


The primary focus of this article is to delineate the essential skills that every manager in clinical operations must possess to effectively oversee and enhance clinical studies. It underscores that skills such as:
are vital for optimizing operational processes and achieving successful research outcomes. Supported by case studies and industry statistics, the article illustrates the significant impact these skills have on efficiency and effectiveness within clinical trials.
In the complex realm of clinical operations, where stakes are elevated and timelines are constrained, the role of a manager is indispensable. With over 80% of clinical studies encountering delays due to recruitment challenges, the demand for proficient management skills has reached a critical juncture. This article explores the ten essential skills that every manager in clinical operations must possess to navigate obstacles, enhance efficiency, and ultimately drive successful outcomes in medical research. What key competencies can transform the management of clinical trials from a formidable task into a streamlined process?
bioaccess® excels in accelerating operational processes for Medtech, Biopharma, and Radiopharma innovators by harnessing critical management skills. These abilities are essential for improving the pace and standard of medical studies, facilitating ethical approvals within 4-6 weeks, and achieving enrollment rates that are 50% quicker than conventional markets.
In 2025, the demand for such agility is underscored by the fact that over 80% of clinical studies encounter delays due to recruitment challenges, potentially costing sponsors between $600,000 and $8 million daily.
Efficient management by the manager clinical operations not only optimizes procedures but also fosters collaboration among various groups to ensure that tests meet their objectives effectively.
Case studies illustrate that organizations employing robust management practices have witnessed significant improvements in timelines and participant engagement.
