ultimately support the successful introduction of innovative therapies to the market.
Key discussions throughout this article emphasize the necessity of understanding factors such as:
Moreover, the risks associated with inadequate sampling practices, particularly in pediatric populations, underscore the need for meticulous planning and strict adherence to regulatory guidelines. The integration of technology emerges as a powerful tool, streamlining processes and improving efficiency in late-phase trials.
In a landscape where timely and accurate data is paramount, prioritizing PK sampling optimization is essential for clinical research directors. By collaborating with expert partners like bioaccess® and embracing innovative solutions, organizations can not only mitigate risks but also enhance their overall research outcomes. The call to action is clear: investing in robust PK sampling strategies will pave the way for more effective drug development and ultimately benefit patients in need of new therapies.
What is bioaccess® and what role does it play in clinical research?
bioaccess® is a clinical research organization based in Latin America that provides swift and effective pharmacokinetic (PK) sampling insights. It leverages the region's cost efficiency, offering savings of over 30% compared to North America and Western Europe, and enables timely access to diverse patient pools.
How does bioaccess® benefit clinical research directors?
bioaccess® empowers clinical research directors by providing timely and relevant information on PK sampling, enhancing site activation and regulatory compliance, and ensuring that IRB/EC and MoH (INVIMA) reviews are completed within 90-120 days.
What are the advantages of conducting clinical research in Colombia?
Colombia offers a high-quality healthcare system, ranked #22 by the World Health Organization, and provides R&D tax incentives, including a 100% tax deduction for investments in science and technology, making it financially advantageous for Medtech startups.
What key factors should be considered when developing PK collection schedules?
Key factors include the drug's pharmacokinetic profile, timing of doses, and expected absorption and elimination rates. These factors should align with the research goals to ensure meaningful data collection.
Why is collaboration with pharmacologists important in PK sampling?
Collaborating with pharmacologists helps refine PK sampling schedules, enhancing the quality of the data gathered and addressing the complexities of clinical research to ensure robust outcomes.
What types of specimens are typically collected for pharmacokinetic analyses?
Common specimen types include blood and urine. Blood specimens measure drug concentrations in systemic circulation, while urine specimens provide insights into renal clearance and drug metabolism. Other types, such as saliva or tissue biopsies, may also be used depending on study objectives.
What are the unique requirements for collecting different specimen types in PK studies?
Each specimen type, such as blood, urine, saliva, or tissue biopsies, has its own specific collection and management requirements that must be evaluated to ensure the integrity of the information gathered.