


The article emphasizes the essential ISO medical standards that manufacturers must comply with for device compliance, underscoring their significance in ensuring safety, quality, and regulatory alignment. Each standard, including ISO 13485 for quality management and ISO 14971 for risk management, establishes a framework that enhances product reliability and facilitates a smoother market entry. This is particularly highlighted in the article's discussion on the benefits of compliance and the evolving regulatory landscape, which further reinforces the necessity for adherence to these standards.
The landscape of medical device manufacturing is increasingly shaped by stringent ISO standards, which serve as the backbone for ensuring product safety and regulatory compliance. As manufacturers navigate a complex web of regulations, understanding and adhering to these essential ISO medical standards becomes crucial for successful market entry and patient safety.
With impending changes and updates to these standards, manufacturers must consider: how can they effectively align their processes to not only meet compliance but also enhance the quality and efficacy of their products?
This article delves into ten vital ISO medical standards that are instrumental in guiding manufacturers through the compliance maze, offering insights into their significance and practical applications.
bioaccess® excels in expediting ISO medical adherence for medical device producers by leveraging its extensive knowledge in navigating regulatory environments across Latin America, the Balkans, and Australia. This strategic focus on early-phase clinical research empowers manufacturers to swiftly align with ISO medical standards, significantly enhancing their market entry speed. By offering comprehensive solutions, bioaccess® enables clients to effectively grasp and apply essential ISO medical requirements, ensuring their products not only meet safety and quality standards but also achieve regulatory adherence efficiently.
With over 50 pre-qualified sites activated in less than eight weeks, bioaccess® facilitates accelerated site activation, allowing for quicker patient recruitment and trial data collection. Recent trends indicate that companies emphasizing ISO medical standards are better positioned to capitalize on emerging market opportunities, reinforcing the critical role of robust quality management systems in the Medtech sector.
Furthermore, with the FDA's final rule on the Quality Management System Regulation (QMSR) set to take effect on February 2, 2026, it is imperative for manufacturers to align their practices with ISO medical standards promptly. As noted by industry leaders, adopting a proactive approach to ISO medical compliance not only facilitates smoother market entry but also enhances the overall quality and safety of products.
ISO 13485 delineates the essential requirements for an ISO medical quality management system (QMS) tailored specifically for the medical equipment sector. This standard promotes a systematic approach to process management, guaranteeing that products consistently meet both customer expectations and regulatory mandates.
Adhering to ISO medical standards like ISO 13485 not only enhances product quality but also significantly elevates customer trust, establishing it as a vital standard for manufacturers striving for success in the competitive medical products arena.
Furthermore, compliance with ISO 13485 facilitates smoother entry into international markets, aligning with various regulatory frameworks, which is crucial for producers seeking to expand their reach and influence.

ISO 14971 establishes a comprehensive framework for risk management in ISO medical equipment, empowering producers to systematically identify hazards, estimate and evaluate associated risks, and implement effective controls to mitigate these risks. This standard is essential for ensuring patient safety and meeting regulatory compliance.
By adhering to the ISO medical standard ISO 14971, manufacturers can proactively manage potential risks throughout the product lifecycle, thereby significantly enhancing the safety and effectiveness of their products. Recent statistics reveal that approximately 70% of medical equipment now complies with ISO 14971, underscoring its increasing importance in the industry.
Safety regulators emphasize that compliance with this ISO medical standard is crucial for protecting patient health, highlighting its pivotal role in the medical equipment sector. Effective risk management approaches, including frequent assessments and revisions of risk management documents, are vital for upholding regulations and ensuring continuous safety in medical product development.
As a specialist in regulatory affairs, Ana Criado underscores the significance of ISO 14971 within the Colombian context, leveraging her extensive experience with INVIMA and international firms to assist producers in navigating the complexities of compliance and risk management.
