


The essential skills for a clinical trial research coordinator are paramount in the realm of clinical research. These include:
Each of these skills plays a critical role in ensuring compliance, enhancing team effectiveness, fostering participant engagement, and navigating the complexities inherent in clinical research. Ultimately, they contribute significantly to successful study outcomes and advancements in medical science. It is essential for professionals in this field to cultivate these competencies to drive progress and innovation.
Navigating the intricate world of clinical trials demands a diverse skill set that transcends basic research knowledge. At the forefront of this dynamic field are clinical trial research coordinators (CRCs), whose expertise plays a crucial role in the success and integrity of medical studies. This article explores ten essential skills that CRCs must master, ranging from regulatory compliance to effective patient recruitment strategies. By honing these competencies, CRCs can significantly enhance research outcomes.
As the landscape of clinical research continues to evolve, what challenges will CRCs encounter in refining these skills, and how can they adapt to ensure the success of their trials?
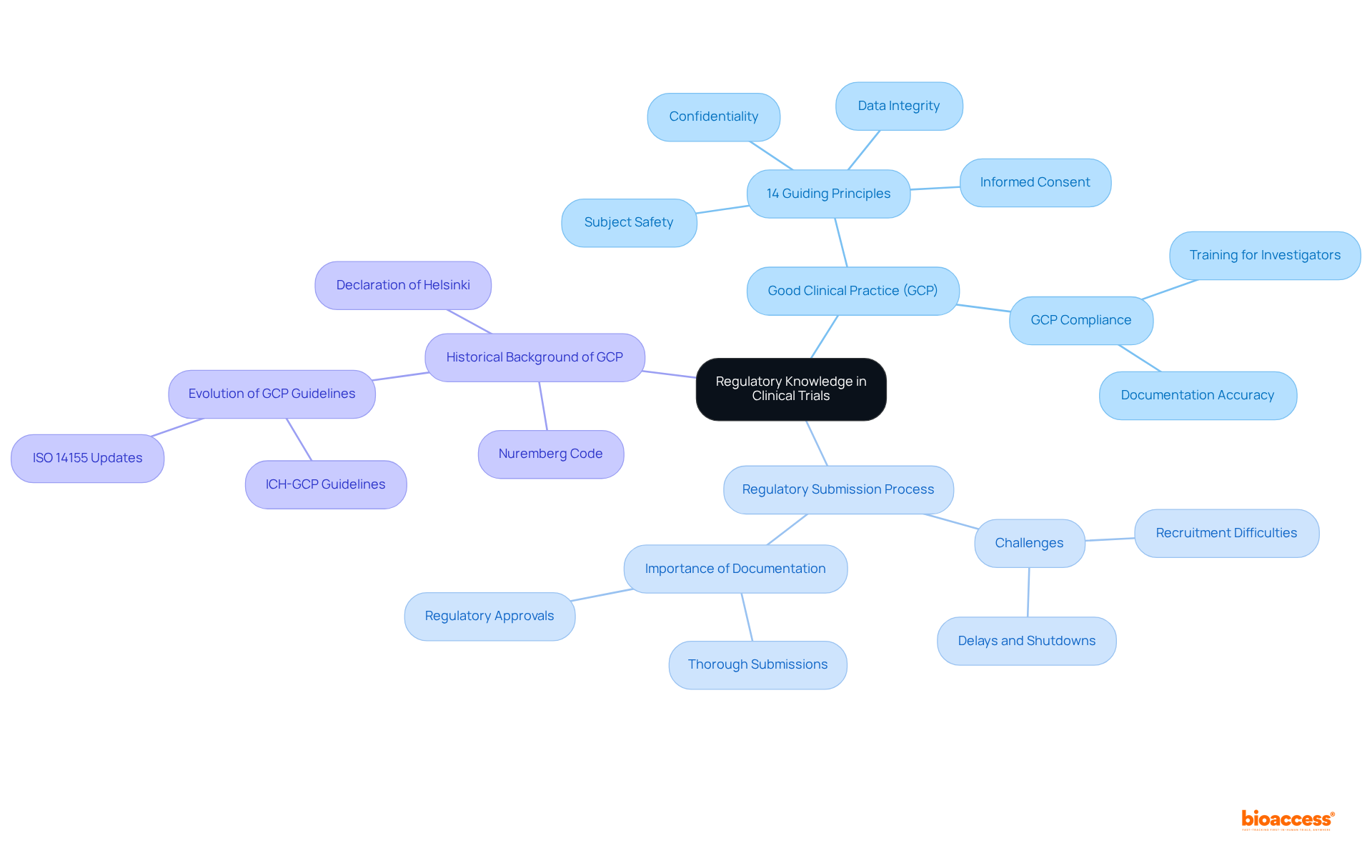
A comprehensive understanding of regulatory requirements, particularly Good Clinical Practice (GCP) and local laws, is vital for a clinical trial research coordinator. Their role includes ensuring that all experimental activities adhere to these regulations, which not only reduces legal risks but also enhances the credibility of the research. Clinical trial research coordinators must be skilled in the regulatory submission process, as around 80% of research studies experience delays or shutdowns due to recruitment difficulties and regulatory obstacles. This underscores the necessity for CRCs to prepare accurate and thorough documentation, which is essential for successful regulatory submissions.
For example, following GCP principles—encompassing informed consent, subject safety, data integrity, and preserving subject confidentiality—is essential for upholding ethical standards in research studies. Moreover, grasping the 14 guiding principles of GCP and the historical background of GCP, grounded in the Nuremberg Code and the Declaration of Helsinki, can significantly improve the effectiveness and success rate of clinical studies, ultimately aiding the progress of medical innovations.
