


The article titled "10 Essential Steps for Successful Device Development" serves to outline the critical steps necessary for effectively developing medical devices. It underscores that successful device development requires a comprehensive approach, which includes:
Furthermore, it highlights the importance of:
These elements are crucial for meeting market demands and ensuring compliance with safety standards.
The landscape of medical device development is evolving at an unprecedented pace, capturing the attention of industry leaders and innovators alike. As the demand for effective healthcare solutions continues to rise, it becomes essential for Medtech companies to grasp the critical steps necessary for successful device development. Understanding these steps is not just beneficial; it’s crucial for thriving in today’s competitive market. However, the journey is fraught with challenges, including:
How can innovators not only meet these demands but also exceed them? This question is pivotal as it paves the way for groundbreaking advancements in healthcare. By addressing these challenges head-on, Medtech companies can position themselves as leaders in the industry, driving innovation and improving patient outcomes. Collaboration and strategic planning will be key in overcoming obstacles and achieving success in this dynamic field.
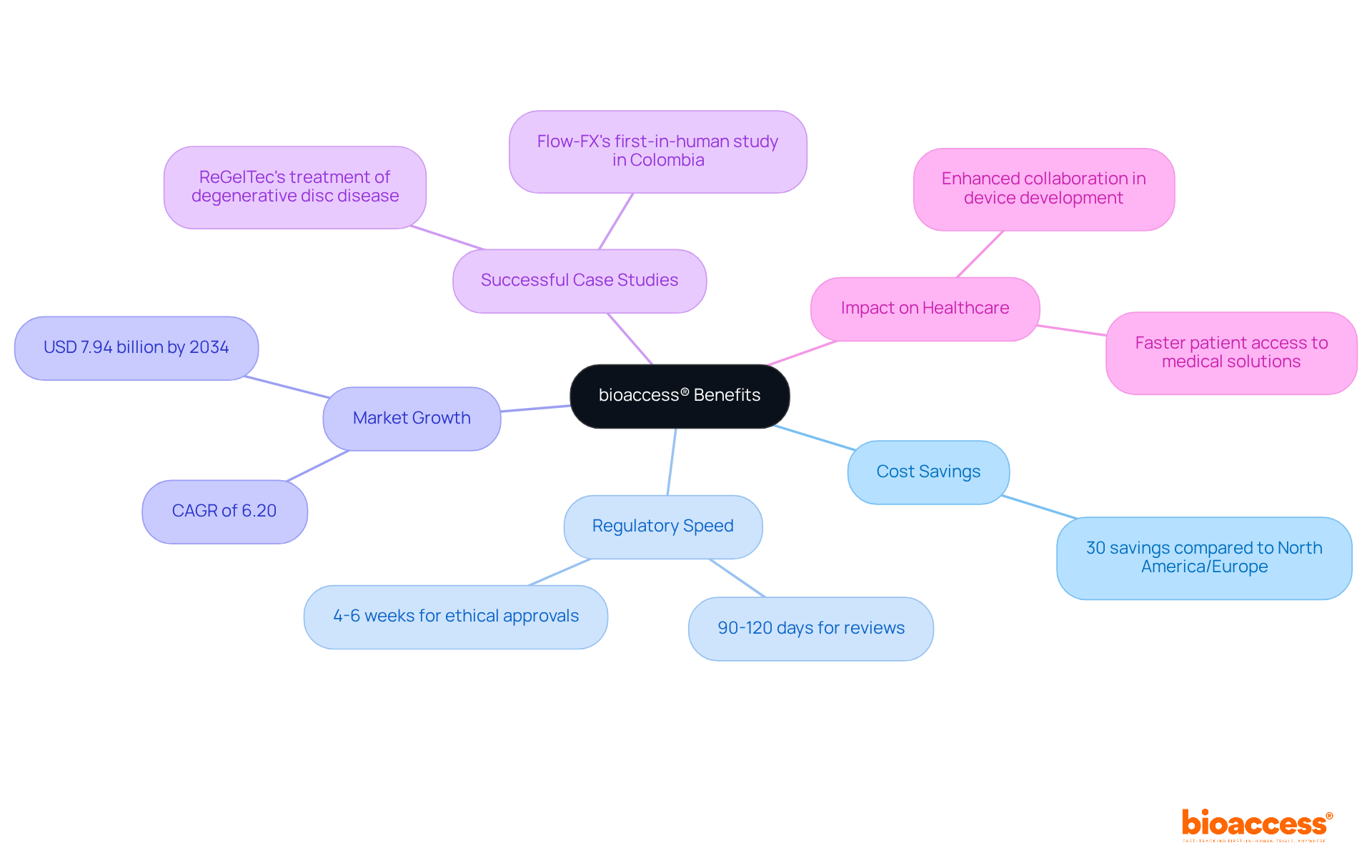