IEC 62304 delineates the essential lifecycle processes for medical equipment software, covering development, maintenance, and risk management requirements. Adhering to this standard is imperative for manufacturers, as it systematically mitigates the risk of software failures that could endanger patient safety. Notably, statistics reveal that user interface (UI) software errors led to 423 medical equipment recalls from 2012 to 2015, underscoring the critical need for compliance with IEC 62304 to enhance software reliability.
As Dr. Stephen G. Odaibo, CEO of RETINA-AI Health, asserts, 'The underlying principles of IEC 62304 are rigorous planning, thorough documentation, testing and verification of everything, and finally, traceability.' This structured approach not only fulfills regulatory requirements but also fosters effective lifecycle management of software-driven products in the Medtech sector.
With updates to IEC 62304 anticipated in 2025, including the elimination of the outdated assumption of 100% defect probability, manufacturers are urged to incorporate these guidelines into their development processes to uphold the highest standards of safety and efficacy in their products.
ISO medical 11135 delineates the essential requirements for the development, validation, and routine control of ethylene oxide (EtO) sterilization processes for medical equipment. This standard is pivotal in ensuring that healthcare products are effectively sterilized, significantly reducing the risk of infection and enhancing patient safety. Adhering to ISO 11135 transcends mere regulatory compliance; it is a fundamental aspect of quality assurance that producers must uphold to demonstrate adherence to sterilization standards and preserve product integrity.
Current adherence rates to ISO 11135 among medical equipment manufacturers reflect an increasing recognition of its importance, with numerous organizations actively integrating these standards into their quality management systems. As we approach 2025, the regulatory landscape continues to evolve, highlighted by the FDA's Ethylene Oxide Sterilization Master File Pilot Program, which aims to streamline documentation processes, foster innovation in sterilization techniques, and mitigate EtO emissions.
Experts in infection control underscore the necessity of adhering to ISO medical 11135 standards, asserting that this compliance is crucial for safeguarding patient health. Successful compliance with sterilization protocols not only ensures the safety of medical instruments but also fosters confidence in the healthcare system, ultimately leading to improved patient outcomes. As Ana Criado, a regulatory affairs expert, articulates, 'Grasping and applying ISO standards is crucial for preserving the integrity of medical equipment.' Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices, further emphasizes, 'The importance of adhering to these practices cannot be overstated.

ISO 15223-1 establishes a critical framework for the symbols utilized in medical equipment labeling, facilitating clear communication of essential safety and usage information. Adhering to this standard is paramount for producers, as it significantly enhances the clarity and effectiveness of their labels, ultimately promoting safer usage of medical equipment.
By following ISO 15223-1, producers can greatly improve interactions with healthcare professionals and patients, ensuring that vital information is conveyed accurately and efficiently. As the landscape of medical equipment regulations evolves, the emphasis on clear labeling becomes increasingly crucial, with experts underscoring that well-defined symbols not only streamline compliance but also bolster user safety.
Notably, there is currently no urgent need to modify device labels, as the updated symbols remain unstandardized, allowing producers to implement changes during regular review cycles rather than as immediate necessities. The updates to ISO 15223-1 in 2025 further reinforce this commitment to clarity, making it imperative for manufacturers to adopt effective labeling strategies that align with these standards.
Moreover, the FDA's acceptance of symbols without nearby explanatory text, provided a glossary is available, presents a practical labeling approach that simplifies adherence. This proactive strategy not only fulfills regulatory requirements but also cultivates trust and understanding among users, ultimately contributing to improved health outcomes.
ISO 11607 establishes rigorous standards for packaging systems designed for terminally sterilized medical instruments, ensuring that these products maintain sterility until their point of use. This standard encompasses essential elements such as materials selection, design concepts, and validation procedures, making compliance crucial for manufacturers committed to safeguarding the integrity of their products. By adhering to ISO 11607, producers significantly mitigate the risk of contamination, thereby enhancing both patient safety and product efficacy.
Recent statistics reveal that the global medical device packaging market is projected to expand from USD 14 billion in 2025 to USD 22.1 billion by 2035, with a compound annual growth rate (CAGR) of 6.1% during this timeframe. This growth underscores the vital role of ISO 11607 in the Medtech sector, as it offers a framework for manufacturers to fulfill international regulatory requirements and facilitate market access.
