


The article outlines the critical steps necessary for effective risk management in the consulting sector for medical devices in Mexico. It underscores the importance of:
These elements are vital for achieving compliance and facilitating successful clinical trials, ultimately enhancing the safety and efficacy of medical devices. Understanding these components is essential for navigating the complexities of the Medtech landscape, where collaboration plays a pivotal role in overcoming challenges and ensuring optimal outcomes.
Navigating the medical device landscape in Mexico presents a unique set of challenges, particularly regarding risk management. With the market projected to grow significantly, it is crucial to understand the intricate regulatory framework and implement effective risk management strategies for success. This article outlines ten essential steps that empower manufacturers and innovators to comply with local regulations and enhance operational efficiency.
How can organizations ensure they are fully equipped to tackle the complexities of risk management while accelerating their market entry?
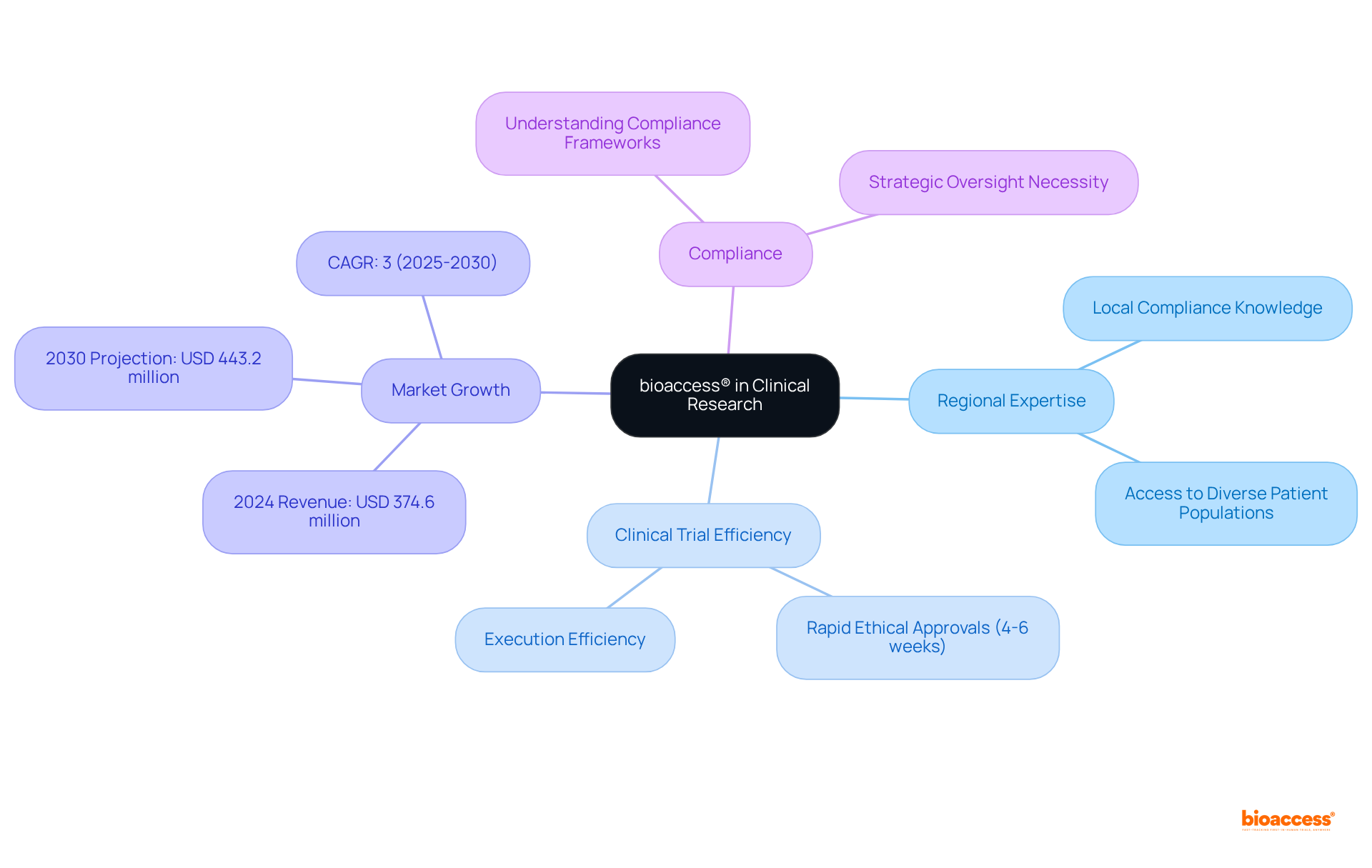
bioaccess® leverages its extensive regional expertise to optimize clinical research for medical devices in Mexico. By utilizing comprehensive local compliance knowledge and access to diverse patient populations, bioaccess® ensures that clinical trials are executed with exceptional efficiency, securing ethical approvals in just 4-6 weeks. This rapid turnaround is vital for Medtech innovators aiming to accelerate their market entry.
In 2024, the medical device clinical trials market in Mexico generated a revenue of USD 374.6 million and is projected to reach USD 443.2 million by 2030, reflecting a compound annual growth rate of 3% from 2025 to 2030. Successful clinical trials in Mexico, particularly pivotal studies that accounted for 42.18% of the revenue share in 2024, have validated the effectiveness of this approach, positioning bioaccess® as a strategic partner in navigating the complexities of the clinical research process.
Innovators recognize that understanding compliance frameworks is essential for success, a sentiment echoed by industry leaders who emphasize the necessity of strategic oversight in clinical trials.