bioaccess® capitalizes on Colombia's competitive advantages, boasting significant cost savings of over 30% compared to North America and Western Europe. With a regulatory speed that facilitates device development alongside IRB/EC and MoH (INVIMA) reviews in just 90-120 days, this global-first healthcare agility empowers Medtech, Biopharma, and Radiopharma innovators to expedite their breakthroughs. As a result, new devices and therapies reach the market faster than ever before.
The Latin America trials market is projected to grow to approximately USD 7.94 billion by 2034, with a compound annual growth rate (CAGR) of 6.20% from 2024 to 2034. This growth underscores the increasing recognition of the region as a pivotal site for research. Companies like ReGelTec and Flow-FX have successfully harnessed this agility, with Flow-FX selecting Colombia for its initial human study, showcasing the tangible benefits of bioaccess®'s approach.
With ethical approvals achieved in as little as 4-6 weeks and an impressive 90.9% of studies receiving ethical approval in first-in-human trials, bioaccess® is revolutionizing the timelines for clinical studies. This transformation enables quicker patient access to groundbreaking medical solutions, highlighting the importance of collaboration in device development and advancing healthcare innovation.
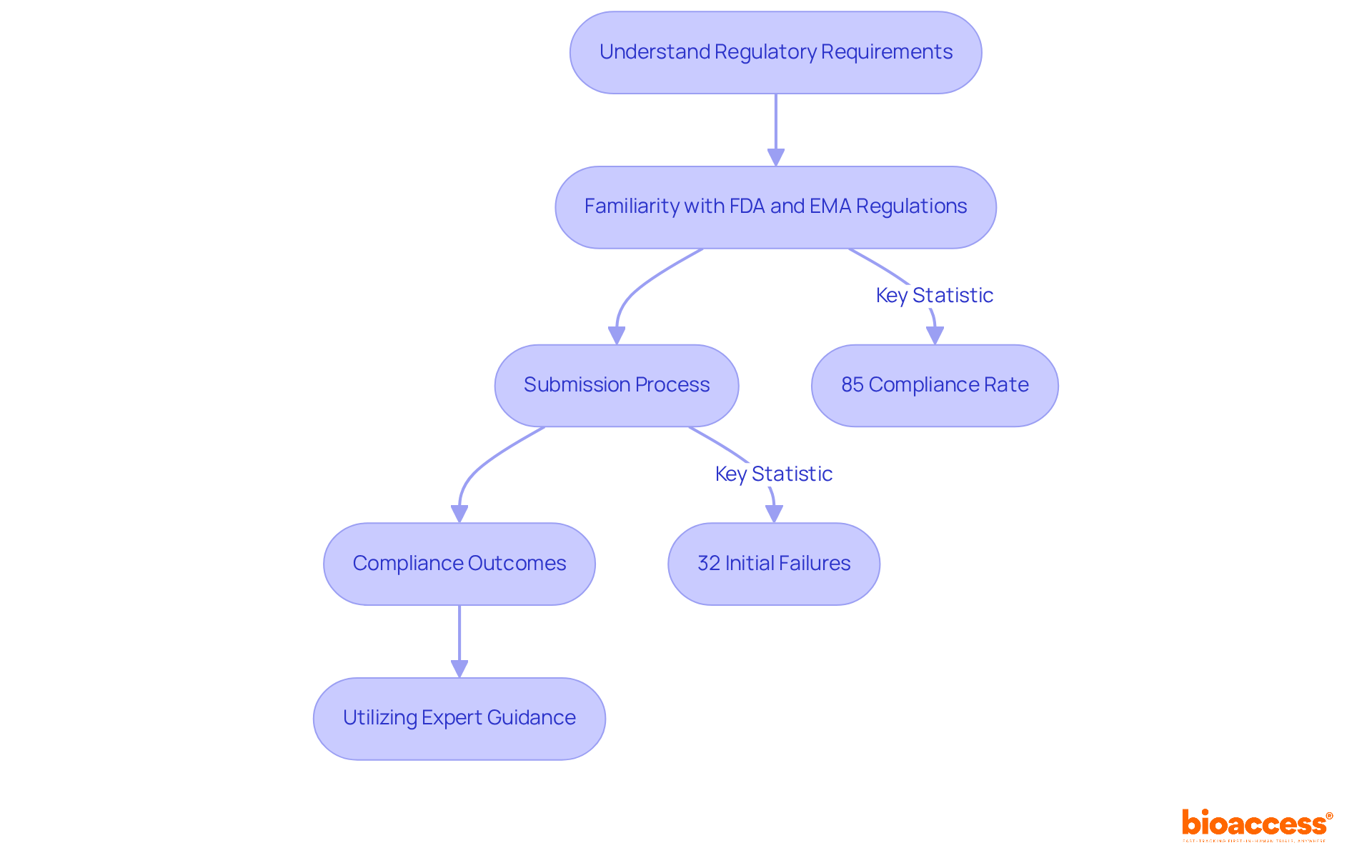
Understanding the compliance environment is crucial before diving into device development. Familiarity with the requirements set by regulatory bodies like the FDA in the U.S. and the EMA in Europe is vital. By adhering to these regulations, you not only ensure the safety and effectiveness of medical equipment but also streamline the approval process, significantly reducing time to market. For example, 85 percent of 510(k) applications received a Substantially Equivalent decision from the FDA, showcasing a favorable compliance rate that aids market entry. Yet, challenges persist; nearly 32 percent of submissions failed the initial acceptance for review check in the year leading up to September 2022, highlighting the necessity for thorough preparation.
Recent updates in medical equipment regulations, particularly the legal effective date for current guidance on companion diagnostics consultation procedures set for December 17, 2024, underscore the importance of staying informed and adapting to changing compliance requirements. Industry leaders stress that viewing compliance as an ongoing commitment to patient safety and product quality can transform challenges into opportunities for innovation, growth, and device development. Successful submissions, including those for Class III devices like ventricular assist devices, which require a premarket approval (PMA) application, illustrate the critical role of comprehensive compliance knowledge in achieving timely approvals.
Moreover, leveraging the expertise of professionals like Ana Criado, Director of Compliance at bioaccess, who possesses extensive experience in compliance and biomedical engineering, can provide invaluable support in navigating these complexities. Bioaccess® offers expedited clinical trial services that connect innovative Medtech, Biopharma, and Radiopharma startups with leading clinical research sites, ensuring a faster path to regulatory approval and entry into the industry.