As industry leaders emphasize, the manager clinical operations plays a vital role in the integration of management capabilities to expedite healthcare processes, ultimately leading to enhanced patient outcomes and increased market competitiveness.
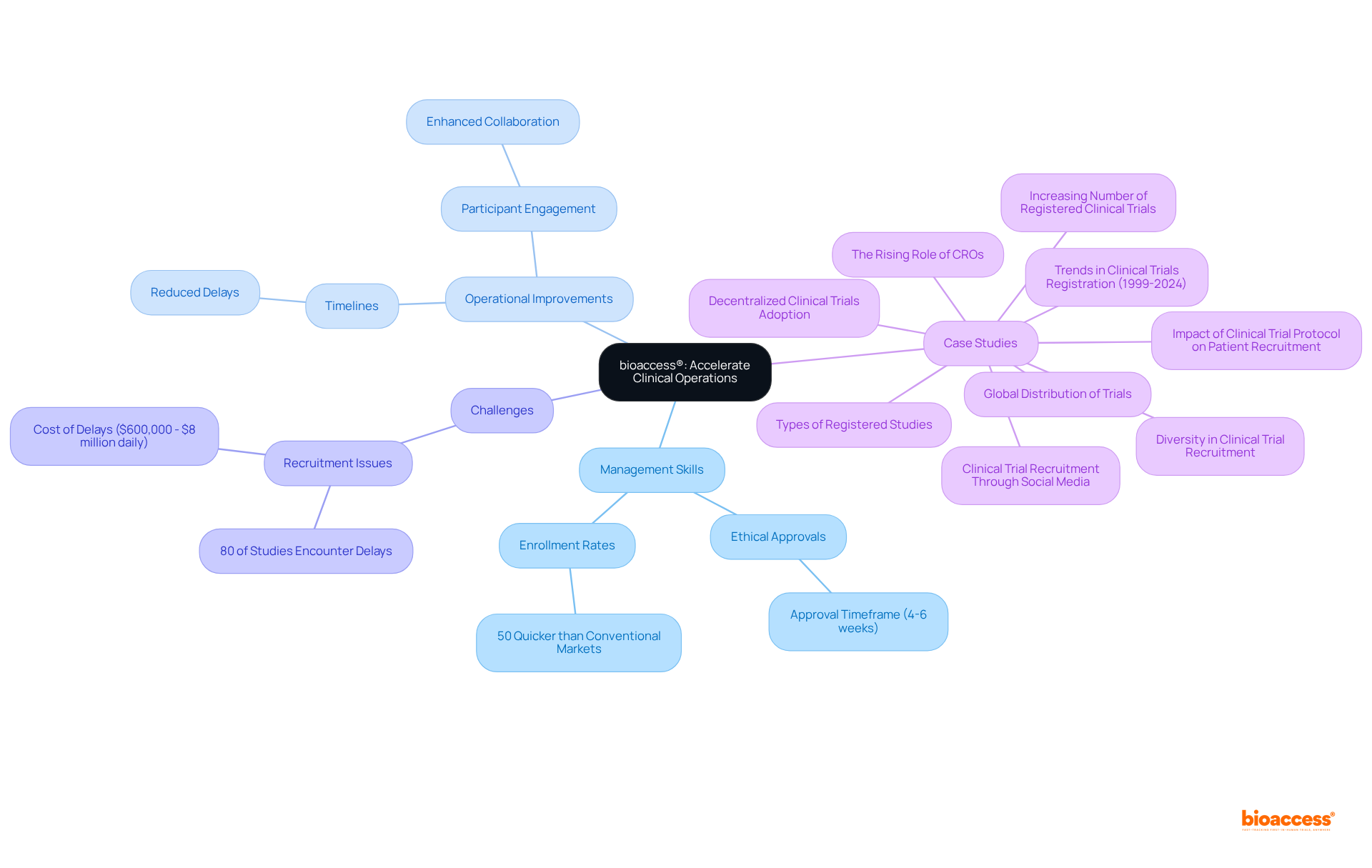
Achieving regulatory compliance is essential for navigating the complex landscape of local and international laws governing medical studies. Manager clinical operations play a pivotal role in ensuring that all studies comply with Good Clinical Practice (GCP) guidelines and other regulatory standards. This responsibility includes:
By prioritizing regulatory adherence, the manager clinical operations ensures that organizations not only mitigate risks but also enhance the credibility of their research efforts.
Statistics indicate that organizations utilizing regulatory analytics can address compliance issues 76% faster than those relying on traditional methods. Additionally, healthcare organizations that adopt automated compliance processes save an average of $3.7 million annually, highlighting the significant financial advantages of effective compliance strategies.
Case studies provide compelling evidence of successful compliance initiatives, such as Memorial Health System, which achieved a remarkable 78% reduction in false-positive compliance alerts and realized $4.2 million in annual savings through the implementation of integrated regulatory analytics. These examples underscore the tangible benefits of adhering to GCP guidelines and emphasize the necessity for continuous improvement in compliance practices.
Current trends reveal a shift towards real-time analytics capabilities, which offer substantial value for regulatory compliance. By embracing these advanced strategies, managers of clinical operations in healthcare can ensure that their teams not only meet but exceed compliance standards, ultimately leading to more successful research studies.
