


This article delves into essential medical abbreviations that are pivotal for success in clinical research. It highlights ten key abbreviations, including CRO (Contract Research Organization) and IRB (Institutional Review Board), and explains their significance in enhancing research efficiency, ensuring ethical standards, and improving data management. These elements collectively support effective clinical trial processes.
Understanding these abbreviations is crucial for professionals navigating the Medtech landscape. As clinical research evolves, the role of these terms becomes increasingly vital in addressing key challenges. By familiarizing oneself with these abbreviations, researchers can streamline communication and enhance collaboration across various stakeholders.
In conclusion, the importance of these medical abbreviations cannot be overstated. They serve as the foundation for effective clinical trials, ensuring that all parties involved are aligned and informed. Moving forward, it is essential for researchers to integrate these terms into their daily practice, fostering a culture of clarity and efficiency in clinical research.
In the fast-paced realm of clinical research, effective communication stands as a pivotal factor that can determine success or failure. For professionals navigating this intricate landscape, grasping essential medical abbreviations is not just beneficial; it’s crucial. These shorthand terms streamline processes and enhance clarity, making them indispensable tools in the field. Yet, with an ever-evolving array of acronyms and their implications for patient safety and regulatory compliance, how can researchers ensure they possess the necessary knowledge for success? This article explores ten key medical abbreviations vital for clinical research, providing insights that empower professionals to navigate their roles with confidence and precision.
bioaccess®: Essential Medical Abbreviations for Clinical Research Success
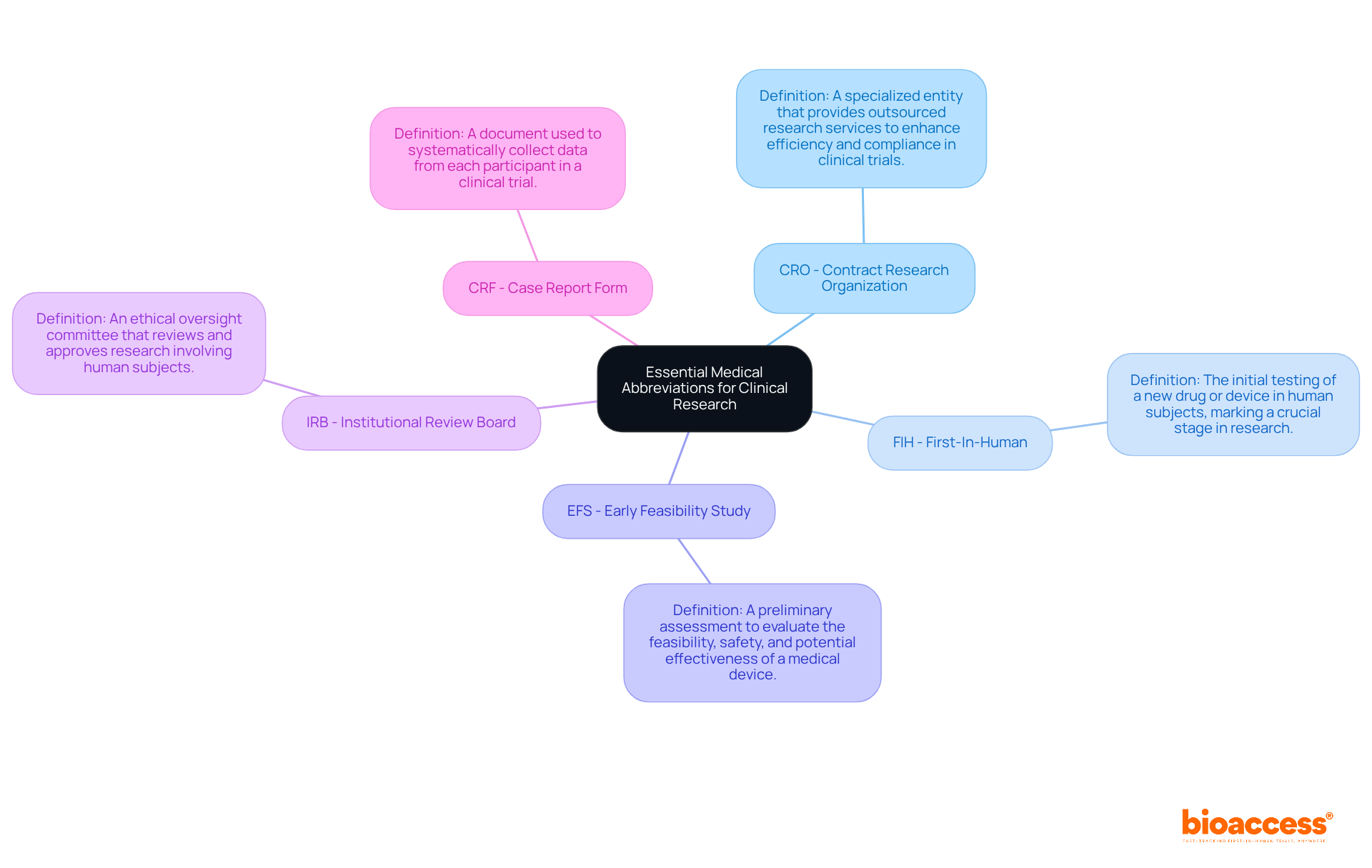
CRO - Contract Research Organization: A specialized entity like bioaccess® that provides outsourced research services to the pharmaceutical, biotechnology, and medical device sectors. This enhances efficiency and compliance in trial processes, making it a vital partner in clinical research.
FIH - First-In-Human: This term signifies the initial testing of a new drug or device in human subjects. It marks a crucial stage in research that CROs facilitate to ensure safety and efficacy, underscoring the importance of rigorous oversight during this phase.
EFS - Early Feasibility Study: A preliminary assessment aimed at evaluating the feasibility, safety, and potential effectiveness of a medical device in human participants. This step is crucial for informed decision-making in product development, allowing stakeholders to gauge the viability of their innovations.
IRB - Institutional Review Board: An ethical oversight committee responsible for reviewing and approving research involving human subjects. This ensures that all studies meet established ethical standards, protecting participants and maintaining the integrity of the research process.