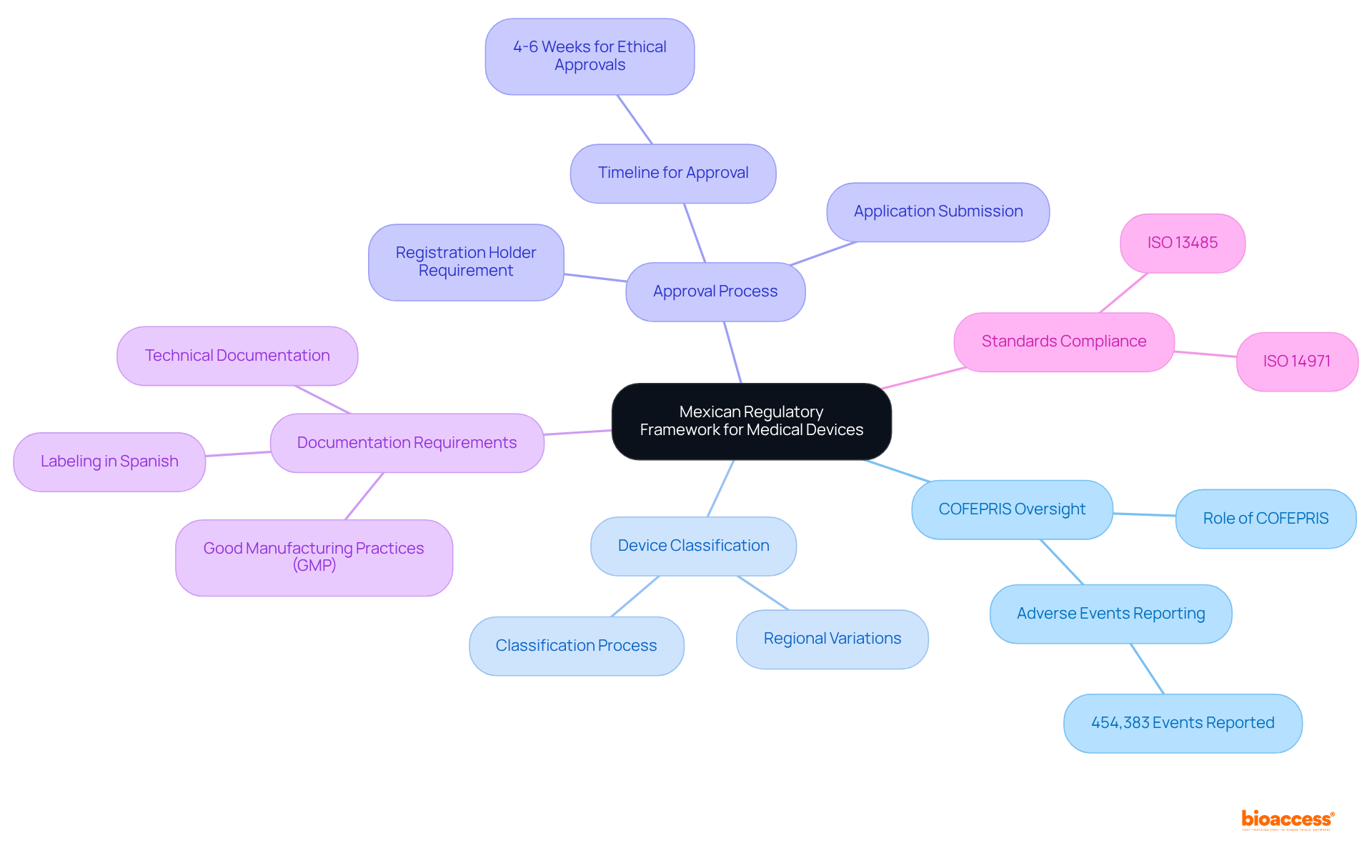
The regulatory landscape for medical devices in Mexico is primarily overseen by COFEPRIS (Federal Commission for Protection against Sanitary Risks). A thorough understanding of device classification, the approval process, and the requisite documentation is essential for compliance.
Manufacturers must demonstrate the safety and efficacy of their products through rigorous testing and comprehensive documentation. Adherence to ISO 14971 for hazard oversight and ISO 13485 for quality control systems is critical, as these standards are vital for meeting COFEPRIS requirements. Notably, 454,383 device-associated adverse events have been reported, underscoring the importance of adhering to these regulations; effective risk management file consulting Mexico devices can help mitigate potential issues.
Furthermore, COFEPRIS has streamlined its guidelines for equipment registration as of July 2025, making it even more crucial for manufacturers to stay informed. Familiarity with the regulatory framework not only simplifies the approval process but also reduces potential adherence challenges, ultimately conserving time and resources for manufacturers. Engaging with local compliance experts can significantly enhance understanding and navigation of these complex requirements, ensuring a smoother path to market entry.