Experts in the field stress the importance of compliance with ISO 11607. For instance, Laura Court emphasizes that the standard mandates that packaging must preserve device sterility until use, thereby reinforcing the commitment to patient safety. Furthermore, the upcoming revisions to ISO 11607 in 2025 are focused on enhancing alignment with international regulatory standards, ensuring that manufacturers are equipped to address evolving challenges in medical packaging.
As manufacturers navigate the complexities of regulations, effective packaging strategies that incorporate ISO medical principles are essential. These strategies not only protect equipment during transportation and storage but also ensure readiness for safe use upon arrival at healthcare facilities. Key testing techniques for seal strength, including ASTM F88, ASTM F1140, and ASTM F2054, are critical for compliance with ISO 11607, ensuring that packaging maintains its integrity. With adherence rates consistently improving, the emphasis on ISO 11607 remains crucial in promoting the quality and safety of medical equipment within the healthcare environment.

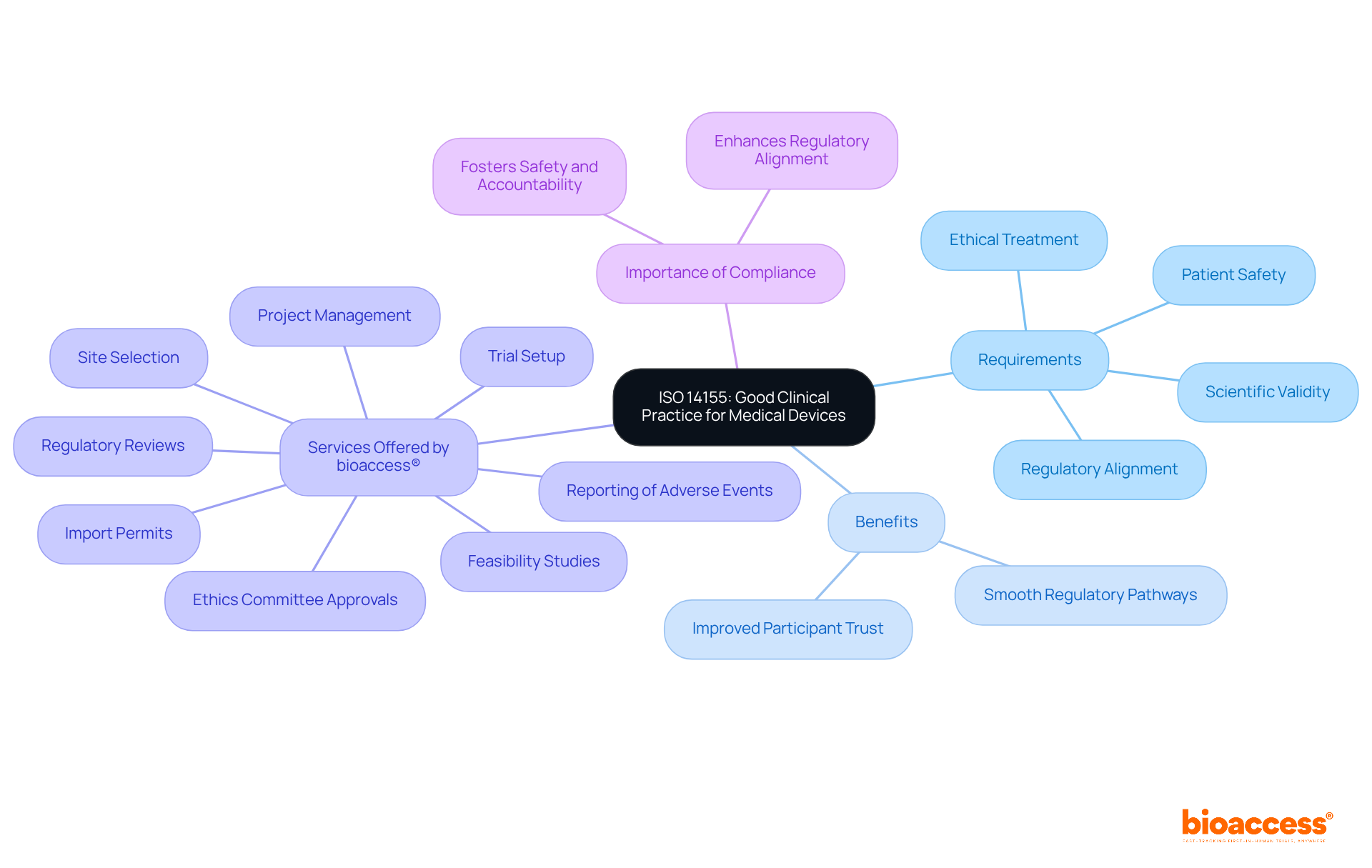
ISO medical 14155 establishes essential requirements for good clinical practice in the design, conduct, recording, and reporting of clinical investigations involving medical equipment. Adhering to this standard is vital for ensuring the ethical treatment of participants and the scientific validity of trial outcomes. Compliance with ISO medical 14155 not only demonstrates a manufacturer's commitment to high ethical standards but also enhances regulatory alignment during clinical trials. Specifically, the standard