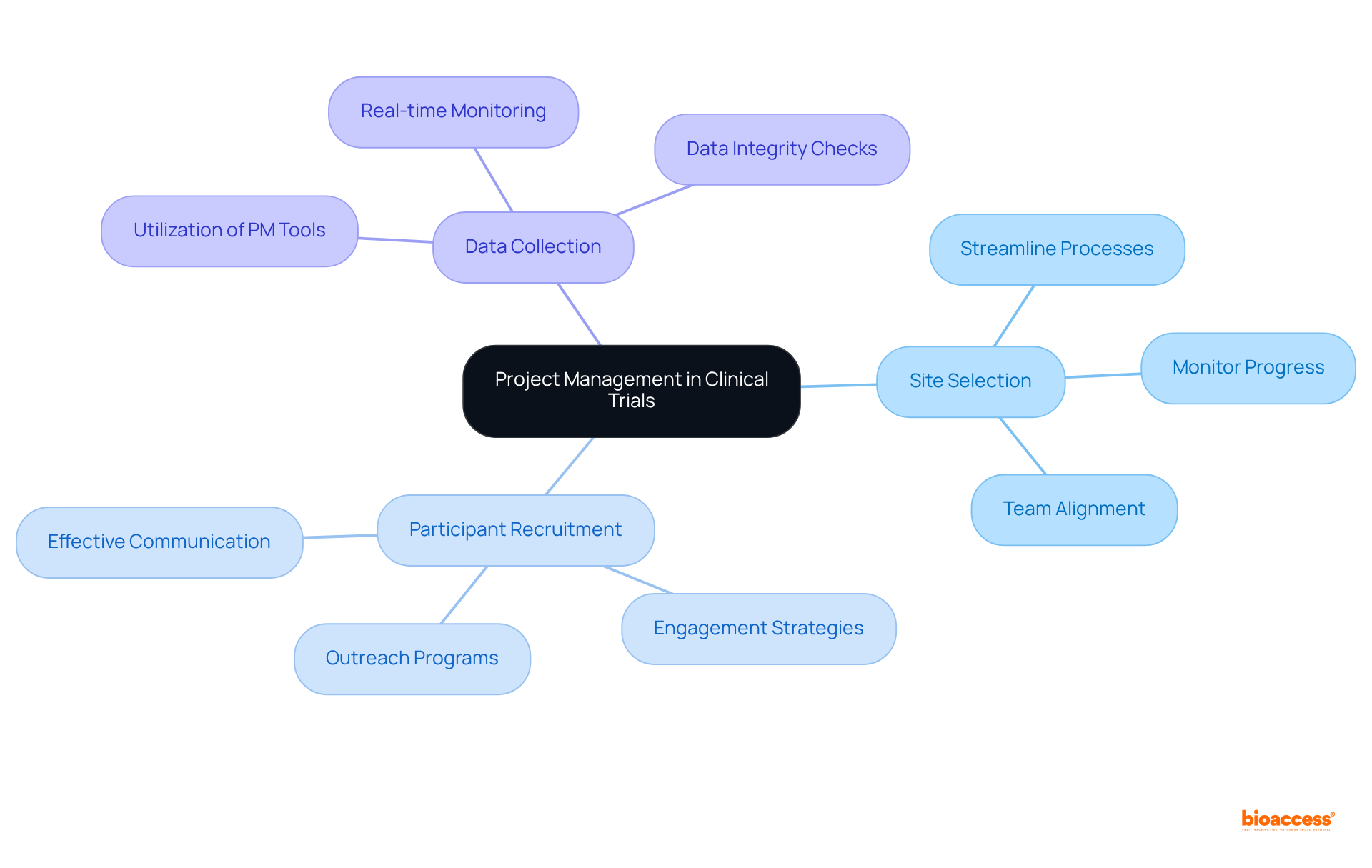
Clinical trial research coordinators must excel in project management, which is a critical competency for coordinating various study activities such as:
By employing project management tools and methodologies, coordinators can:
Regular meetings and updates are essential, as they keep everyone informed and engaged, fostering a collaborative environment that drives success.
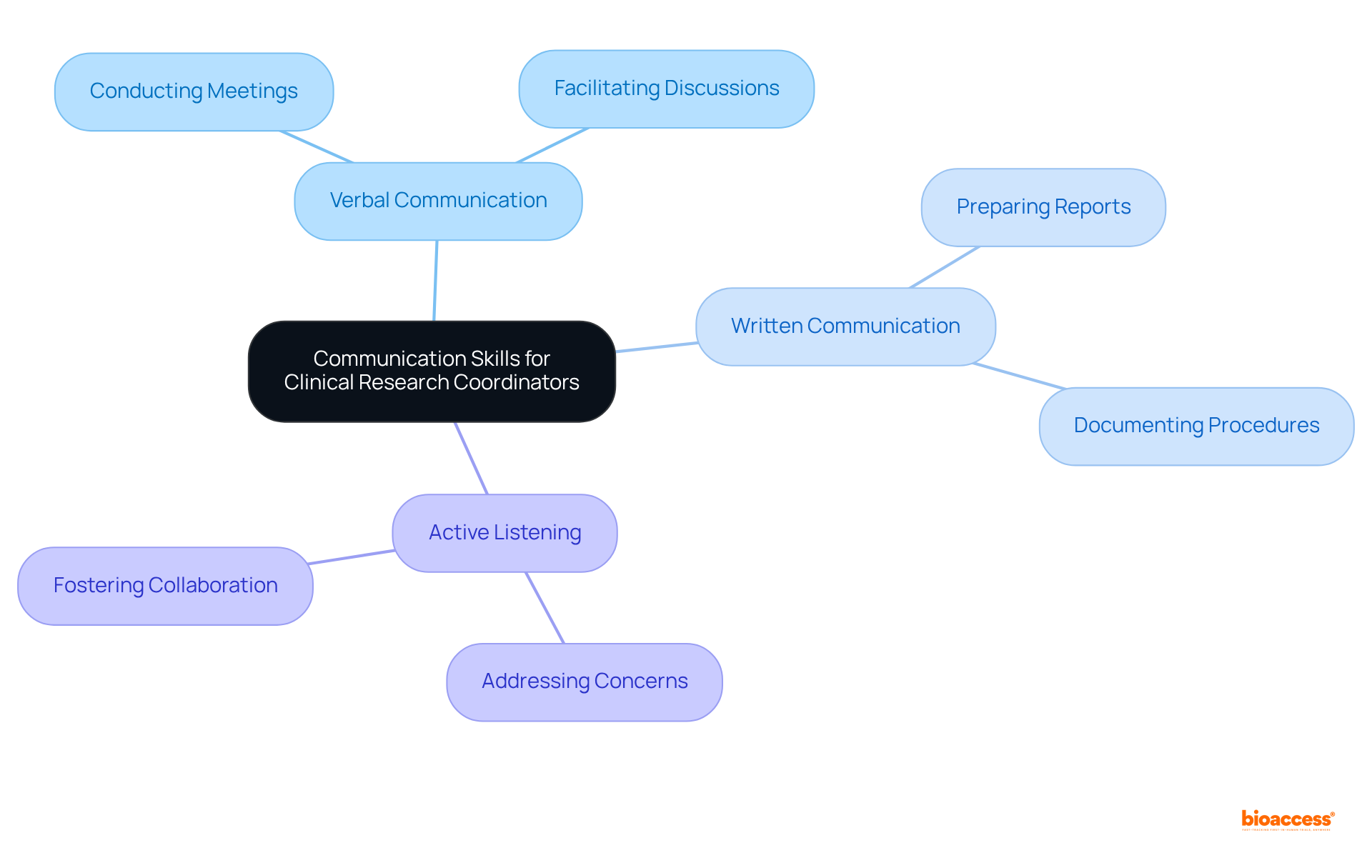
Clinical research coordinators must possess excellent verbal and written communication skills to convey study objectives, instructions, and updates clearly. This includes:
Active listening is also crucial for addressing concerns and fostering a collaborative environment. Research indicates that effective communication can significantly enhance stakeholder engagement. In fact, studies show that organizations