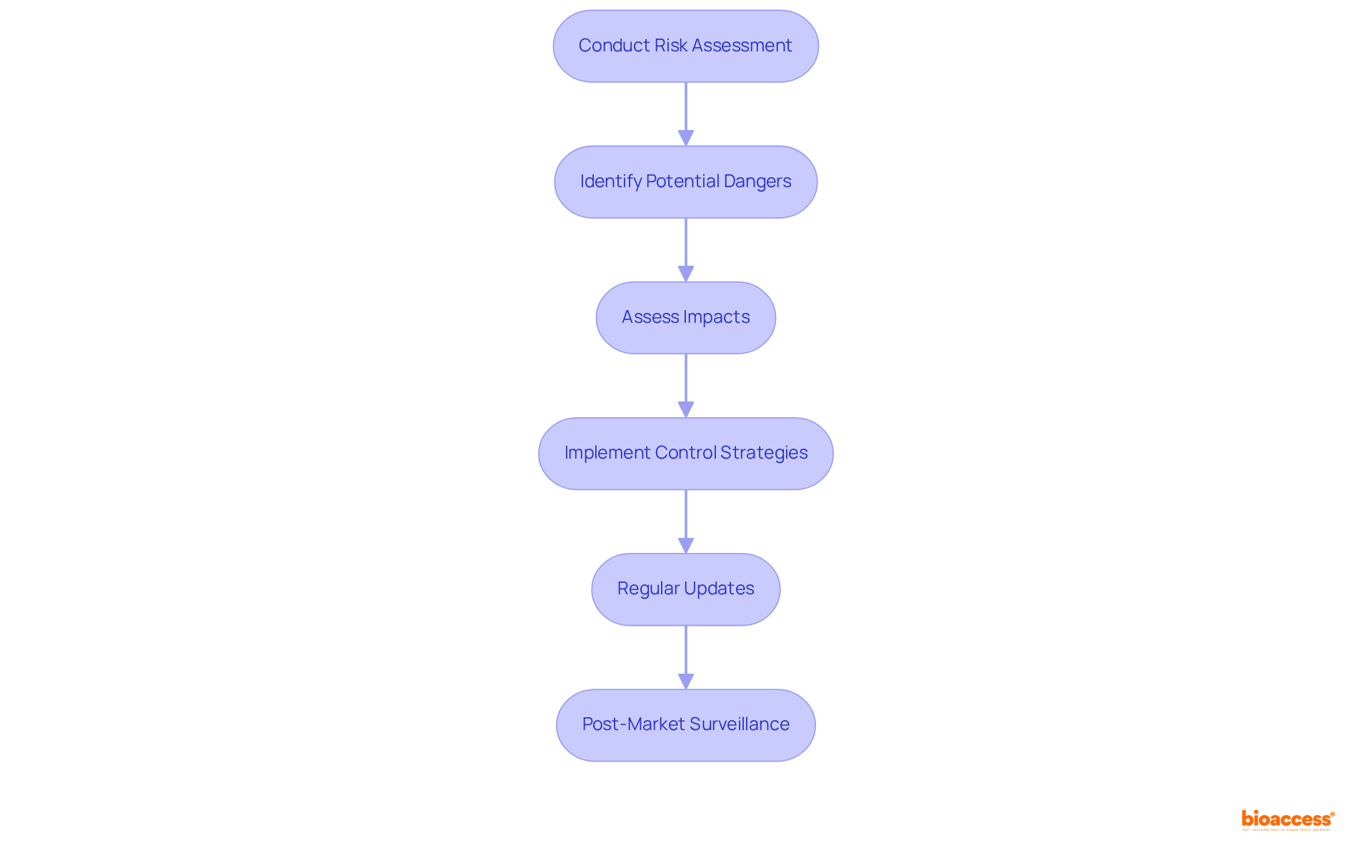
Conducting thorough evaluations of potential dangers is essential for identifying possible threats, assessing their impacts, and implementing effective control strategies. This process must align with ISO 14971, which outlines a structured framework for managing uncertainties in medical devices. By systematically examining uncertainties, manufacturers can proactively mitigate potential issues, ensuring that their devices are safe for use.
Regular updates to evaluations are critical, particularly as new information emerges or as devices undergo changes. For instance, manufacturers adhering to ISO 14971 have reported significant improvements in their hazard oversight processes, leading to enhanced product safety and compliance with regulatory standards.
As of 2025, compliance rates among medical device manufacturers have shown a positive trend, reflecting an increasing commitment to these vital guidelines. Experts in the field emphasize that hazard oversight should be regarded as a continuous, iterative process throughout the entire lifecycle of a device, underscoring the need for ongoing vigilance and adaptation in response to evolving threats.
Moreover, the significance of post-market surveillance and hazard monitoring is paramount, as these practices are crucial for ensuring device safety and effectiveness following market entry.