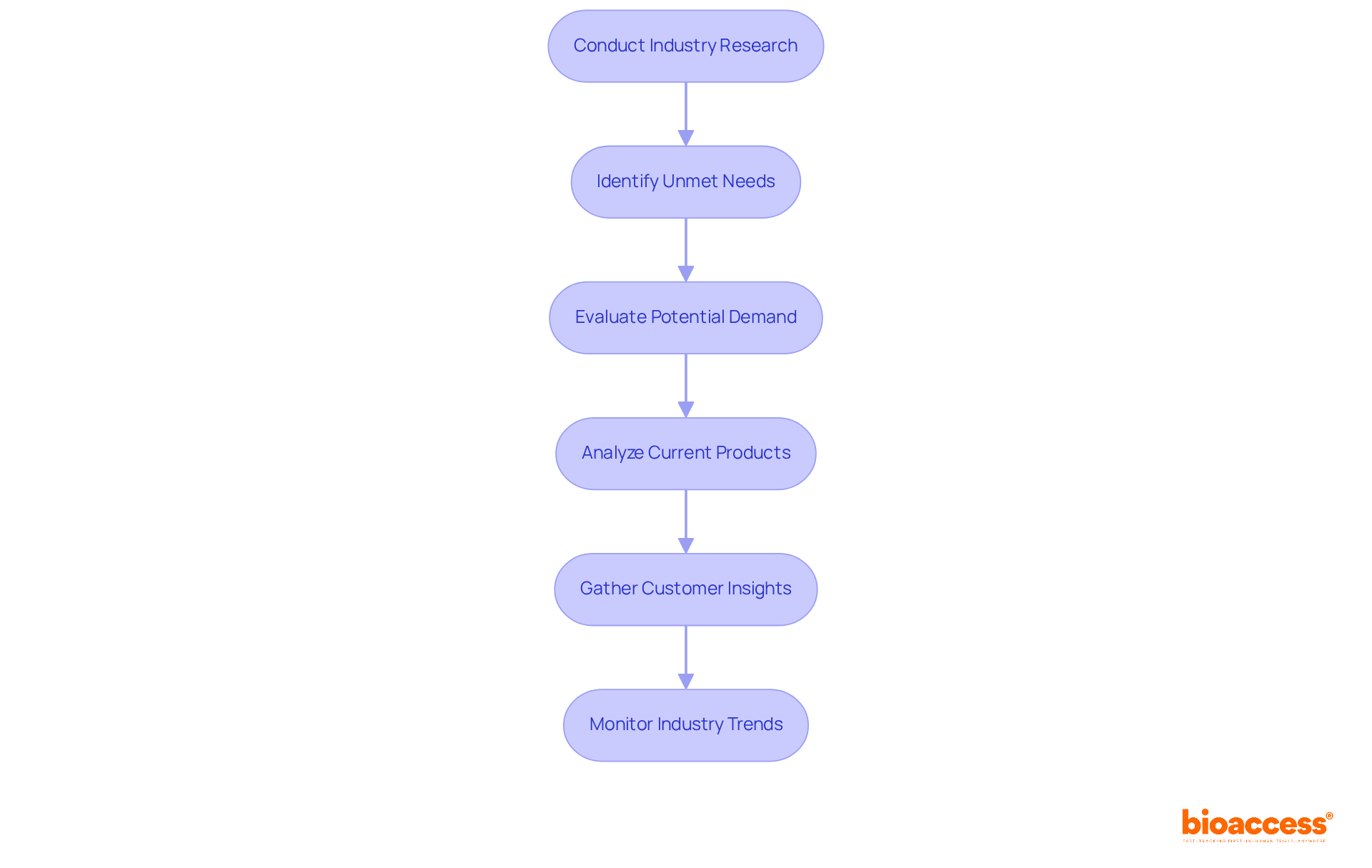
Conducting thorough industry research is crucial for innovators aiming to navigate the competitive landscape of the Medtech sector. This process not only identifies unmet needs but also evaluates the potential demand for device development. By analyzing current products, gathering customer insights, and monitoring industry trends, companies can ensure that their device development effectively addresses real-world challenges. For example, the increasing prevalence of chronic diseases has led to a substantial demand for advanced healthcare solutions, particularly in wearable technology and personalized medicine.
Effective analysis often involves the strategic use of AI tools, which enhance data precision and segmentation. This enables companies to pinpoint specific gaps within the industry. Expert insights underscore that understanding these unmet needs is vital for aligning device development with consumer demands. Ultimately, this alignment results in more effective healthcare solutions, reinforcing the importance of collaboration in addressing key challenges in clinical research.