closely aligns with the Medical Devices Regulation (MDR), which mandates that clinical investigations for implantable and class III medical devices adhere to rigorous ethical oversight and data management protocols. This alignment is crucial, as it ensures that clinical investigations prioritize patient safety and data integrity.
The benefits of adhering to ISO 14155 are evident in successful clinical trials across the Medtech sector. Companies that follow these guidelines often experience smoother regulatory pathways and improved participant trust. For instance, bioaccess® provides extensive clinical trial management services, including:
Their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) positions them as a crucial partner for U.S. medical technology companies navigating the complexities of clinical investigations in Latin America.
Experts in the field underscore the significance of ISO medical standards, particularly ISO 14155, which sets the global gold standard for conducting clinical investigations. As Lucy Jung, CEO of bioaccess®, stated, 'ISO medical 14155:2020 ensures that studies are ethically sound, scientifically valid, and regulatory compliant while prioritizing patient safety and data integrity.' This standard not only facilitates adherence to regulatory requirements but also fosters a culture of safety and accountability in clinical research. As the Medical Device Coordination Group (MDCG) continues to release guidance on clinical investigations, the importance of ISO 14155 remains paramount in shaping the future of ethical clinical research.
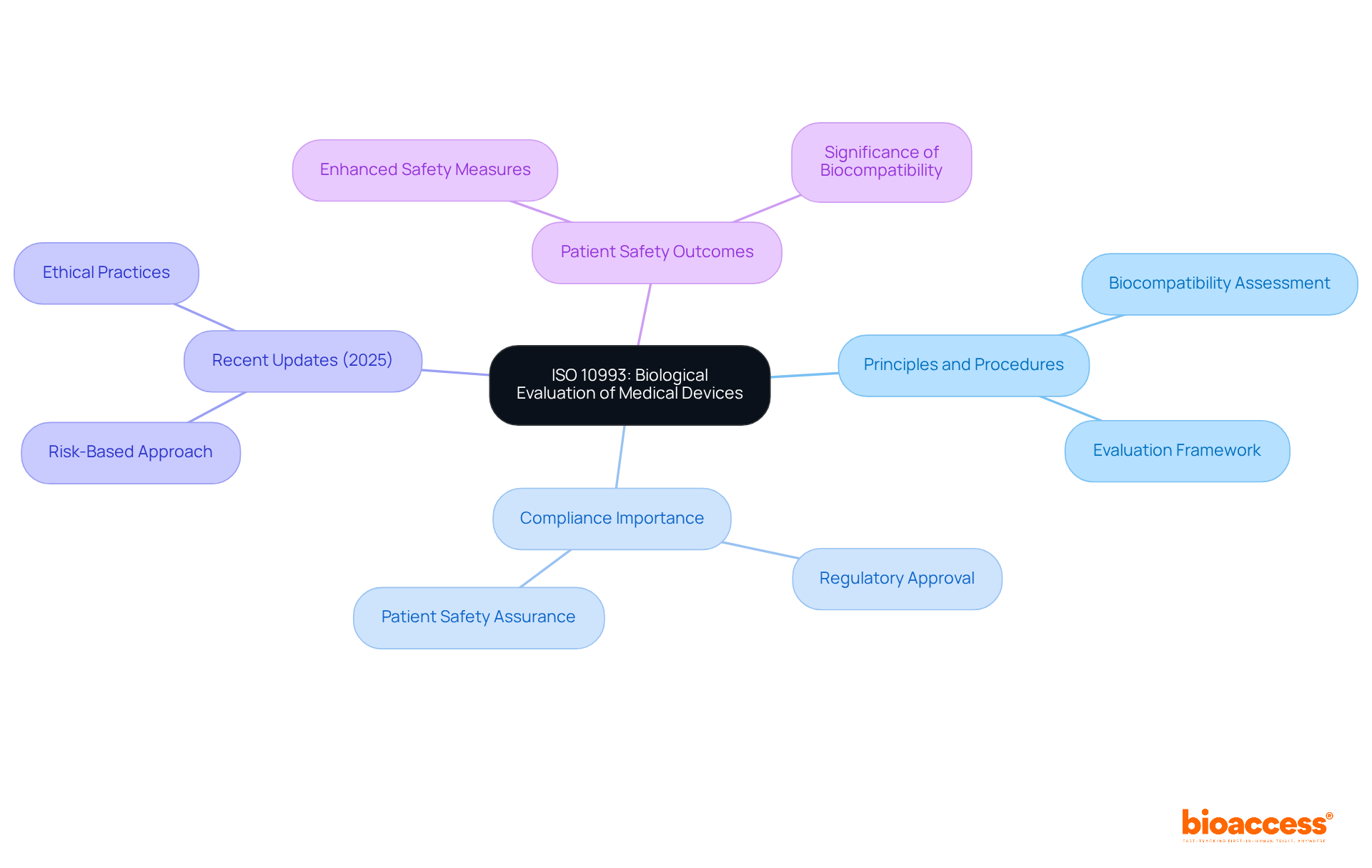