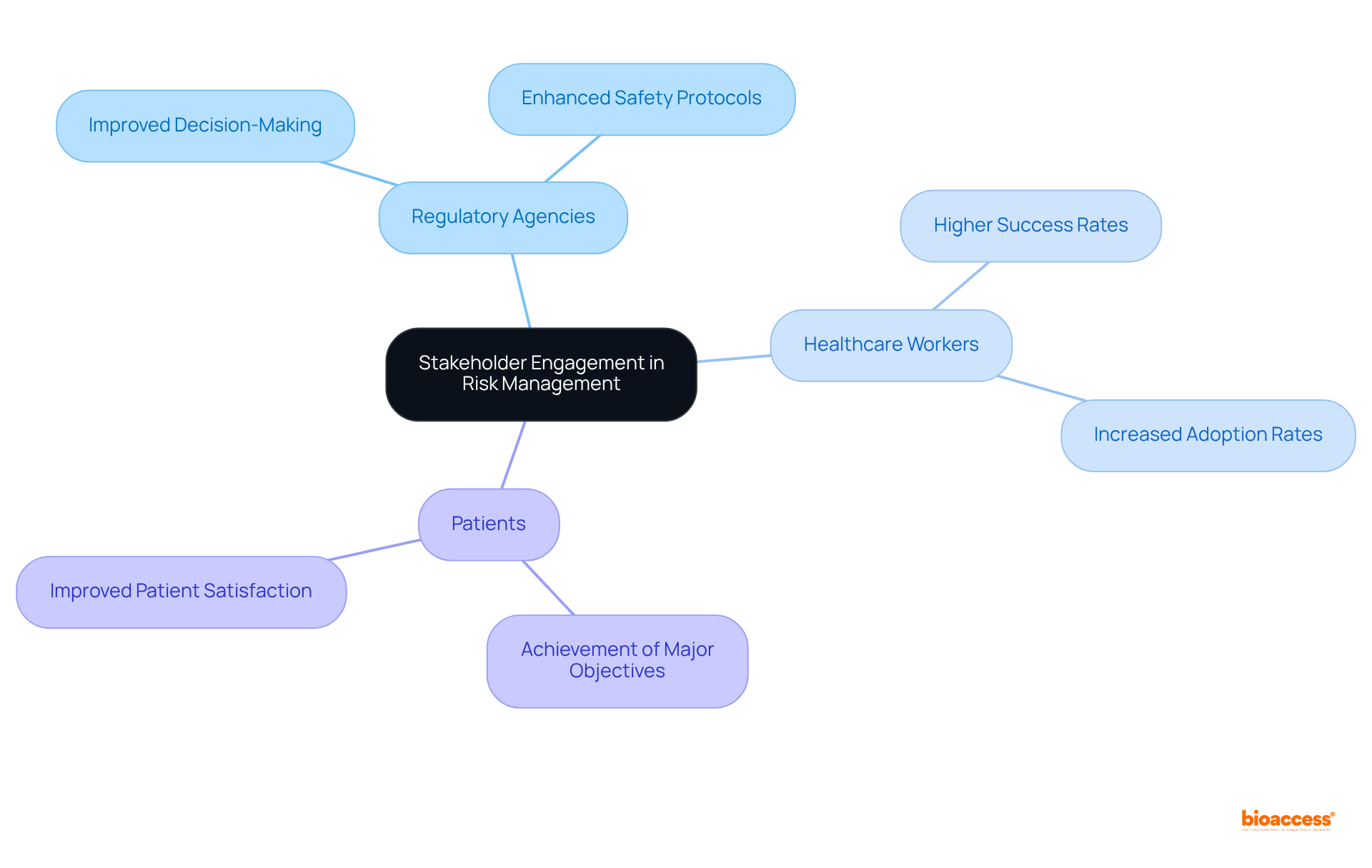
Effective hazard oversight hinges on the active participation of all relevant stakeholders, including regulatory agencies, healthcare workers, and patients. Engaging these parties early in the process allows manufacturers to gather essential insights into potential challenges and devise appropriate strategies to address them.
Research shows that projects with robust stakeholder engagement plans succeed 83% of the time, particularly in clinical trials, compared to a mere 32% for those lacking such plans. Consistent communication and teamwork foster trust and ensure that diverse viewpoints are integrated into the oversight process.
Involving stakeholders not only improves the quality of decision-making but also significantly enhances the success rates of clinical trials. Businesses that prioritize stakeholder input in product development experience a 30% higher adoption rate, which is crucial for effective oversight and achieving favorable outcomes in clinical trials.
Moreover, companies that actively engage with stakeholders are 50% more likely to achieve their major objectives, highlighting the vital role of collaboration in navigating the complexities of the medical device sector. As Barbara Hoey, an expert in safety assessment, notes, proactive involvement can lead to improved safety protocols and superior overall healthcare delivery.
Thus, a comprehensive strategy for stakeholder collaboration is essential for addressing the intricacies of uncertainty oversight in the medical device industry.
Establishing thorough training programs for all team members engaged in clinical research is crucial for guaranteeing adherence to management protocols. These programs must encompass regulatory requirements, risk assessment techniques, and best practices for managing risks associated with risk management file consulting Mexico devices. Regular refresher courses and updates on evolving regulations are essential for maintaining high levels of awareness and preparedness among staff. For instance, UC San Diego mandates that personnel involved in animal research complete orientation and refresher courses every three years, exemplifying best practices in training. This proactive strategy not only improves adherence but also significantly contributes to the overall success of clinical trials.
As industry expert Lucy Parker observes, effective training promotes a culture of adherence, which is essential for managing the complexities of medical device regulations and ensuring the integrity of clinical research. Moreover, with the U.S. Bureau of Labor Statistics anticipating an 8.2% rise in jobs for regulatory officers by 2026, the significance of strong training programs cannot be overstated. However, it is important to acknowledge the challenges faced in training compliance, as only 10% of employees report that compliance training has impacted their work practices. Addressing these challenges is essential for maximizing the effectiveness of training initiatives.