CRF - Case Report Form: A vital document utilized to systematically collect data from each participant in a clinical trial. It serves as a key tool for data integrity and regulatory compliance, ensuring that the information gathered is both accurate and reliable.
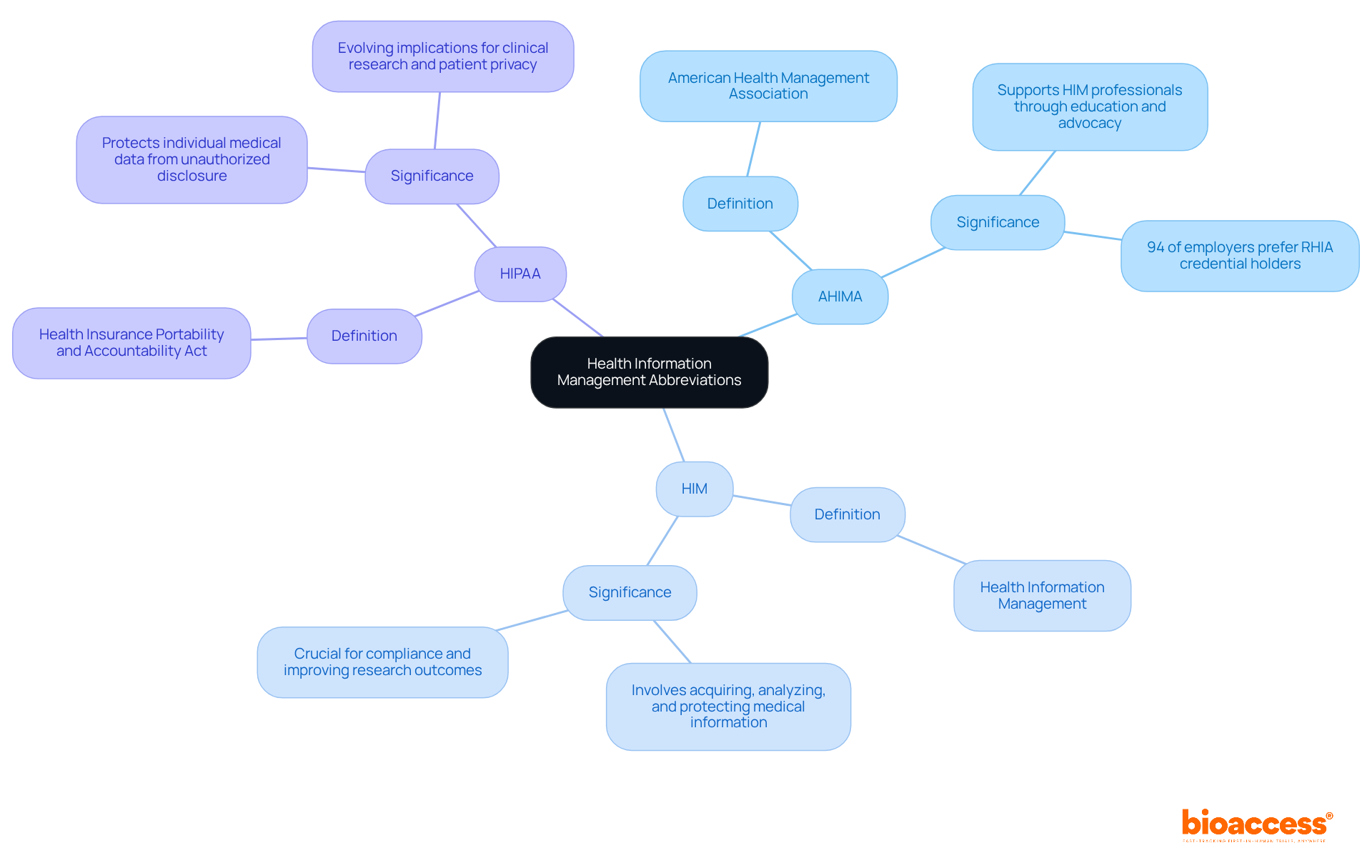
AHIMA - American Health Management Association: This professional association is dedicated to health data management (HIM) professionals. AHIMA plays a pivotal role in supporting HIM professionals through education, advocacy, and resources that significantly enhance the quality of healthcare data management. Notably, a survey reveals that 94 percent of employers prefer hiring candidates with the RHIA credential, underscoring the critical importance of professional qualifications in this field.
HIM - Health Information Management: This practice involves acquiring, analyzing, and protecting both digital and traditional medical information, which is essential for delivering quality patient care. Effective HIM practices are crucial for ensuring compliance with healthcare regulations and improving research outcomes, particularly in the realm of comprehensive trial management services. These services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all vital for successful research in the medical sector.
HIPAA - Health Insurance Portability and Accountability Act: This federal statute protects individual medical data from unauthorized disclosure. As we approach 2025, the implications of HIPAA are evolving, influencing how clinical research is conducted and ensuring patient privacy is maintained across all healthcare settings. Experts like William Schweinle III emphasize that understanding these regulations is essential for HIM professionals to navigate the complexities of health management, especially as international collaboration and innovation in Medtech drive advancements in global health.