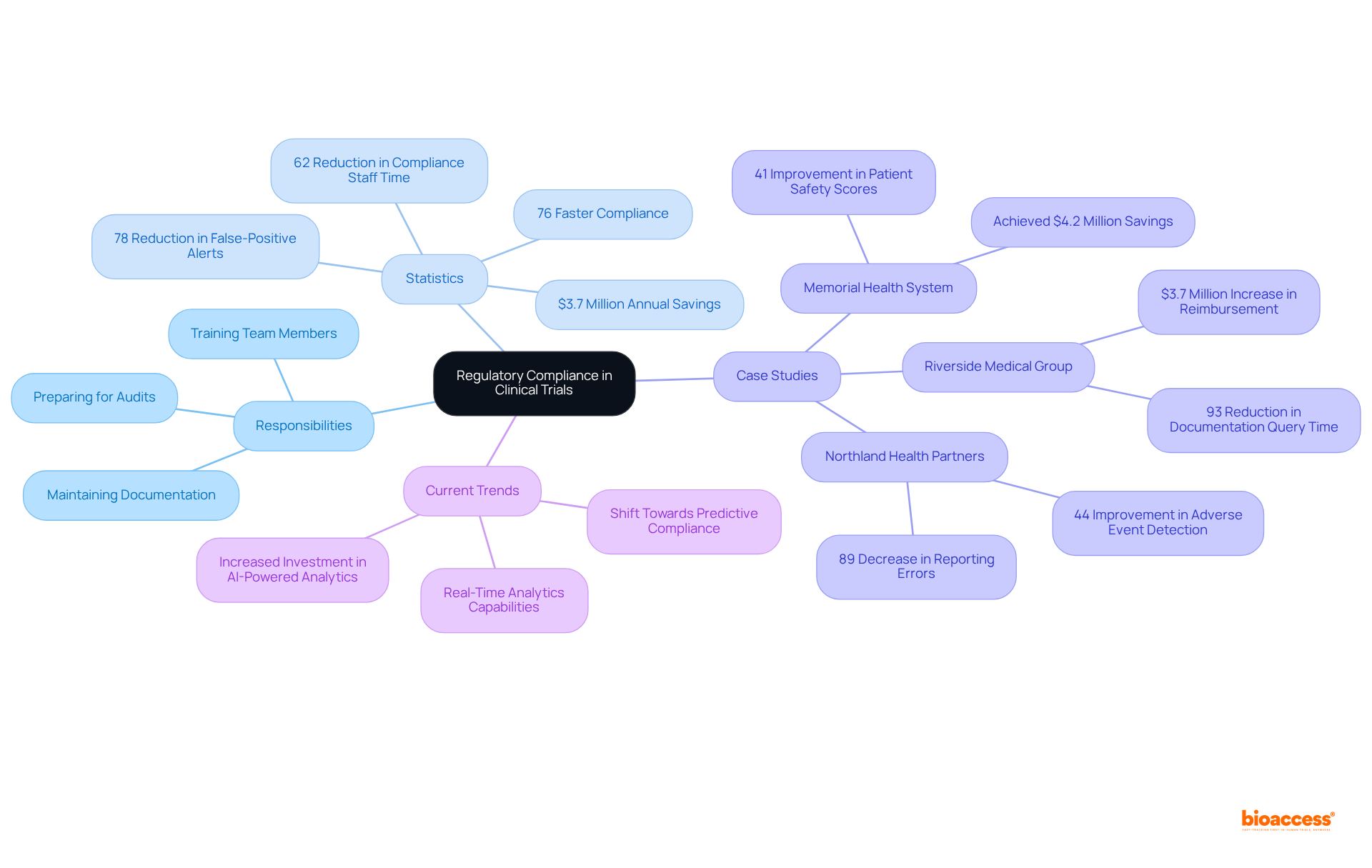
Leadership abilities are crucial for the manager of clinical operations, who guides their teams through the challenges of research studies. Effective leaders cultivate a collaborative environment, establishing clear expectations that align team efforts and enhance resource utilization. They provide constructive feedback and empower team members by recognizing their contributions, fostering a sense of ownership and accountability.
Research indicates that organizations with leaders who prioritize psychological safety see a 40% reduction in preventable errors, underscoring the importance of a supportive atmosphere. Moreover, leaders who participate in regular feedback loops are twice as nimble in addressing challenges, which is essential in the fast-paced research environment.
By investing in professional growth and promoting ongoing education, the manager of clinical operations can significantly enhance team unity and drive successful execution. Leaders trained in change management improve initiative success rates by 38%, while those who champion continuous learning drive 41% higher innovation output.
Case studies show that transformational leadership styles are positively associated with improved performance in studies, illustrating that effective leadership not only motivates teams but also directly influences outcomes. As Tacy Byham highlights, it is crucial for organizations to restore confidence and trust in their leadership, which is essential for nurturing a productive healthcare environment.
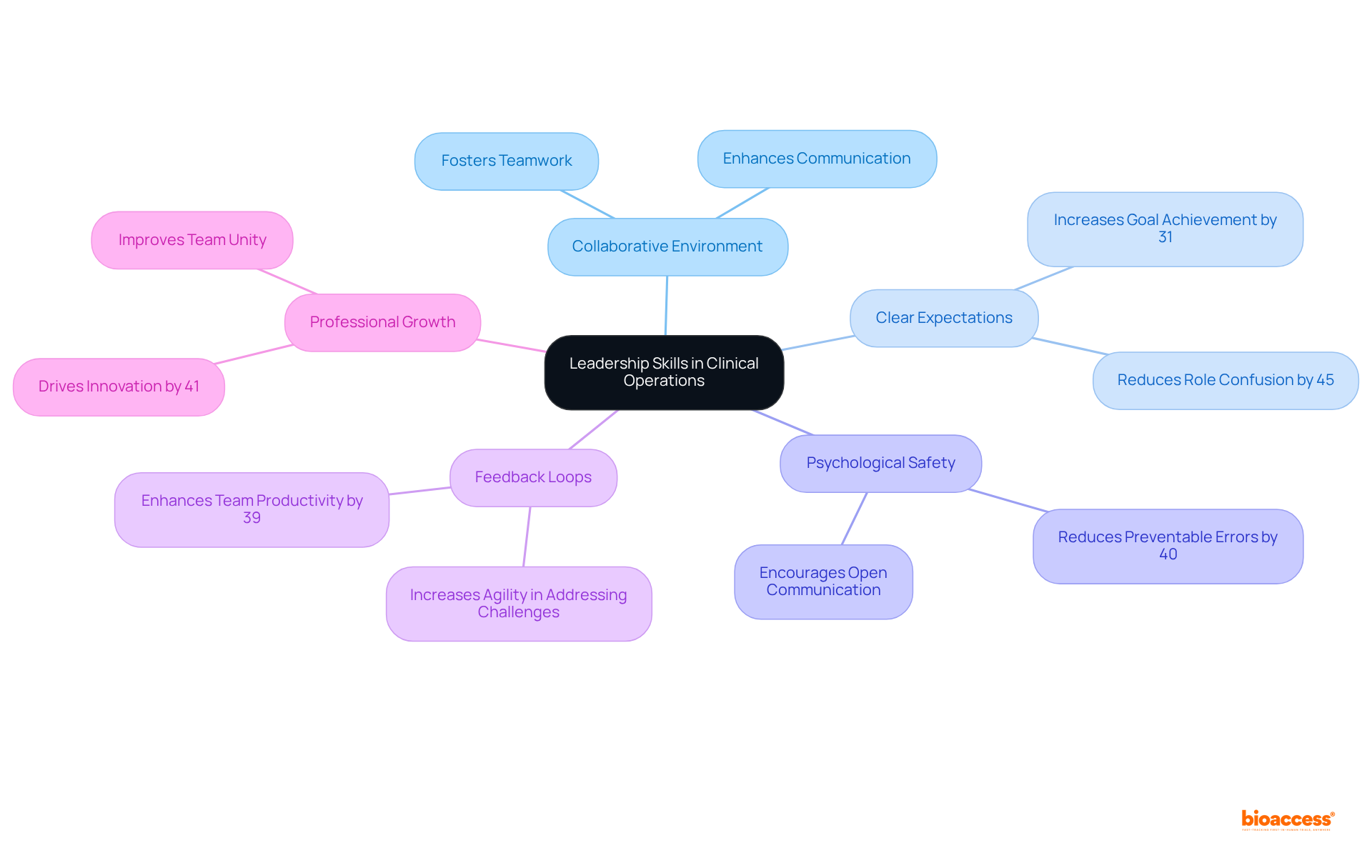