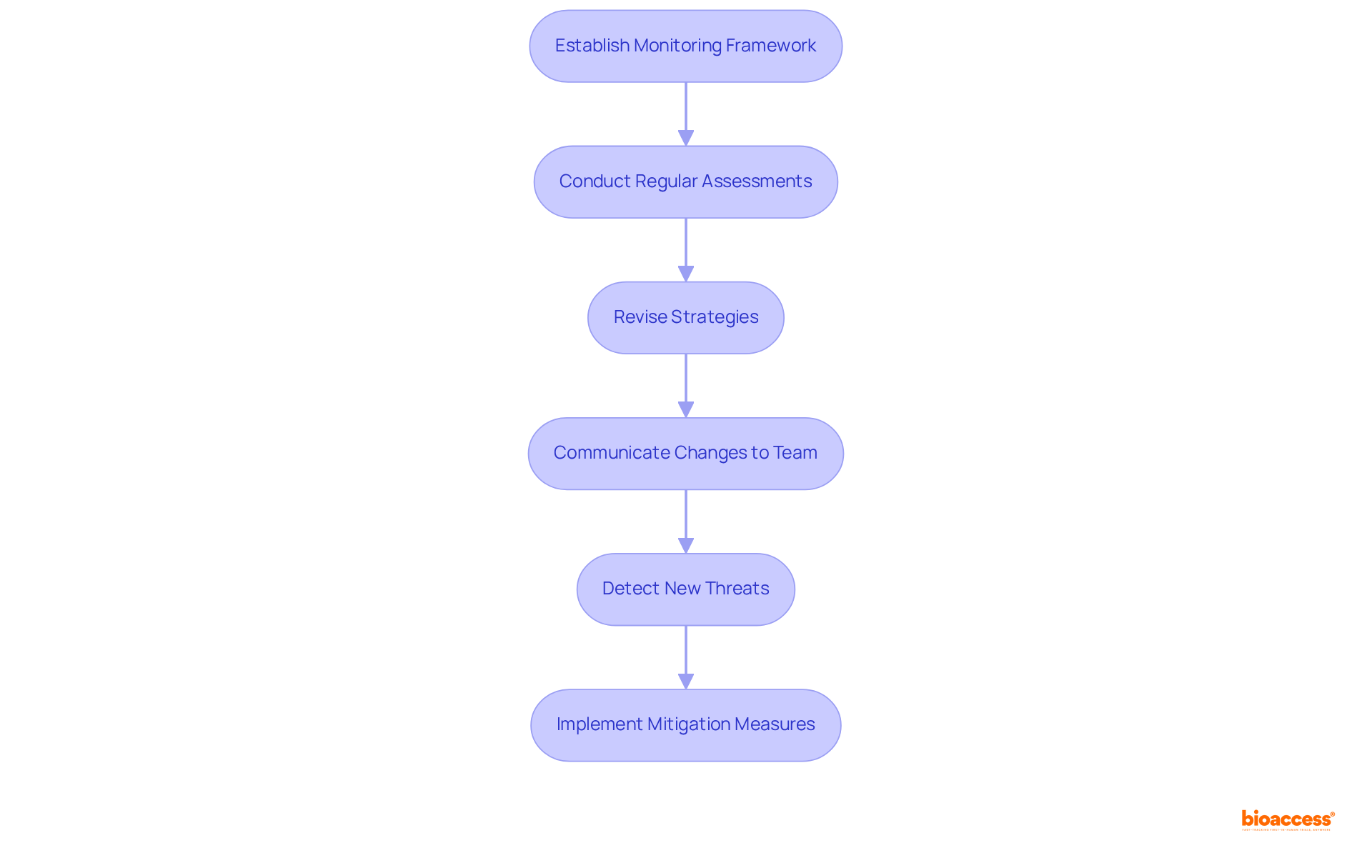
Establishing a robust framework for the continuous observation of threat handling approaches is essential for adapting to evolving situations and emerging challenges. This process necessitates regular scrutiny of assessments regarding potential issues, the revision of strategies for addressing them, and ensuring that all team members are informed of any changes. By sustaining an active monitoring system, organizations can promptly detect new threats and implement effective measures to mitigate them, thus ensuring the safety and efficacy of medical devices throughout their lifecycle.
Recent advancements in threat assessment monitoring systems have empowered organizations to automate data collection and analysis, significantly enhancing their ability to respond to real-time danger indicators. For instance, automated monitoring systems can issue alerts when critical danger indicators surpass predefined thresholds, facilitating immediate investigation and action. As industry specialists emphasize, the primary objective of hazard oversight is to minimize harm to patients and users by proactively identifying risks and implementing preventive measures.
Organizations that have successfully adapted their threat handling strategies in real-time exemplify the importance of continuous observation. By integrating insights from ongoing evaluations, they can refine their strategies to address emerging threats, ensuring compliance with standards, including oversight for approvals by the FDA and EU MDR. This proactive approach not only protects patient welfare but also equips companies to navigate the complexities of medical device development with increased agility and confidence. Furthermore, evaluating residual threats is crucial to ascertain whether remaining uncertainties after implementing controls are acceptable according to industry standards. Incorporating risk management file consulting Mexico devices into the development process is vital for ensuring safer products and expediting approval processes.