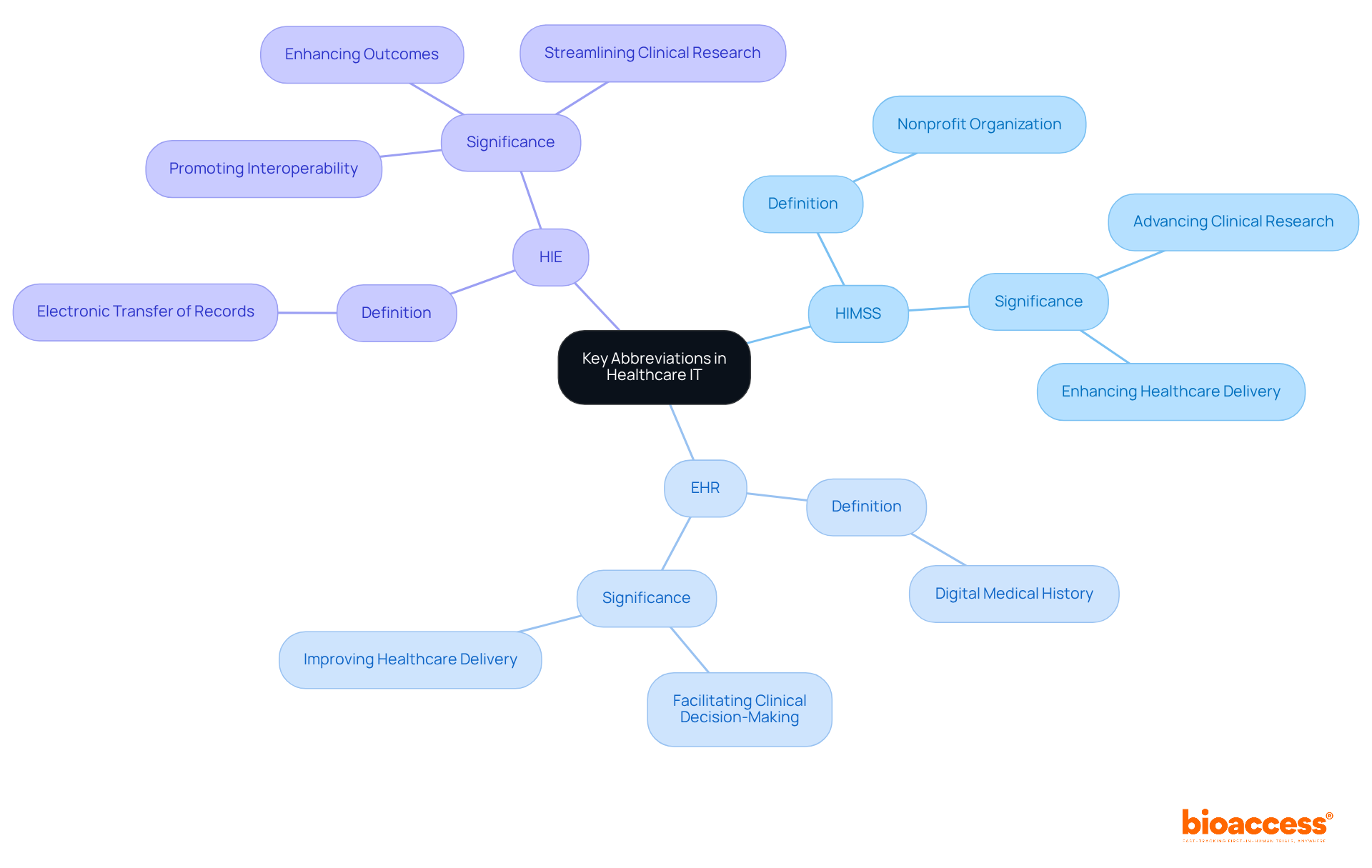
HIMSS - The Healthcare Information and Management Systems Society is a nonprofit organization dedicated to enhancing healthcare delivery through the effective use of technology. This commitment is crucial for advancing clinical research and improving patient outcomes.
EHR - An Electronic Health Record serves as a digital representation of an individual's medical history. It provides a comprehensive overview of care, facilitating better clinical decision-making and ultimately leading to improved healthcare delivery.
HIE - Health Information Exchange refers to the electronic transfer of medical records among various healthcare organizations. This process promotes interoperability, which is essential for enhancing outcomes for individuals and streamlining clinical research efforts.
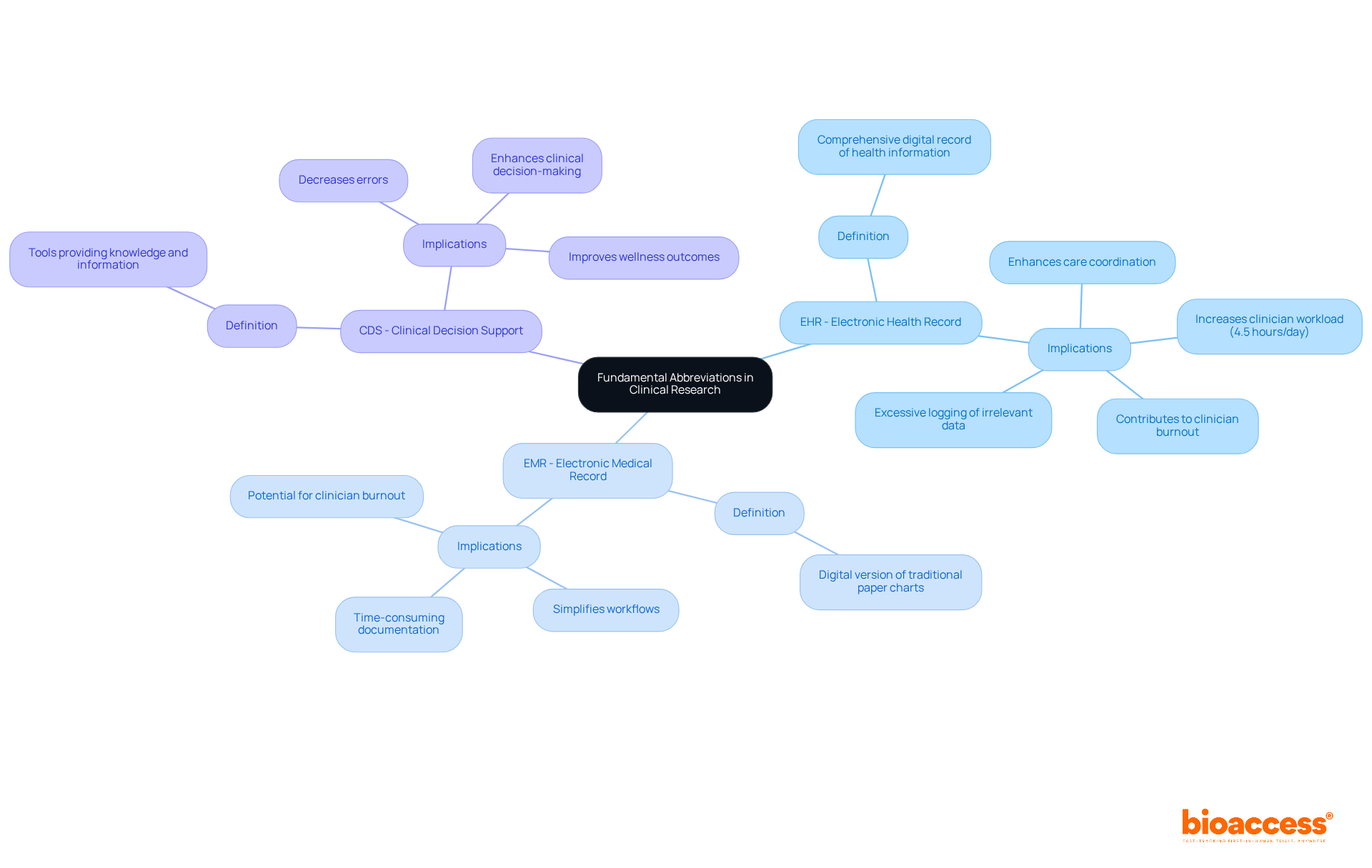
EHR - Electronic Health Record: This comprehensive digital record of individual health information facilitates sharing across various healthcare settings, enhancing care coordination and improving outcomes. Studies indicate that physicians spend an estimated 4.5 hours daily on EHRs, significantly impacting the time available for direct patient care (JAMA). Such extensive use can lead to clinician burnout. As noted by James Kahn, MD, MPH, excessive logging of medically irrelevant data negatively impacts focus and outcomes.