Efficient project management in clinical operations, led by a manager clinical operations, is essential for the careful planning, execution, and monitoring of clinical study activities. Managers must develop comprehensive project plans that detail timelines, resource allocation, and risk management strategies. The incorporation of project management tools and methodologies, such as Agile and Six Sigma, significantly enhances efficiency and adaptability in project management. Agile methodologies, for instance, demonstrate a failure rate of only 9%, compared to 29% for traditional Waterfall approaches, underscoring their effectiveness in achieving project goals.
By maintaining a clear emphasis on project goals and outcomes, supervisors can ensure that tests progress seamlessly and achieve important milestones. Successful case studies illustrate that organizations employing robust project management practices experience 28 times less financial waste and achieve a 92% success rate in meeting project goals. Furthermore, the shift towards hybrid methodologies that combine traditional and Agile approaches is increasingly prevalent, allowing for greater flexibility and responsiveness to changing project dynamics.
Incorporating expert insights, it is evident that effective project planning not only aligns with organizational goals but also fosters stakeholder engagement and enhances overall project outcomes. As the demand for qualified project leaders continues to rise, particularly in the realm of intricate medical studies, the manager clinical operations must focus on ongoing education and the utilization of sophisticated project management applications, which will be crucial for success in this evolving environment.
Effective communication serves as the cornerstone of successful healthcare operations. The manager clinical operations is tasked with facilitating open dialogue among team members, sponsors, and regulatory bodies to ensure alignment and transparency. This involves conducting regular meetings, maintaining clear documentation, and utilizing collaborative tools. By fostering a culture of communication, the manager clinical operations can enhance collaboration, swiftly address issues, and greatly improve process efficiency.