promoting collaborative working are five times more likely to achieve high performance. Moreover, encouraging open communication among multidisciplinary teams not only boosts data precision but also increases participant retention, ultimately resulting in more successful research trials. By mastering these communication skills, a clinical trial research coordinator can navigate the complexities of clinical research and drive project success.
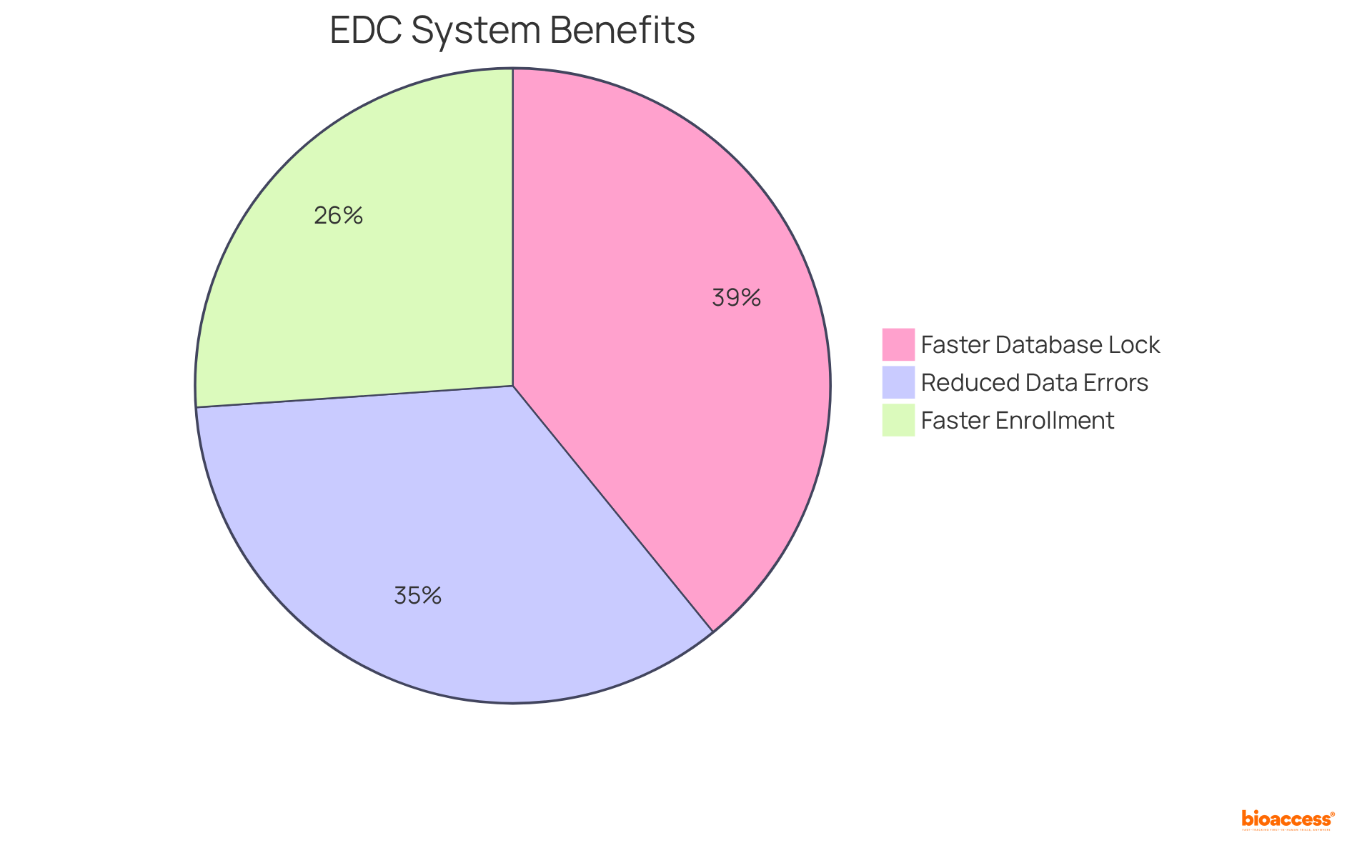
Clinical Research Coordinators (CRCs) play a pivotal role in ensuring the success of clinical research through their expertise in data management techniques, which encompass data entry, validation, and analysis. Proficiency in electronic data capture (EDC) systems is not just beneficial; it is vital, as these platforms significantly enhance data accuracy and security.