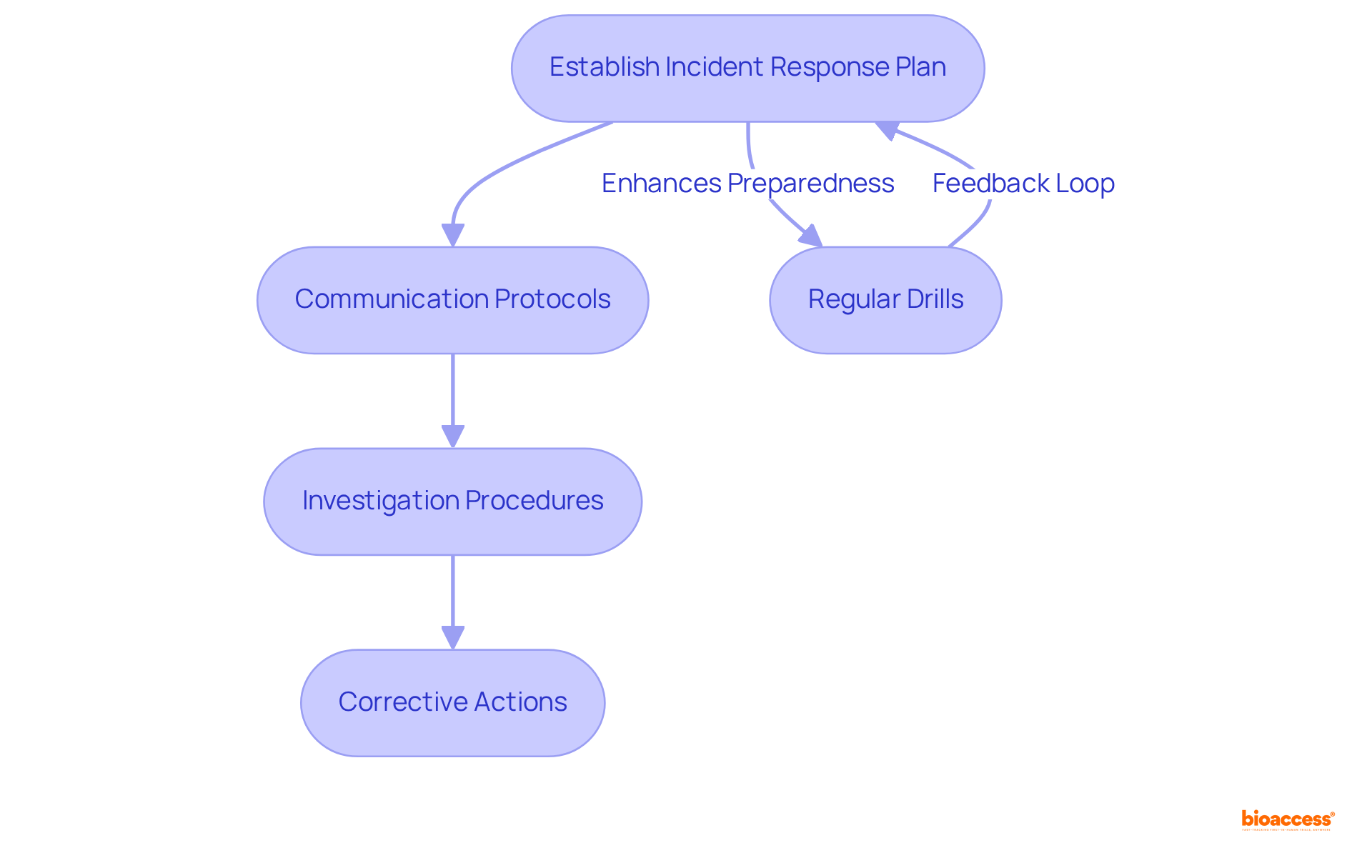
Establishing a comprehensive incident response plan is crucial for managing potential incidents involving medical devices. This plan must clearly delineate the necessary steps to take in the event of an adverse event, encompassing:
Regular drills are vital; they not only prepare team members to respond swiftly and effectively but also help identify areas for improvement in the plan. Updating the response strategy based on these drills ensures compliance with regulatory requirements and minimizes potential harm to patients.
Safety specialists stress that effective incident coordination depends on preparedness and clear communication, both of which are essential for preserving trust and safety in clinical research. Notably, organizations without an incident response plan face an average breach lifecycle of 258 days, underscoring the urgency for improvement in this area. Moreover, the typical expense of a healthcare data breach is $4.88 million, emphasizing the financial consequences of insufficient incident handling.
Incorporating regular drills into the incident response plan is essential, as they enhance preparedness and ensure that all team members are equipped to handle incidents effectively.
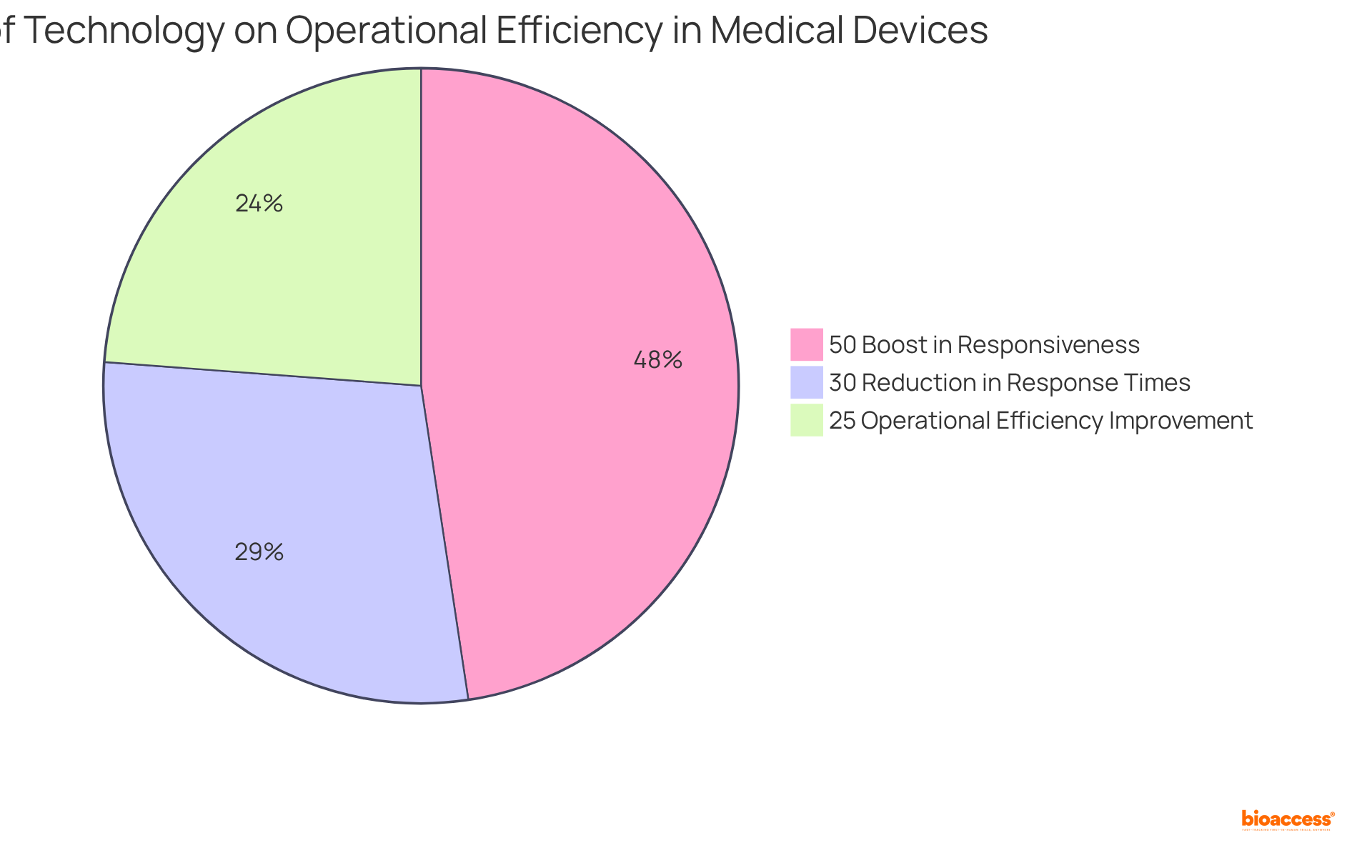
Utilizing technology and data analysis significantly enhances management efforts within the medical device industry. Sophisticated analytical instruments play a crucial role in recognizing patterns and trends in data, empowering organizations to proactively address potential challenges. Firms that leverage data analysis report a remarkable 25% improvement in operational efficiency, underscoring the effectiveness of these tools in optimizing assessment procedures.