EMR - Electronic Medical Record: A digital version of traditional paper charts used in a clinician's office, EMRs primarily focus on diagnosis and treatment. While they simplify workflows in medicine, the considerable time needed for documentation can also contribute to clinician burnout.
CDS - Clinical Decision Support: These tools are designed to provide clinicians with knowledge and individual-specific information, thereby enhancing care. Efficient CDS systems can improve clinical decision-making and decrease errors, ultimately resulting in better wellness outcomes.
Actionable Tip: To alleviate the challenges associated with EHR usage, consider implementing training programs that focus on optimizing EHR workflows and enhancing user experience. Such initiatives can help reduce clinician burnout and improve care for individuals.
RHIA - Registered Health Information Administrator: This certification is vital for information management professionals, highlighting their expertise in managing patient data. RHIA-certified individuals are recognized for their comprehensive understanding of clinical, administrative, technical, ethical, and legal standards, making them invaluable assets in healthcare organizations. Notably, the Bureau of Labor Statistics projects a 20% increase in employment for medical and wellness services managers from 2016 to 2026, underscoring the growing demand for professionals in this field. As UIC Clinical Assistant Professor Gideon Ramirez emphasizes, employers often prefer candidates with RHIA certification due to their proven proficiency in medical data management. However, achieving this certification necessitates a CAHIIM-accredited degree, reflecting its importance and the dedication required. For those aiming to boost their career prospects, pursuing a CAHIIM-accredited program is a strategic move.
CAHIIM - Commission on Accreditation for Health Informatics and Management Education: This organization is responsible for accrediting medical data management programs, ensuring they adhere to stringent quality standards. CAHIIM accreditation is essential for educational institutions, as it not only validates the quality of their programs but also fosters continuous improvement, thereby enhancing the employability of graduates in the competitive healthcare job market.
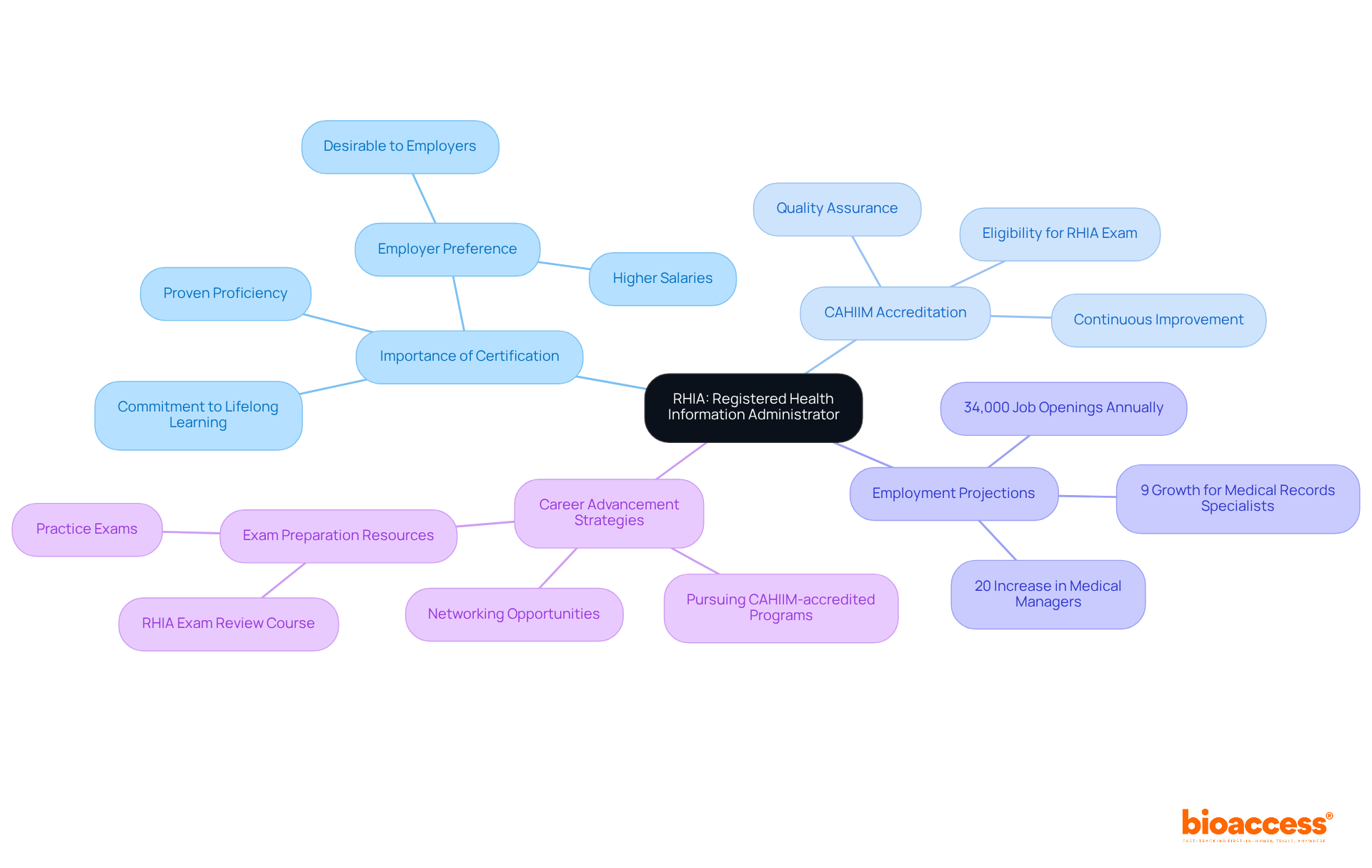
RHIT - Registered Health Information Technician: This certification signifies professionals who are pivotal in managing patient records, ensuring their accuracy, security, and compliance with regulations. With employment for healthcare data technologists projected to rise by 15% from 2024 to 2034, according to the U.S. Bureau of Labor Statistics, the demand for precise data management is clear. This growth reflects the increasing reliance on advanced medical technologies and regulatory requirements. Furthermore, the median yearly salary for medical data specialists was $67,310 in May 2024, underscoring the critical role this position plays in the healthcare system.