Research indicates that studies utilizing integrated EDC solutions can:
Regular audits and quality checks are essential to identify and rectify discrepancies, with findings demonstrating that effective data management can lead to a 40% reduction in data entry errors. Furthermore, the adoption of robust EDC systems is increasingly recognized as a strategic asset, directly influencing the reliability of research outcomes and the overall success of study initiatives.
As the landscape of clinical research evolves, it is imperative for clinical trial research coordinators to remain well-informed about advancements in EDC technology and its implications for data integrity and compliance.
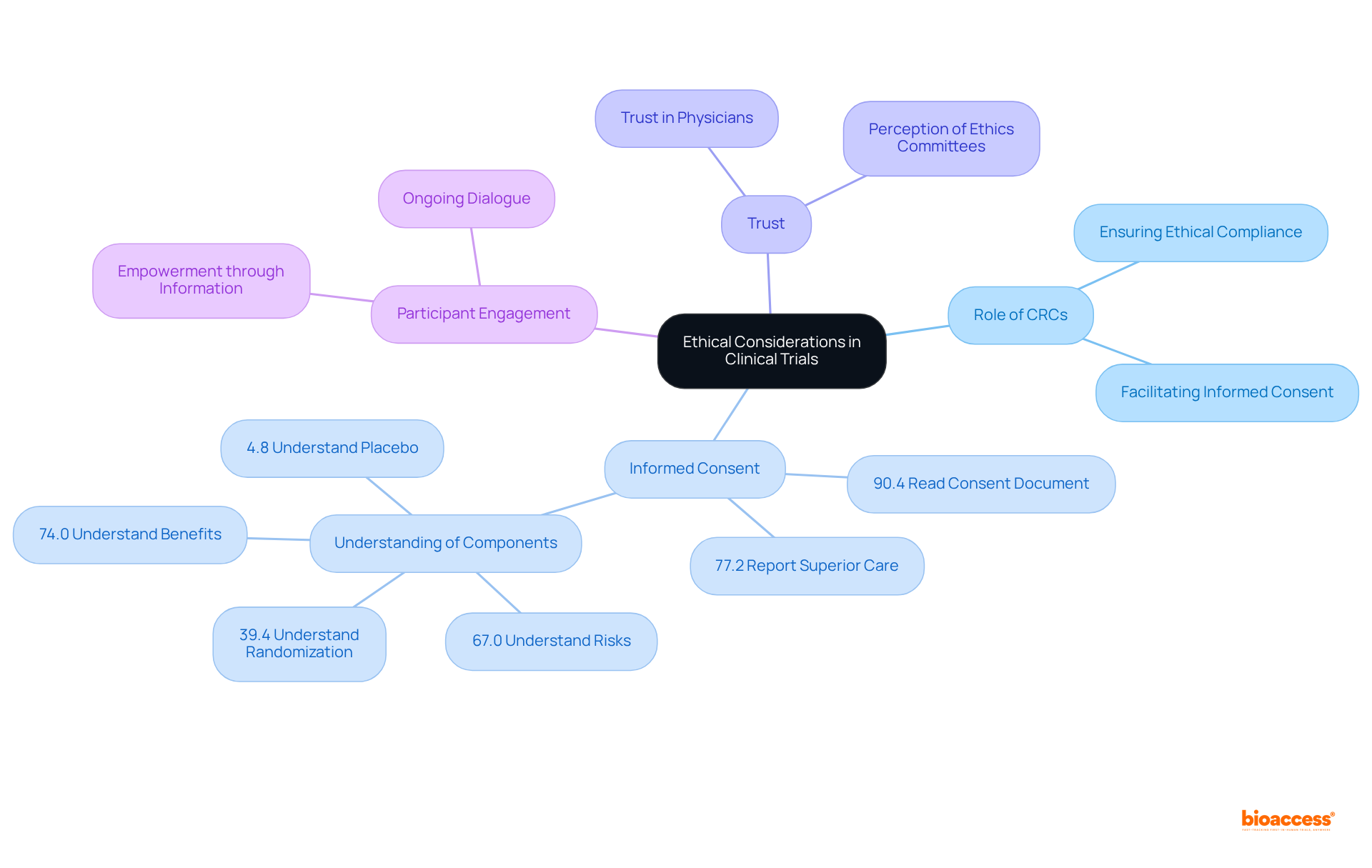
Clinical trial research coordinators (CRCs) are integral to ensuring ethical compliance within clinical studies, particularly through the informed consent process. Their expertise in ethical standards governing research is vital, as it ensures that all participants are fully aware of the trial's risks and benefits, with their involvement being entirely voluntary. This process transcends mere formality; it is essential for honoring individual autonomy and fostering trust. Notably, a study indicated that 90.4% of individuals thoroughly read the informed consent document, showcasing strong engagement when the process is clearly articulated and effectively communicated.
Furthermore, the informed consent process has a profound effect on trust among participants. Research indicates that individuals who feel well-informed about their rights and the study's nature are more likely to view medical studies as a valuable means of obtaining healthcare. In fact, 77.2% of individuals involved in industry-sponsored research trials reported that the care they received was superior to their usual medical care, underscoring the positive influence of effective informed consent practices.