Furthermore, the integration of real-time monitoring systems can result in a 30% reduction in response times during incidents, while companies that employ real-time dashboards can boost responsiveness by up to 50%. As Amlan Shome aptly notes, 'One of the greatest benefits of data-driven hazard oversight is the capability to track threats in real-time.'
With 41% of organizations reporting three or more significant threat occurrences in the past year, the need for effective oversight is clear. By incorporating these technologies into their risk management file consulting Mexico devices, organizations can significantly enhance their ability to navigate potential challenges and maintain regulatory compliance.
To fully realize these benefits, organizations must continuously assess and adapt their risk management file consulting Mexico devices strategies, ensuring they remain effective in identifying and mitigating risks.
Engaging with local experts is essential for comprehending the intricate regulatory landscape and market dynamics in Mexico. These professionals possess the expertise necessary to navigate local regulations effectively, ensuring compliance and facilitating a smoother market entry process. Companies that collaborate with local partners can significantly enhance their risk management file consulting Mexico devices, ensuring their operations align with local practices and expectations. This partnership simplifies adherence to standards while promoting a deeper understanding of consumer preferences and legal nuances, ultimately fostering more informed decision-making.
As noted by Ongresso, "Partnering with local experts is essential for navigating the complexities of domiciliation in Mexico." Moreover, with companies in Mexico averaging 20 administrative procedures annually and the oversight burden accounting for roughly 3.4% of GDP, leveraging local expertise is crucial for Medtech companies aiming to thrive in this market, where adherence to regulations can directly impact operational efficiency and market responsiveness.