HIT - Health Information Technology: This encompasses the tools and systems used to manage and exchange medical information efficiently. As healthcare continues to embrace digital solutions, HIT professionals are essential in facilitating seamless data flow, enhancing care quality, and supporting clinical research initiatives. The integration of advanced technologies, such as electronic medical records (EHRs), is revolutionizing the utilization of medical data, leading to improved outcomes for patients and operational efficiencies. However, professionals in this field encounter challenges, including evolving technology, regulatory compliance, and data security risks, necessitating ongoing education and adaptability. Thus, certifications like RHIT are vital for career advancement and meeting the demands of this ever-evolving landscape.
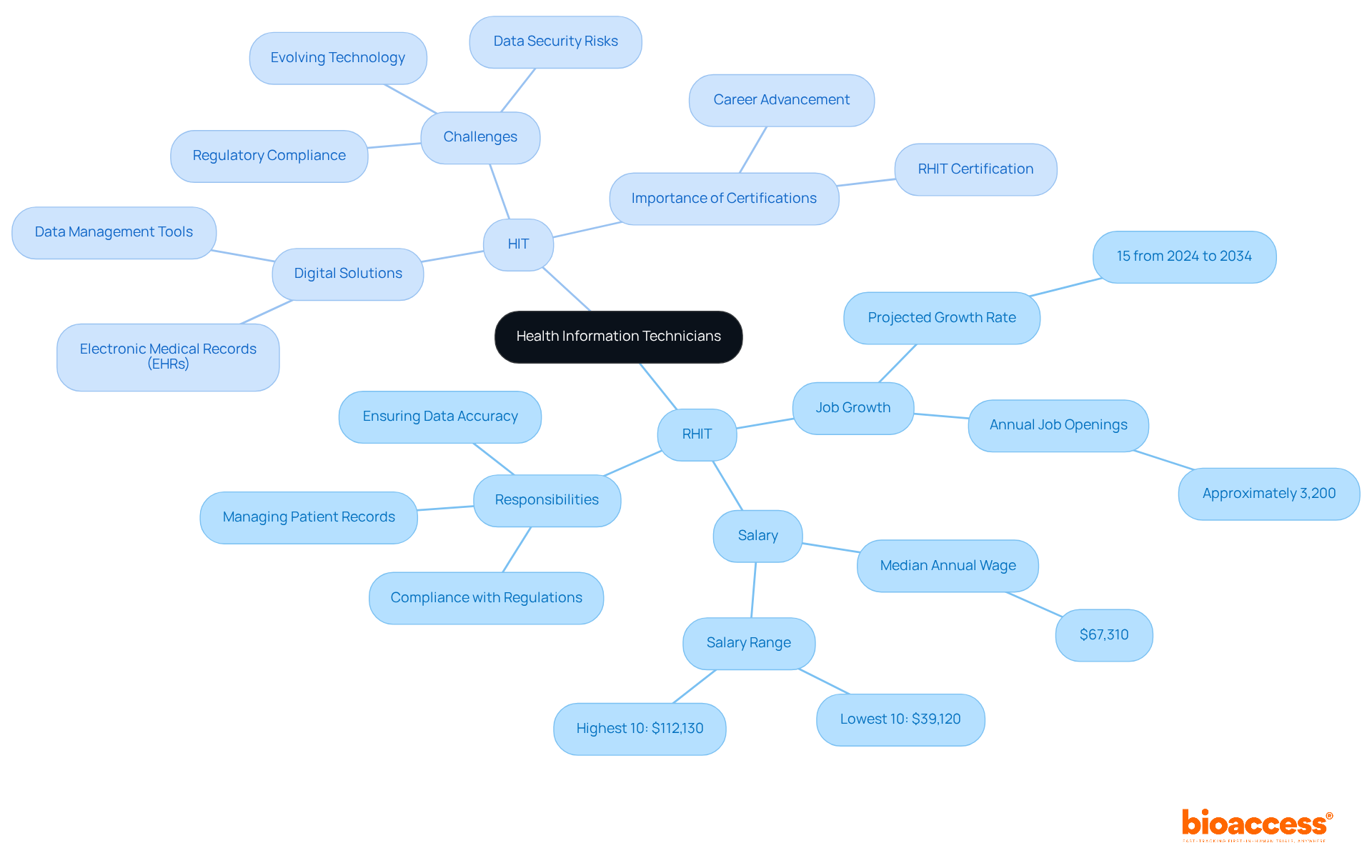
HIT - Health Information Technology: Health Information Technology encompasses a range of technologies designed for the storage, sharing, and analysis of health information. This technology is pivotal in medical research, as it streamlines data management processes, enhances the accuracy of health records, and fosters improved communication among healthcare providers. The integration of HIT has demonstrated its ability to elevate patient outcomes and operational efficiency, establishing it as a cornerstone of modern healthcare systems. In the realm of bioaccess, HIT is instrumental in managing trial data, ensuring compliance with regulatory requirements, and boosting the overall efficiency of trial processes.