ISO medical 10993 serves as a vital framework for the biological assessment of medical instruments, ensuring their safety for patient use. This iso medical standard outlines the principles and procedures essential for evaluating biocompatibility, which is crucial for meeting regulatory requirements. Adhering to iso medical guidelines such as ISO 10993 allows manufacturers to substantiate that their products do not present undue risks to patients, thereby bolstering the safety and efficacy of their offerings.
Recent updates in 2025 emphasize a risk-based approach, allowing the use of existing data to minimize unnecessary animal testing, aligning with the industry's commitment to ethical practices. Experts in biocompatibility stress that compliance with iso medical standards, including ISO 10993, is not merely a regulatory checkbox; it is essential for safeguarding patient health and facilitating smoother regulatory approvals.
Successful biocompatibility evaluations based on ISO 10993 standards have highlighted the importance of iso medical practices, resulting in considerable enhancements in patient safety outcomes and emphasizing the significance of biocompatibility in advancing medical products.
The iso medical standard ISO 80369-1 establishes essential requirements for small-bore connectors utilized in healthcare settings, aiming to avert misconnections that could result in patient harm. It is imperative for producers to adhere to this standard, ensuring that their products are both safe and compatible with existing systems. By complying with iso medical standards such as ISO 80369-1, manufacturers not only enhance patient safety but also significantly reduce the likelihood of adverse outcomes associated with medical device usage.