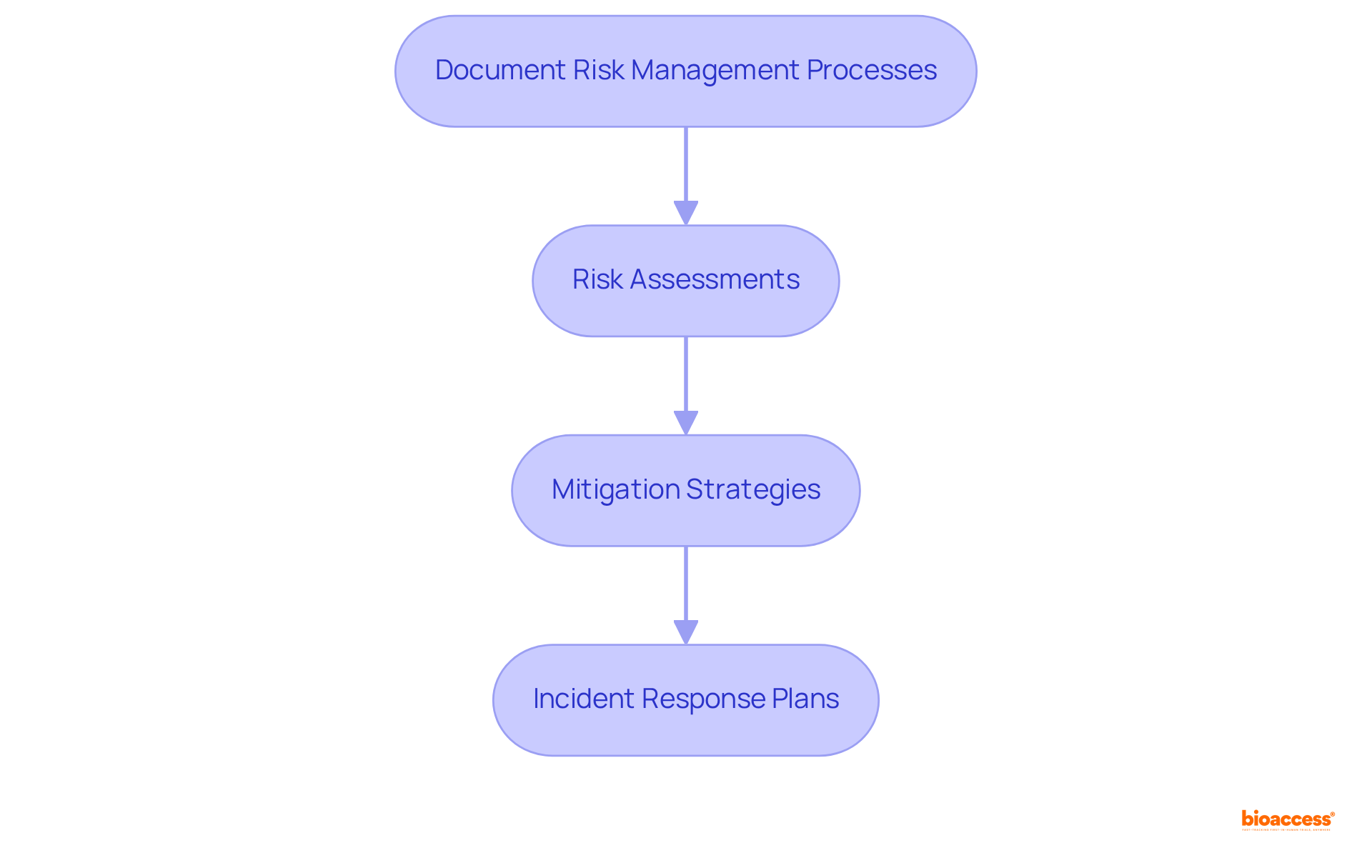
Recording management procedures is essential for demonstrating adherence to regulatory obligations and facilitating audits. This documentation must include risk management file consulting Mexico devices, covering:
to ensure that all actions taken are transparent and accountable. Regular reviews and updates to these documents are crucial for maintaining their relevance and accuracy, providing a dependable reference for stakeholders involved in the clinical research process.
Navigating risk management in the medical device sector in Mexico requires a structured approach that incorporates several essential steps. By prioritizing compliance with local regulations, conducting thorough risk assessments, and engaging stakeholders, organizations can significantly enhance their operational efficiency and market responsiveness. Insights gained from leveraging local expertise and technology not only streamline processes but also ensure that medical devices meet safety standards while expediting their time to market.
The key arguments presented throughout this article underscore the importance of understanding the Mexican regulatory framework, the necessity of continuous monitoring, and the value of training programs in fostering a culture of compliance. Establishing a robust incident response plan further solidifies an organization's preparedness for potential challenges, while documenting risk management processes serves as a vital reference for maintaining accountability and transparency.
Ultimately, the significance of effective risk management in the medical device industry cannot be overstated. By adopting these essential steps, organizations can safeguard patient welfare and position themselves for success in a competitive market. Embracing a proactive approach to risk management empowers companies to navigate complexities with confidence, fostering innovation and ultimately improving healthcare outcomes in Mexico.
What is bioaccess® and how does it contribute to clinical research for medical devices in Mexico?
bioaccess® leverages regional expertise to optimize clinical research for medical devices in Mexico, ensuring efficient execution of clinical trials with ethical approvals secured in just 4-6 weeks. This rapid turnaround is crucial for Medtech innovators looking to accelerate their market entry.
What was the revenue generated by the medical device clinical trials market in Mexico in 2024, and what is the projected growth?
In 2024, the medical device clinical trials market in Mexico generated a revenue of USD 374.6 million, projected to reach USD 443.2 million by 2030, reflecting a compound annual growth rate of 3% from 2025 to 2030.
What role does COFEPRIS play in the regulation of medical devices in Mexico?
COFEPRIS (Federal Commission for Protection against Sanitary Risks) oversees the regulatory landscape for medical devices in Mexico, requiring manufacturers to demonstrate the safety and efficacy of their products through rigorous testing and documentation.
What standards are critical for compliance with COFEPRIS requirements?
Adherence to ISO 14971 for hazard oversight and ISO 13485 for quality control systems is critical for meeting COFEPRIS requirements.
Why is understanding the regulatory framework important for medical device manufacturers?
Familiarity with the regulatory framework simplifies the approval process, reduces adherence challenges, and conserves time and resources, ultimately facilitating smoother market entry.
What is the significance of conducting comprehensive risk assessments for medical devices?
Conducting thorough evaluations of potential dangers aligns with ISO 14971 and is essential for identifying threats, assessing impacts, and implementing control strategies to ensure device safety.
How should manufacturers approach hazard oversight throughout the lifecycle of a medical device?
Hazard oversight should be regarded as a continuous, iterative process, with regular updates and adaptations in response to new information or changes to the device, ensuring ongoing safety and compliance.
What is the importance of post-market surveillance and hazard monitoring for medical devices?
Post-market surveillance and hazard monitoring are crucial for ensuring the safety and effectiveness of devices after they have entered the market, helping to mitigate potential issues.