Expert insights highlight the ongoing significance of informed consent, particularly in the work of a clinical trial research coordinator in clinical research. It is perceived as a continuous dialogue rather than a one-time event, emphasizing the necessity for sustained communication between researchers and participants. This approach not only enhances understanding but also cultivates a collaborative relationship, empowering individuals to engage actively in the research process. Research committees must prioritize these ethical considerations to uphold the integrity of studies and ensure that participants feel valued and respected throughout their involvement.
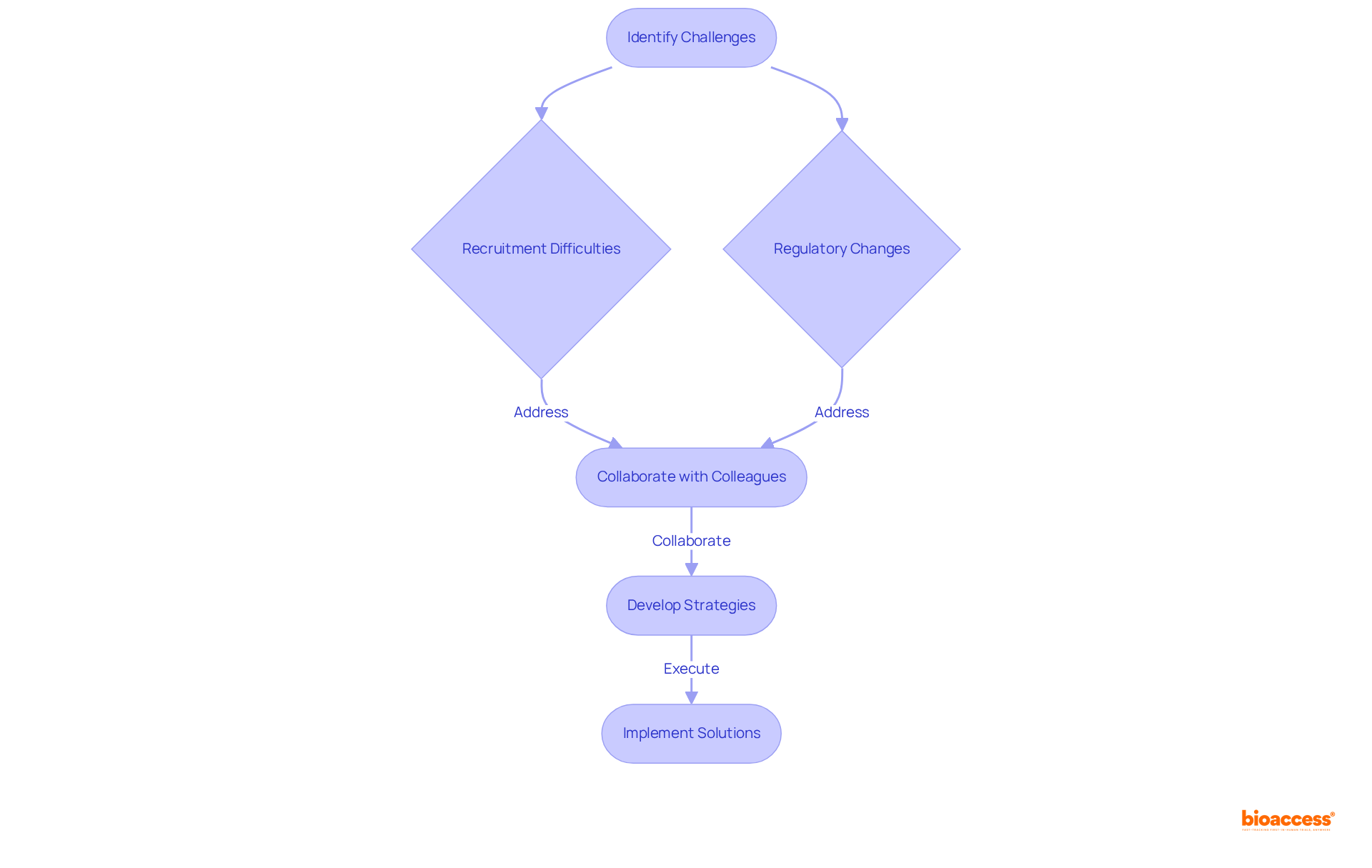
Clinical Research Coordinators (CRCs) must cultivate robust problem-solving skills to proactively identify potential issues and implement effective solutions. This adaptability is crucial, particularly when confronted with unexpected challenges such as recruitment difficulties or regulatory changes. Notably, approximately 80% of research studies experience delays due to recruitment challenges, with 37% of locations under-enrolling volunteers and 11% failing to enroll a single patient. Therefore, CRCs play a pivotal role in formulating strategies to enhance participant enrollment. Collaborating with colleagues to generate creative methods can lead to efficient solutions, fostering a culture of resilience within the management process.
As the complexity of clinical studies escalates—exacerbated by the COVID-19 pandemic, which has emerged as the second-most reported challenge—CRCs are tasked with navigating multifaceted issues, including regulatory hurdles and operational inefficiencies. By leveraging robust communication abilities and engaging in stakeholder evaluations and discussions, the clinical trial research coordinator can ensure that studies remain on course. Furthermore, integrating contingency plans into research protocols is vital for preserving study timelines. Ultimately, these efforts contribute significantly to the advancement of medical science and the enhancement of patient care.