Studies show that effective communication can result in a 50% quicker enrollment rate compared to conventional markets, highlighting its significance in research success. Moreover, the average expense incurred for each day of delay ranges from $600,000 to $8 million, emphasizing the financial consequences of inadequate communication. A case study revealed that hospitals implementing modern communication platforms saw a marked increase in patient safety and a reduction in medical errors, demonstrating the tangible benefits of improved communication strategies.
Furthermore, specialist suggestions highlight the necessity for continuous development in communication abilities for healthcare teams. This training not only promotes teamwork but also improves the overall quality of patient care, ultimately resulting in better research outcomes. Notably, 27% of medical malpractice cases are attributed to communication failures, reinforcing the critical nature of effective communication in preventing adverse outcomes. As the environment of medical research changes, especially with the emergence of decentralized studies, the capacity to communicate efficiently will be essential for the manager clinical operations to manage the intricacies of contemporary medical activities.
Data evaluation in healthcare activities is essential for deriving valuable insights from study data. Managers must possess expertise in utilizing statistical tools and software to analyze data trends, assess effectiveness, and identify areas for improvement. By harnessing the power of data analytics, the manager clinical operations in healthcare can make informed decisions that enhance study design, optimize resource allocation, and ultimately improve patient outcomes.
Rigorous statistical analysis not only supports the evaluation of treatment safety and effectiveness but also plays a crucial role in understanding patient characteristics and quality of life enhancements. Effective communication of statistical results, including clear visualizations and context, is vital for disseminating findings and ensuring compliance with regulatory standards.
As Mercedes Ovejero Bruna emphasizes, "trial statisticians must be involved from the beginning of protocol development according to ICH guidelines," highlighting the importance of aligning statistical analysis with trial objectives. This strategic approach to data analysis is revolutionizing medical research, enabling quicker ethical approvals in 4-6 weeks and enrollment that is 50% faster than traditional markets.
With bioaccess®'s FDA-ready data, medical processes can achieve substantial cost reductions of $25K per patient, effectively addressing recruitment challenges faced by medtech and biopharma startups.
Managers in clinical operations must cultivate robust problem-solving skills to adeptly navigate the complexities of study management. This involves the early identification of potential issues, a thorough analysis of their root causes, and the formulation of actionable solutions. A proactive problem-solving culture within teams is vital, transforming challenges into opportunities for improvement.
For instance, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier hub for medical research in Latin America, with the support of Colombia's Minister of Health. This initiative not only enriches the research landscape but also exemplifies how innovative strategies can enhance participant recruitment and retention rates, achieving a reported 50% reduction in recruitment time and 95% retention rates.
The COVID-19 pandemic accelerated the acceptance of decentralized research studies, further demonstrating how such approaches can enhance participant diversity and expedite enrollment, recognizing that 70% of the population lives far from academic medical facilities. By proactively addressing issues, the manager of clinical operations can mitigate disruptions and sustain study momentum, ultimately leading to a reduction of the estimated 40% of overall budgets allocated for patient recruitment, which totals approximately $1.89 billion.
Additionally, it is crucial to acknowledge that around 80% of medical studies are delayed or terminated due to recruitment challenges, which underscores the necessity for effective problem-solving by a manager in clinical operations. Industry leaders stress the importance of a structured problem-solving approach, with Allison Dunn highlighting that strong problem-solving skills enable leaders to make informed decisions and navigate obstacles effectively.
Successful case studies illustrate that involving statisticians early in the data management process can streamline data collection, ensuring that only critical data points are gathered, thereby improving the quality of the final database used for analysis. This strategic focus on problem-solving not only enhances operational efficiency but also contributes significantly to the overall success of medical studies.