HISP - Health Information Service Provider: A Health Information Service Provider is an organization dedicated to facilitating the exchange of health information among various stakeholders within the healthcare ecosystem. By providing essential infrastructure and services, HISPs enable secure data sharing, which is vital for effective medical research. Their significance is growing as healthcare organizations strive for interoperability and improved patient data management, ensuring that critical information is readily accessible when needed. In the context of bioaccess, HISPs play a crucial role in ensuring that data related to clinical trials is shared securely and efficiently, thereby supporting the overall success of advancements in medical devices.

CAHIIM - The Commission on Accreditation for Health Informatics and Data Management plays a pivotal role in ensuring that medical data management programs meet rigorous educational standards. This accreditation is vital for maintaining the quality and integrity of medical education, which is essential for cultivating skilled professionals in the field. Recognized as the gold standard for HIM programs, CAHIIM accreditation guarantees that graduates are thoroughly prepared to tackle the demands of the healthcare industry.
HIM - Health Information Management encompasses the systematic oversight of individual medical information, ensuring its accuracy, accessibility, and security. HIM professionals are indispensable to clinical research, as they manage the lifecycle of medical data, ensuring compliance with regulatory standards while enhancing the quality of patient care through effective data governance. As Dr. Steven J. Stack points out, the challenges posed by electronic medical records (EHRs) significantly impact healthcare provision, underscoring the importance of proficient HIM experts in navigating these complexities. Moreover, with over 95% of hospitals utilizing certified EHR technology as of 2017, the role of HIM in managing this data has never been more critical.
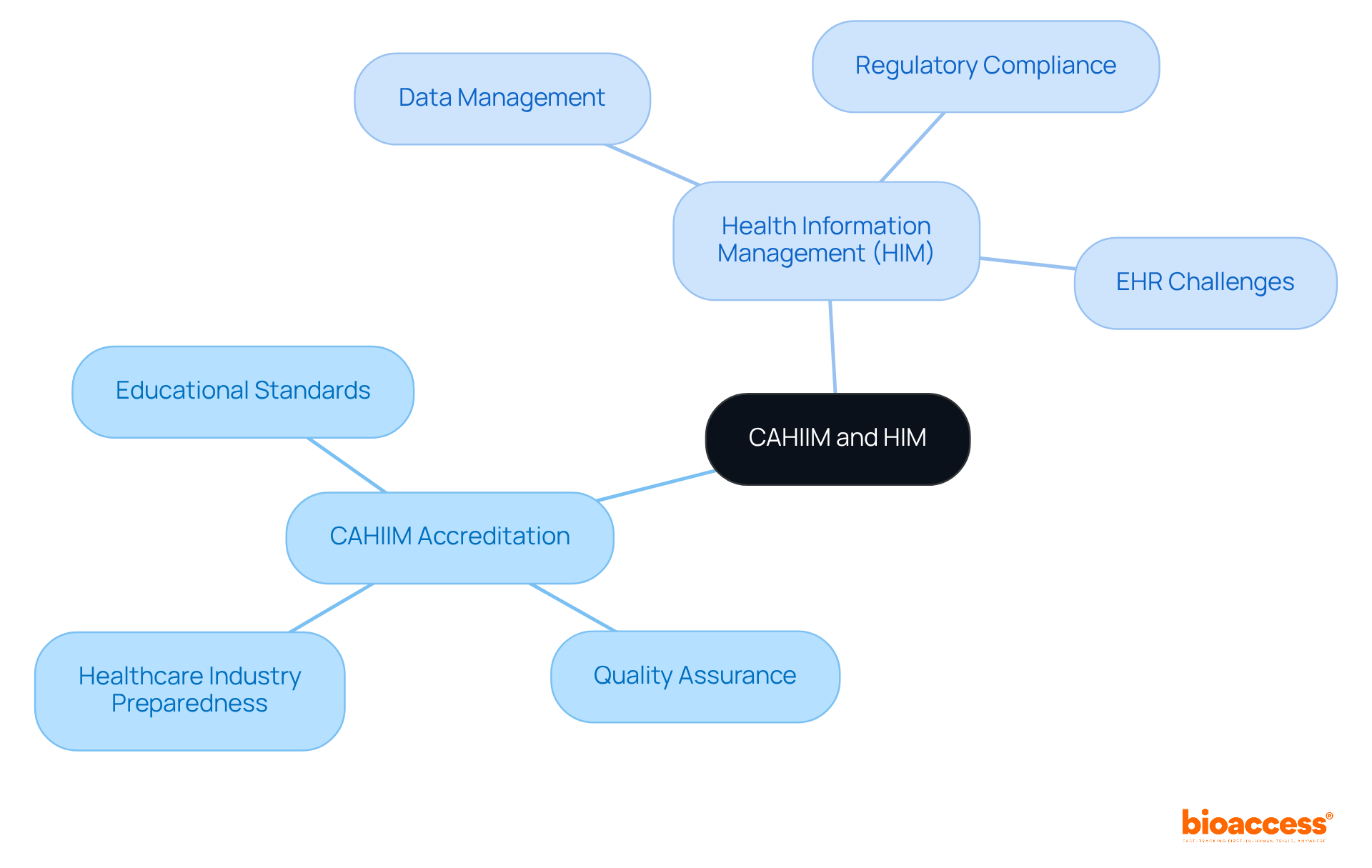
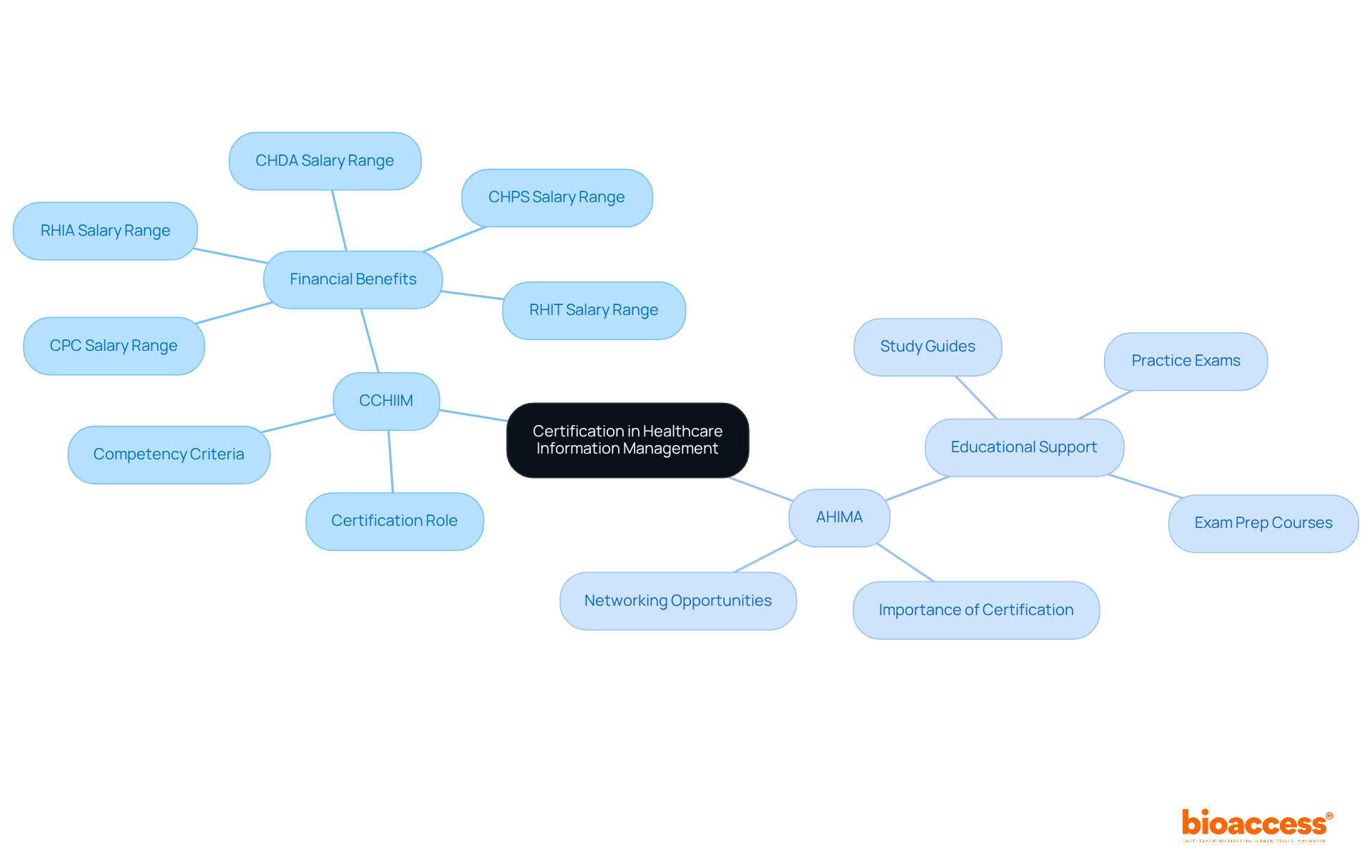
CCHIIM - The Commission on Certification for Informatics and Information Management plays a pivotal role in certifying informatics professionals. By establishing stringent criteria for competency, CCHIIM ensures that certified individuals possess the essential skills needed to navigate the complexities of medical data management effectively. This certification not only validates expertise but also significantly enhances career prospects in a rapidly evolving field. For instance, RHIA-certified professionals can earn between $75,000 and $100,000 per year, underscoring the financial advantages of obtaining certification.
AHIMA - The American Health Data Management Association serves as a vital resource for healthcare professionals. AHIMA offers a wealth of support, including educational materials, certification preparation resources, and networking opportunities. As the healthcare landscape continues to expand, AHIMA's resources become increasingly essential for professionals aiming to advance their careers and stay informed about industry developments. Their commitment to education and certification emphasizes the importance of maintaining high standards in information management. As AHIMA states, "Healthcare Information Management certifications are more important than ever in 2025," reflecting the growing demand for HIM professionals due to evolving regulatory requirements.