Recent statistics reveal an encouraging trend: as of 2025, approximately 75% of manufacturers now meet this standard, reflecting improved compliance rates. Experts underscore the pivotal role of iso medical standards, specifically ISO 80369-1, in preventing misconnections, noting that its implementation has been correlated with a marked decrease in adverse events. A particularly alarming case involved a patient who tragically suffered a fatal air embolus due to a misconnection, highlighting the severe consequences of non-compliance.
Continuous revisions to the ISO 80369 series further underscore its significance, ensuring that it adapts to technological advancements and best practices in healthcare. Manufacturers are urged to remain informed about these revisions and proactively implement the necessary changes to bolster patient safety.

The importance of adhering to ISO medical standards cannot be overstated; these guidelines serve as a cornerstone for ensuring the safety, quality, and efficacy of medical devices. By aligning with essential ISO standards, manufacturers enhance product reliability and facilitate smoother market entry, ultimately benefiting both the healthcare sector and patient outcomes.
This article has discussed key ISO standards such as ISO 13485 for quality management, ISO 14971 for risk management, and ISO 11135 for sterilization in detail. Each standard plays a crucial role in addressing specific aspects of medical device compliance, from managing risks and ensuring product safety to maintaining rigorous quality assurance processes. The proactive adoption of these standards, especially in light of upcoming regulatory changes, positions manufacturers to navigate the complexities of the Medtech landscape effectively.
In conclusion, embracing ISO medical standards is essential for manufacturers aiming to thrive in the competitive healthcare market. By prioritizing compliance and quality, stakeholders uphold the integrity of their products and contribute to the overall safety and trust in medical devices. It is imperative for manufacturers to stay informed about these standards and actively integrate them into their practices, ensuring they are well-prepared to meet the challenges of tomorrow’s healthcare environment.
What is bioaccess® and how does it assist medical device manufacturers?
bioaccess® specializes in accelerating ISO compliance for medical device manufacturers by leveraging its expertise in regulatory environments across Latin America, the Balkans, and Australia. It helps manufacturers align with ISO medical standards quickly, enhancing their market entry speed.
How does bioaccess® facilitate site activation for clinical trials?
bioaccess® has activated over 50 pre-qualified sites in less than eight weeks, which accelerates site activation, allowing for quicker patient recruitment and trial data collection.
Why is compliance with ISO medical standards important for companies?
Companies that emphasize ISO medical standards are better positioned to capitalize on emerging market opportunities and ensure robust quality management systems, which are critical in the Medtech sector.
What is the significance of the FDA's final rule on the Quality Management System Regulation (QMSR)?
The FDA's final rule on QMSR, effective February 2, 2026, emphasizes the need for manufacturers to align their practices with ISO medical standards promptly to facilitate smoother market entry and enhance product quality and safety.
What does ISO 13485 entail for medical device manufacturers?
ISO 13485 outlines the essential requirements for a quality management system (QMS) specifically for the medical equipment sector, promoting a systematic approach to process management to ensure products meet customer expectations and regulatory mandates.
How does ISO 13485 benefit manufacturers?
Compliance with ISO 13485 enhances product quality, elevates customer trust, and facilitates smoother entry into international markets, which is crucial for manufacturers looking to expand their reach.
What is the purpose of ISO 14971 in the medical device industry?
ISO 14971 provides a framework for risk management in medical equipment, enabling manufacturers to identify hazards, evaluate risks, and implement controls to mitigate those risks, ensuring patient safety and regulatory compliance.
Why is compliance with ISO 14971 critical for manufacturers?
Compliance with ISO 14971 allows manufacturers to manage potential risks throughout the product lifecycle, significantly enhancing the safety and effectiveness of their products, which is crucial for protecting patient health.
What role does risk management play in medical product development?
Effective risk management, including frequent assessments and revisions of risk management documents, is vital for upholding regulations and ensuring continuous safety in medical product development.
How does Ana Criado contribute to compliance and risk management in the Colombian context?
Ana Criado, a specialist in regulatory affairs, leverages her extensive experience with INVIMA and international firms to assist producers in navigating the complexities of compliance and risk management in Colombia.