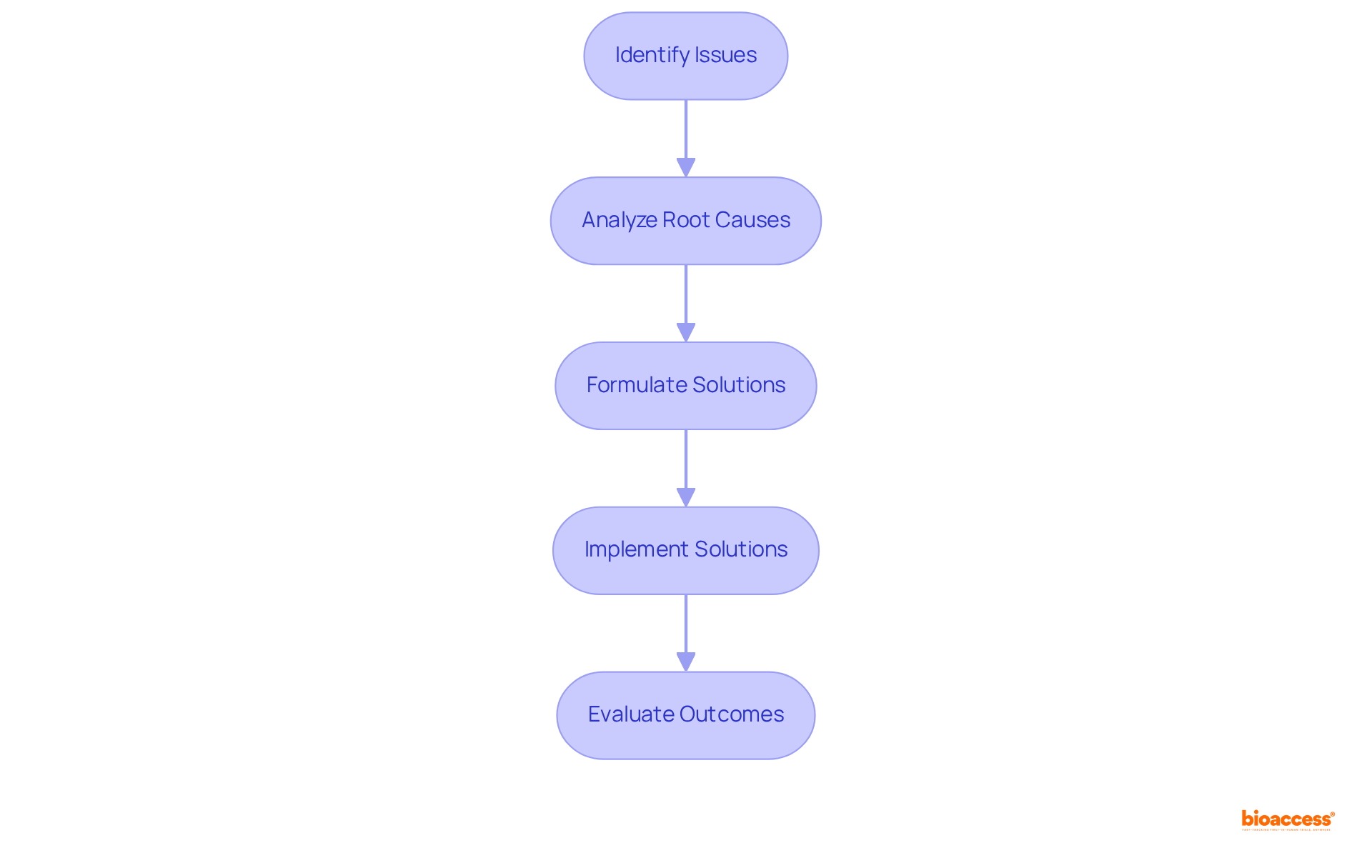
In the fast-paced realm of medical research, adaptability emerges as an essential skill for operations supervisors. These professionals must be prepared to recalibrate their strategies in response to evolving regulations, technological innovations, and fluctuating market demands. By cultivating a culture of adaptability within their teams, managers can enhance operational agility, which is crucial for achieving successful experimentation outcomes.
Comprehensive clinical trial management services offered by bioaccess—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—play a vital role in this process. For example, adaptive study designs allow for real-time modifications based on accumulating data, significantly enhancing efficiency and reducing expenses.
The BATTLE study, which utilized a Bayesian adaptive randomization strategy, exemplifies how such flexibility can lead to improved patient outcomes by personalizing treatment based on genetic information. Furthermore, insights from healthcare administrators highlight the importance of embracing change; they emphasize that thriving in a dynamic environment requires not only strategic vision but also a commitment to ongoing education and teamwork.
As the landscape of medical studies continues to evolve, those who prioritize flexibility will be better positioned to tackle challenges and seize opportunities, ultimately fostering innovation in the sector and contributing to local economic development through job creation and enhanced healthcare.