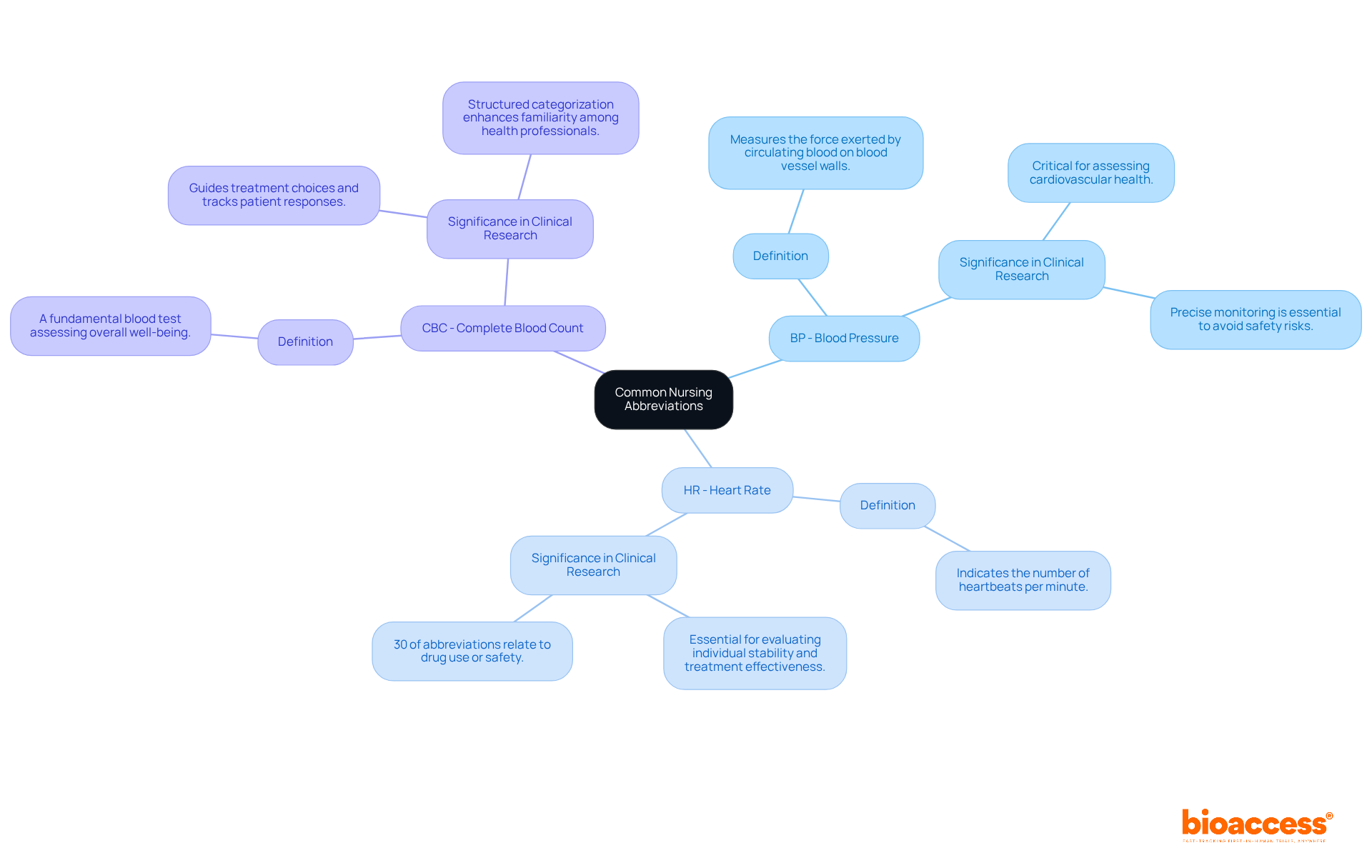
BP - Blood Pressure: This vital sign measures the force exerted by circulating blood on the walls of blood vessels, providing critical insights into cardiovascular health and potential complications in medical studies. Precise monitoring of BP is crucial; miscommunication about its abbreviation can lead to significant safety risks for individuals. The feasibility studies and compliance reviews conducted by bioaccess ensure that such vital signs are monitored accurately, enhancing the reliability of trial outcomes.
HR - Heart Rate: Indicating the number of heartbeats each minute, HR is an essential measure of overall health and physiological response, frequently observed during research studies to evaluate individual stability and treatment effectiveness. Understanding HR is vital, especially since 30% of the 300 commonly used medical abbreviations relate to drug use or safety, underscoring the need for precise communication in clinical settings. The project management services provided by bioaccess facilitate efficient monitoring and reporting of HR, contributing to improved safety for individuals and the integrity of studies.
CBC - Complete Blood Count: A fundamental blood test that assesses overall well-being and identifies various conditions, including anemia and infection. The results of CBC tests can significantly influence research outcomes by guiding treatment choices and tracking patient responses. The structured categorization of abbreviations, including CBC, is expected to enhance familiarity among health professionals, promoting safer and more efficient clinical communications. By ensuring thorough trial setup, feasibility studies, and compliance reviews, bioaccess supports the accurate interpretation of CBC results, ultimately benefiting the healthcare system and local economies.
Understanding essential medical abbreviations is crucial for success in clinical research. These abbreviations streamline communication among healthcare professionals and enhance the efficiency and accuracy of research processes. Familiarity with terms like CRO, FIH, and IRB is vital for anyone involved in clinical trials, ensuring that key protocols and ethical standards are upheld.
The article provides a comprehensive overview of critical abbreviations across various domains, including clinical research, health information management, and healthcare technology. Each abbreviation serves a specific purpose, from facilitating the initial testing of new therapies to ensuring the integrity of patient data. Organizations like AHIMA and CAHIIM play a significant role in promoting high standards and certifications, underscoring the importance of these abbreviations in maintaining quality healthcare services.