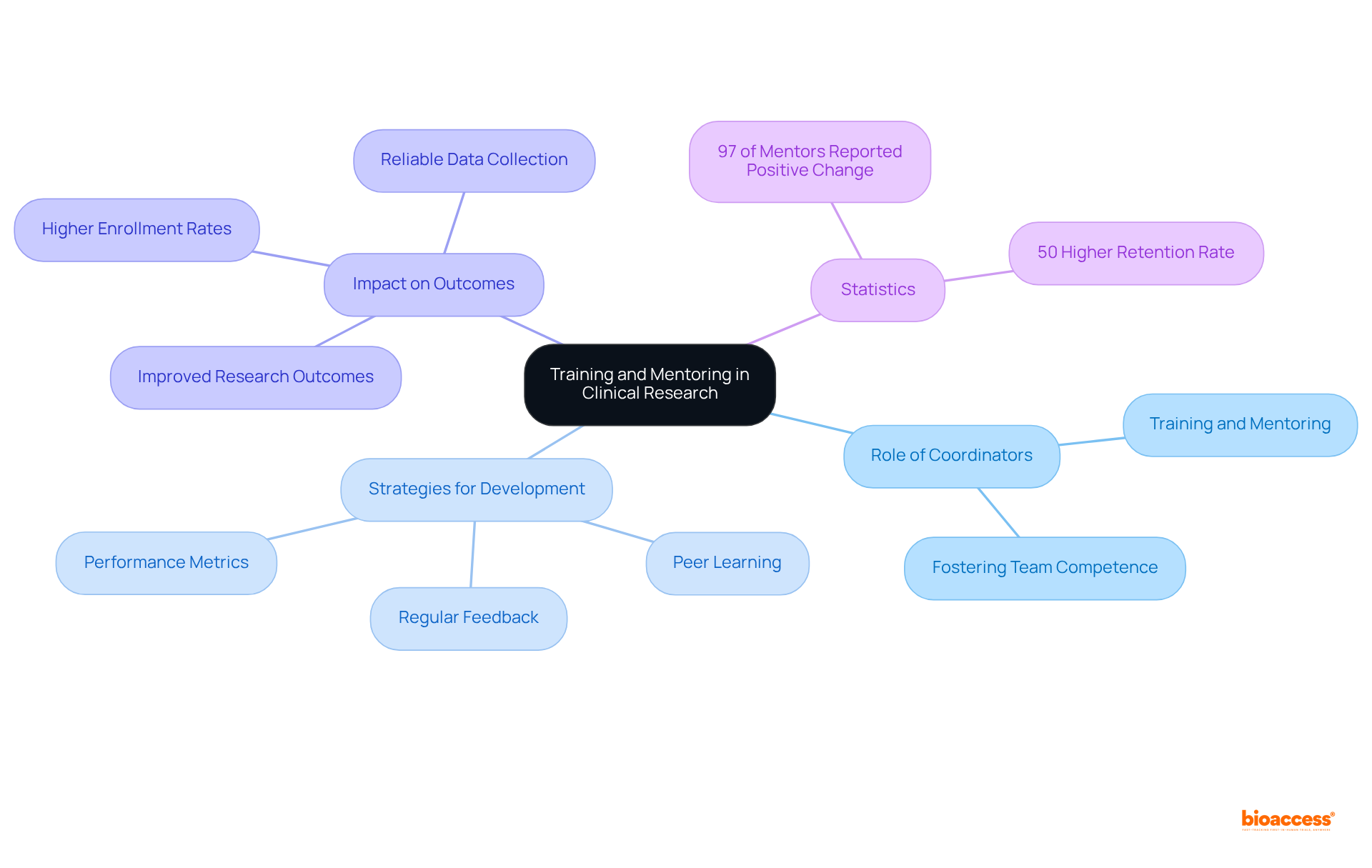
Clinical trial research coordinators play a crucial role in fostering competence within their teams by actively engaging in training and mentoring new members. This responsibility encompasses not only conducting training sessions but also sharing valuable resources and insights on best practices. By cultivating an environment of ongoing education and teamwork, clinical trial research coordinators can significantly enhance team proficiency, directly correlating with improved research outcomes.
Research indicates that teams with higher competence levels conduct experiments more efficiently, leading to faster enrollment and more reliable data collection. Furthermore, expert opinions suggest that structured mentoring initiatives can elevate the skills of clinical trial research coordinators, ensuring they are well-equipped to navigate the complexities of modern research environments. Notably, 97% of mentors in the intervention group reported increased awareness, intent to change, or actual behavioral change, underscoring the effectiveness of organized mentoring.
Successful strategies for team development include:
Importantly, employees engaged in mentoring programs enjoy a 50% higher retention rate compared to their non-mentoring counterparts, highlighting the long-term advantages of mentoring for team stability and competence. By implementing these practices, community resource centers not only facilitate the professional growth of their peers but also drive the overall success of research studies. As Christine Pfund aptly stated, "Effective mentoring is critical to the success of early-career investigators.