Financial expertise is paramount for the manager clinical operations tasked with overseeing budgets and ensuring effective resource distribution in clinical research. This critical responsibility involves:
Efficient budget oversight is essential, especially considering that the typical expense of phase 1 studies can reach $4 million, while phase 2 and phase 3 studies average $13 million and $20 million, respectively. By maintaining a strong emphasis on financial oversight, supervisors can ensure that experiments are conducted sustainably, utilizing resources effectively to support research objectives.
Successful strategies include:
For instance, organizations that utilize machine learning for healthcare cost forecasting can enhance their financial planning, leading to more precise budget allocations and reduced variances. Ultimately, the financial decisions made by the manager clinical operations profoundly impact study outcomes, influencing not only the efficiency of resource utilization but also the overall success of research initiatives in medicine.
In the evolving landscape of medical research, technological expertise is paramount for manager clinical operations. Knowledge of tools such as Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) systems is vital for optimizing study processes and enhancing data precision. The effective utilization of these technologies can significantly elevate operational efficiency, facilitating real-time data access and informed decision-making.
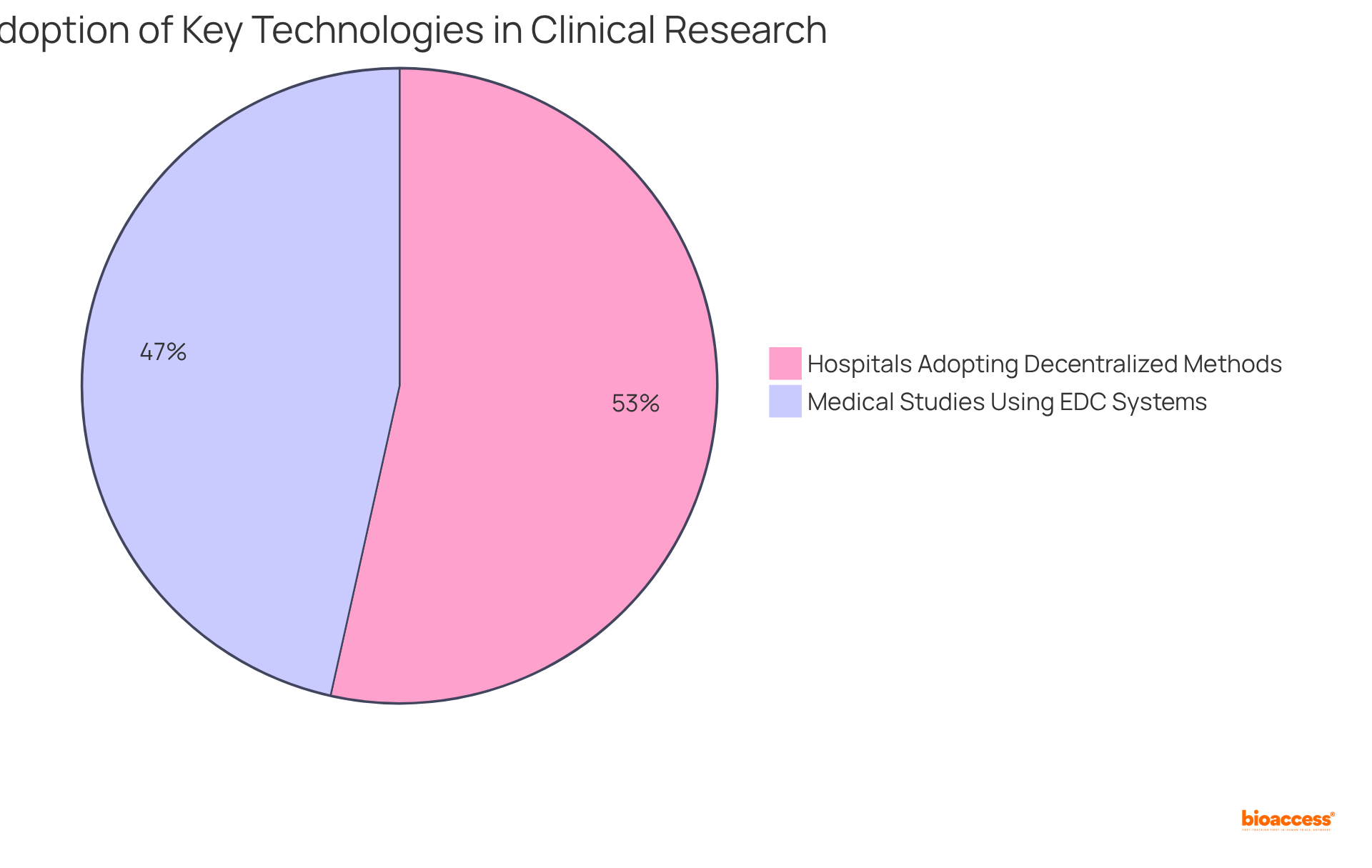
Notably, approximately 80% of medical studies now employ EDC systems, marking a shift towards digital data management that bolsters study integrity and efficiency. Furthermore, the integration of advanced technologies like AI and data analytics is transforming research methodologies, enabling faster patient recruitment and improved study outcomes.