As the healthcare landscape continues to evolve, staying informed about essential medical abbreviations becomes increasingly important. Professionals in the field should prioritize mastering these terms to enhance their communication and effectiveness in clinical research. Embracing ongoing education and certification will not only improve individual career prospects but also contribute to the overall advancement of healthcare practices.
What is a Contract Research Organization (CRO)?
A Contract Research Organization (CRO) is a specialized entity, such as bioaccess®, that provides outsourced research services to the pharmaceutical, biotechnology, and medical device sectors, enhancing efficiency and compliance in trial processes.
What does First-In-Human (FIH) mean?
First-In-Human (FIH) refers to the initial testing of a new drug or device in human subjects, marking a crucial stage in research that CROs facilitate to ensure safety and efficacy.
What is an Early Feasibility Study (EFS)?
An Early Feasibility Study (EFS) is a preliminary assessment aimed at evaluating the feasibility, safety, and potential effectiveness of a medical device in human participants, crucial for informed decision-making in product development.
What role does an Institutional Review Board (IRB) play in clinical research?
An Institutional Review Board (IRB) is an ethical oversight committee responsible for reviewing and approving research involving human subjects to ensure that studies meet established ethical standards and protect participants.
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is a vital document used to systematically collect data from each participant in a clinical trial, serving as a key tool for data integrity and regulatory compliance.
What does the American Health Management Association (AHIMA) do?
The American Health Management Association (AHIMA) is a professional association dedicated to health data management (HIM) professionals, supporting them through education, advocacy, and resources to enhance healthcare data management quality.
What is Health Information Management (HIM)?
Health Information Management (HIM) involves acquiring, analyzing, and protecting both digital and traditional medical information, which is essential for delivering quality patient care and ensuring compliance with healthcare regulations.
What is the Health Insurance Portability and Accountability Act (HIPAA)?
The Health Insurance Portability and Accountability Act (HIPAA) is a federal statute that protects individual medical data from unauthorized disclosure, influencing how clinical research is conducted and ensuring patient privacy.
What is the Healthcare Information and Management Systems Society (HIMSS)?
The Healthcare Information and Management Systems Society (HIMSS) is a nonprofit organization focused on enhancing healthcare delivery through the effective use of technology.
What is an Electronic Health Record (EHR)?
An Electronic Health Record (EHR) is a digital representation of an individual's medical history, providing a comprehensive overview of care to facilitate better clinical decision-making.
What does Health Information Exchange (HIE) refer to?
Health Information Exchange (HIE) refers to the electronic transfer of medical records among various healthcare organizations, promoting interoperability essential for enhancing outcomes and streamlining clinical research efforts.