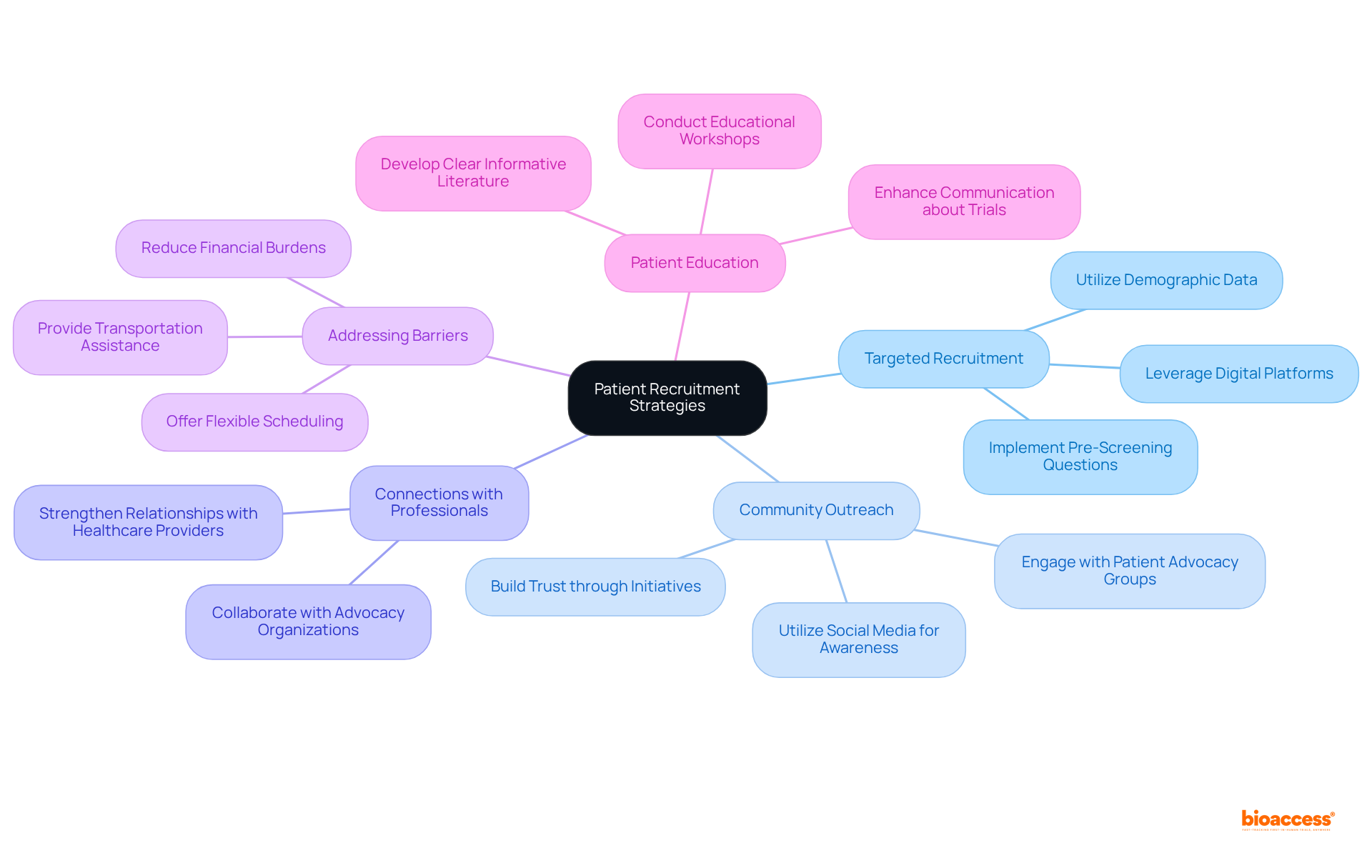
To effectively engage potential participants in clinical studies, the clinical trial research coordinator must implement targeted recruitment strategies. Understanding the demographics of the target population is essential, as it facilitates tailored outreach efforts.
By integrating these strategies, clinical trial research coordinators at research centers can improve enrollment rates and ensure a more diverse participant pool, ultimately enhancing the quality and relevance of clinical research.
Clinical trial research coordinators must exhibit strong financial management capabilities to effectively develop and oversee budget plans. This requires a thorough understanding of the primary cost categories associated with various experimental activities, which typically include:
For example, federally funded studies incur substantial costs in personnel, patient care, and data management, all of which can significantly influence the overall budget.
Instances of effective budget management in medical research underscore the importance of negotiating agreements with suppliers and meticulously monitoring costs throughout the study. Regular financial assessments are vital for a clinical trial research coordinator, as they help in the early identification of potential issues, ensuring that the project remains within budget. Research indicates that delays in clinical studies can cost sponsors between $600,000 and $8 million for each day of postponement, emphasizing the critical nature of effective budget management.
Expert opinions suggest that a clinical trial research coordinator can enhance efficiency in the process by integrating budgeting with project management. By leveraging historical data, cost review committees can forecast expenses and avert overspending, thereby reinforcing effective budget management. Furthermore, budget management tools can streamline processes, optimize resource allocation, and maintain precise financial records. This proactive approach not only mitigates financial risks but also improves the return on investment (ROI) for research studies, ultimately contributing to the advancement of medical knowledge and superior healthcare outcomes.
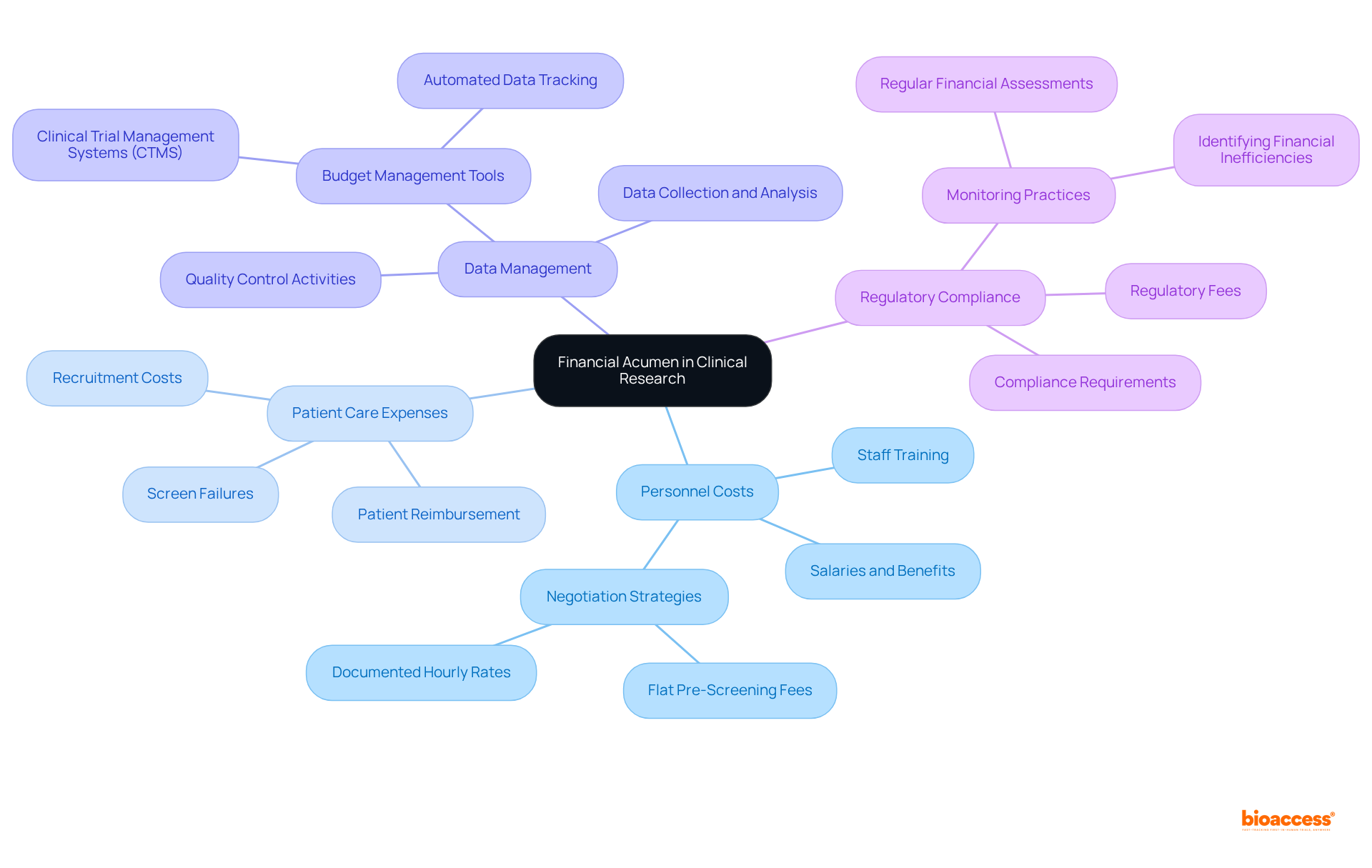
Clinical trial research coordinators must exhibit agility in navigating the ever-evolving landscape of medical research, increasingly shaped by regulatory changes, technological advancements, and innovative study designs. Staying informed about industry trends is crucial; for instance, the FDA's proposed regulation for single IRB review, expected to be implemented in 2025, is fundamentally reshaping research protocols and operational practices, necessitating revisions in standard operating procedures.
The adoption of innovations such as artificial intelligence and decentralized studies can significantly enhance efficiency and participant engagement, especially considering that over 80% of traditional studies fail to meet enrollment targets. Furthermore, the integration of real-world evidence emerged as a pivotal aspect of medical studies in 2024, facilitating more personalized and effective treatment approaches.
Clinical trial research coordinators who actively seek knowledge and adapt to these developments can foster innovative solutions, ultimately improving study outcomes and enhancing participant satisfaction. As the clinical trial environment continues to transform, those prioritizing adaptability will be strategically positioned to overcome challenges and capitalize on opportunities for success.
The role of a clinical trial research coordinator is multifaceted, requiring a diverse set of essential skills that contribute to the success of clinical studies. Mastery of the following skills is crucial for navigating the complexities of clinical research:
Each of these competencies plays a significant role in ensuring trials are conducted efficiently, ethically, and in compliance with established standards.
Key insights regarding the importance of each skill have been highlighted:
In light of these insights, it is clear that the effectiveness of clinical trial research coordinators directly impacts the advancement of medical science and patient care. As the landscape of clinical research continues to evolve, embracing these essential skills will enhance individual performance and drive the success of clinical trials. Stakeholders in the field should prioritize the development of these competencies to ensure that research efforts yield meaningful results, ultimately contributing to improved healthcare outcomes and innovations in treatment.
Why is regulatory knowledge important for clinical trial research coordinators?
Regulatory knowledge, particularly of Good Clinical Practice (GCP) and local laws, is vital for clinical trial research coordinators as it ensures that all experimental activities comply with regulations, reducing legal risks and enhancing research credibility.
What are the key responsibilities of clinical trial research coordinators regarding regulatory compliance?
Clinical trial research coordinators are responsible for ensuring adherence to regulatory requirements, preparing accurate and thorough documentation for regulatory submissions, and understanding the regulatory submission process to avoid delays or shutdowns in research studies.
What principles are encompassed in Good Clinical Practice (GCP)?
GCP principles include informed consent, subject safety, data integrity, and preserving subject confidentiality, which are essential for upholding ethical standards in research studies.
How can understanding the historical background of GCP improve clinical studies?
Grasping the historical background of GCP, which is rooted in the Nuremberg Code and the Declaration of Helsinki, can enhance the effectiveness and success rate of clinical studies, aiding the progress of medical innovations.
What project management skills are essential for clinical trial research coordinators?
Clinical trial research coordinators must excel in project management skills to efficiently coordinate activities such as site selection, participant recruitment, and data collection.
How can project management tools benefit clinical trial research coordinators?
Project management tools can help coordinators streamline processes, monitor progress, and ensure that all team members align with the project's objectives.
Why are regular meetings important in clinical trial management?
Regular meetings are essential as they keep everyone informed and engaged, fostering a collaborative environment that drives success in clinical trials.
What communication skills are necessary for clinical trial research coordinators?
Clinical trial research coordinators need excellent verbal and written communication skills to convey study objectives, prepare reports, conduct meetings, and ensure that all stakeholders understand their roles and responsibilities.
How does effective communication impact stakeholder engagement in clinical trials?
Effective communication can significantly enhance stakeholder engagement, as organizations that promote collaborative working are five times more likely to achieve high performance.
What is the effect of open communication among multidisciplinary teams in clinical trials?
Encouraging open communication among multidisciplinary teams boosts data precision and increases participant retention, ultimately resulting in more successful research trials.