As highlighted by industry specialists, the ability to effectively leverage these tools is not merely advantageous but essential for success in healthcare initiatives. The growing trend of decentralized medical studies, with 92% of hospitals adopting such approaches, underscores the necessity for administrators to be adept in utilizing technology to enhance accessibility and efficiency in research.
By prioritizing technological proficiency, the manager of clinical operations can adeptly navigate the complexities of modern trials and significantly contribute to the advancement of medical research.
The role of a manager in clinical operations is pivotal in ensuring the success and efficiency of medical studies. Mastering essential skills such as:
Empowers these managers to navigate the complexities of clinical trials and drive positive outcomes. Each skill uniquely enhances operational processes, improves patient engagement, and ultimately accelerates the pace of research.
Key insights throughout the article highlight how effective management practices can:
The integration of advanced technologies and data analytics not only improves study integrity but also streamlines processes, leading to significant cost savings. Furthermore, the emphasis on leadership and communication fosters a supportive team environment, vital for achieving project goals and ensuring compliance with stringent regulatory standards.
As the landscape of clinical research continues to evolve, the importance of these management skills cannot be overstated. Embracing these competencies will enhance the efficiency of clinical operations and contribute to the overall advancement of medical research. Stakeholders in the healthcare industry are encouraged to prioritize the development of these essential skills within their teams to remain competitive and responsive to the dynamic challenges of clinical trials. Investing in these capabilities today will pave the way for improved patient outcomes and a more agile research environment in the future.
What is bioaccess® and how does it benefit clinical operations?
bioaccess® accelerates operational processes for Medtech, Biopharma, and Radiopharma innovators by utilizing essential management skills, improving the pace and standard of medical studies, facilitating ethical approvals within 4-6 weeks, and achieving enrollment rates that are 50% quicker than conventional markets.
What are the challenges faced in clinical studies regarding recruitment?
Over 80% of clinical studies encounter delays due to recruitment challenges, which can potentially cost sponsors between $600,000 and $8 million daily.
What role does the manager of clinical operations play in improving clinical studies?
The manager of clinical operations optimizes procedures, fosters collaboration among various groups, and ensures that tests meet their objectives effectively, leading to enhanced patient outcomes and increased market competitiveness.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is essential for navigating local and international laws governing medical studies and ensures that all studies adhere to Good Clinical Practice (GCP) guidelines, mitigating risks and enhancing research credibility.
What responsibilities does the manager of clinical operations have regarding regulatory compliance?
Responsibilities include preparing for audits, maintaining accurate documentation, and ensuring that all team members are thoroughly trained in compliance protocols.
How do organizations benefit from automated compliance processes?
Healthcare organizations that adopt automated compliance processes can save an average of $3.7 million annually and address compliance issues 76% faster than those relying on traditional methods.
What are current trends in regulatory compliance for clinical operations?
There is a shift towards real-time analytics capabilities, which provide substantial value for regulatory compliance, helping managers ensure their teams exceed compliance standards.
What leadership skills are crucial for the manager of clinical operations?
Effective leadership skills include cultivating a collaborative environment, establishing clear expectations, providing constructive feedback, and empowering team members to foster ownership and accountability.
How does psychological safety impact clinical operations?
Organizations with leaders who prioritize psychological safety experience a 40% reduction in preventable errors, highlighting the importance of a supportive atmosphere in research environments.
What is the impact of continuous learning and professional growth on clinical operations teams?
Investing in professional growth and promoting ongoing education enhances team unity, drives successful execution, and improves initiative success rates by 38% and innovation output by 41%.
How does transformational leadership influence clinical study performance?
Transformational leadership styles are positively associated with improved performance in studies, motivating teams and directly influencing outcomes.