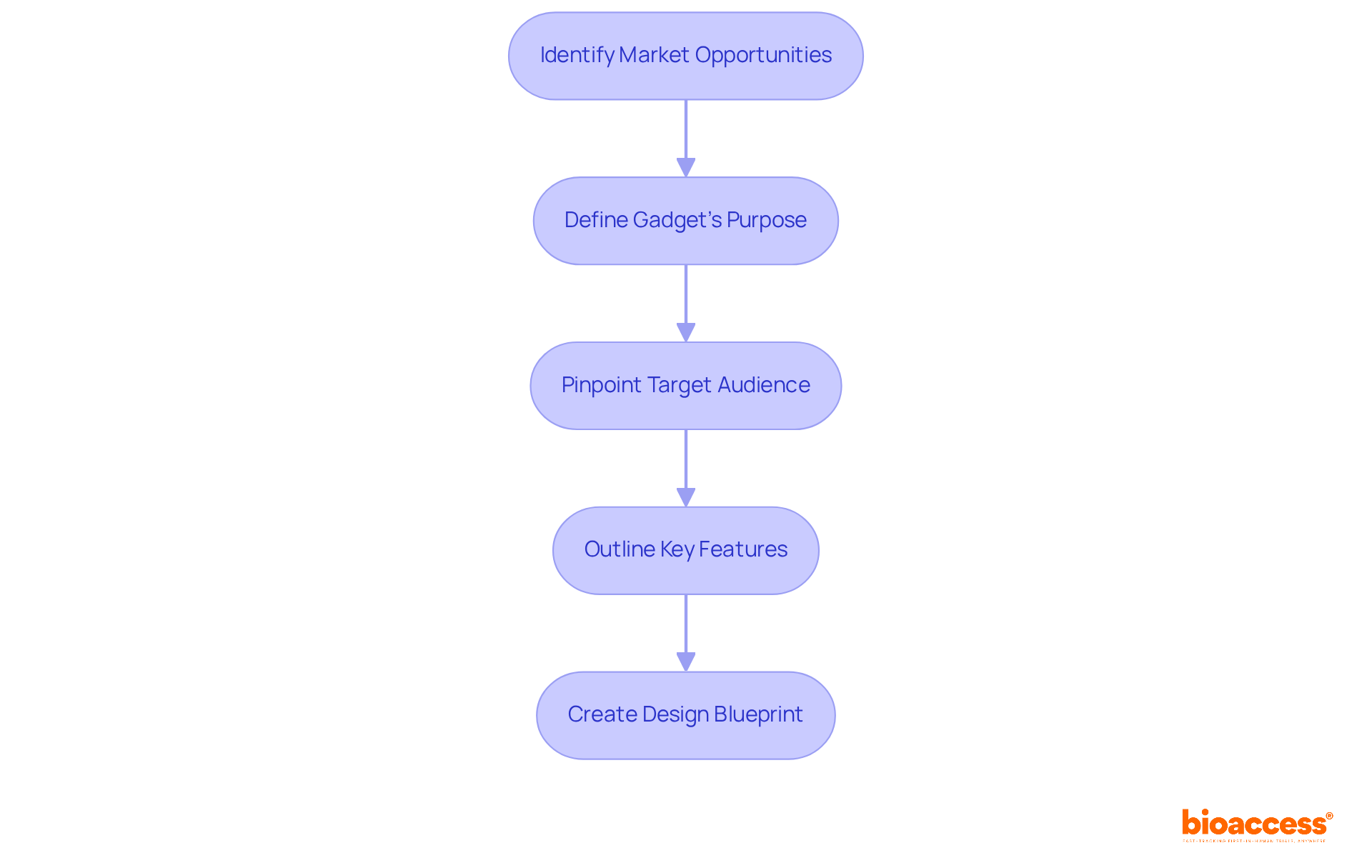
Identifying market opportunities is just the beginning; the next crucial step is to develop a clear product concept. This process involves:
A comprehensive design blueprint must be created to guide the development process. This blueprint ensures that all team members are aligned and focused on the same goals, fostering collaboration and efficiency. By establishing a solid foundation, you set the stage for successful product development.
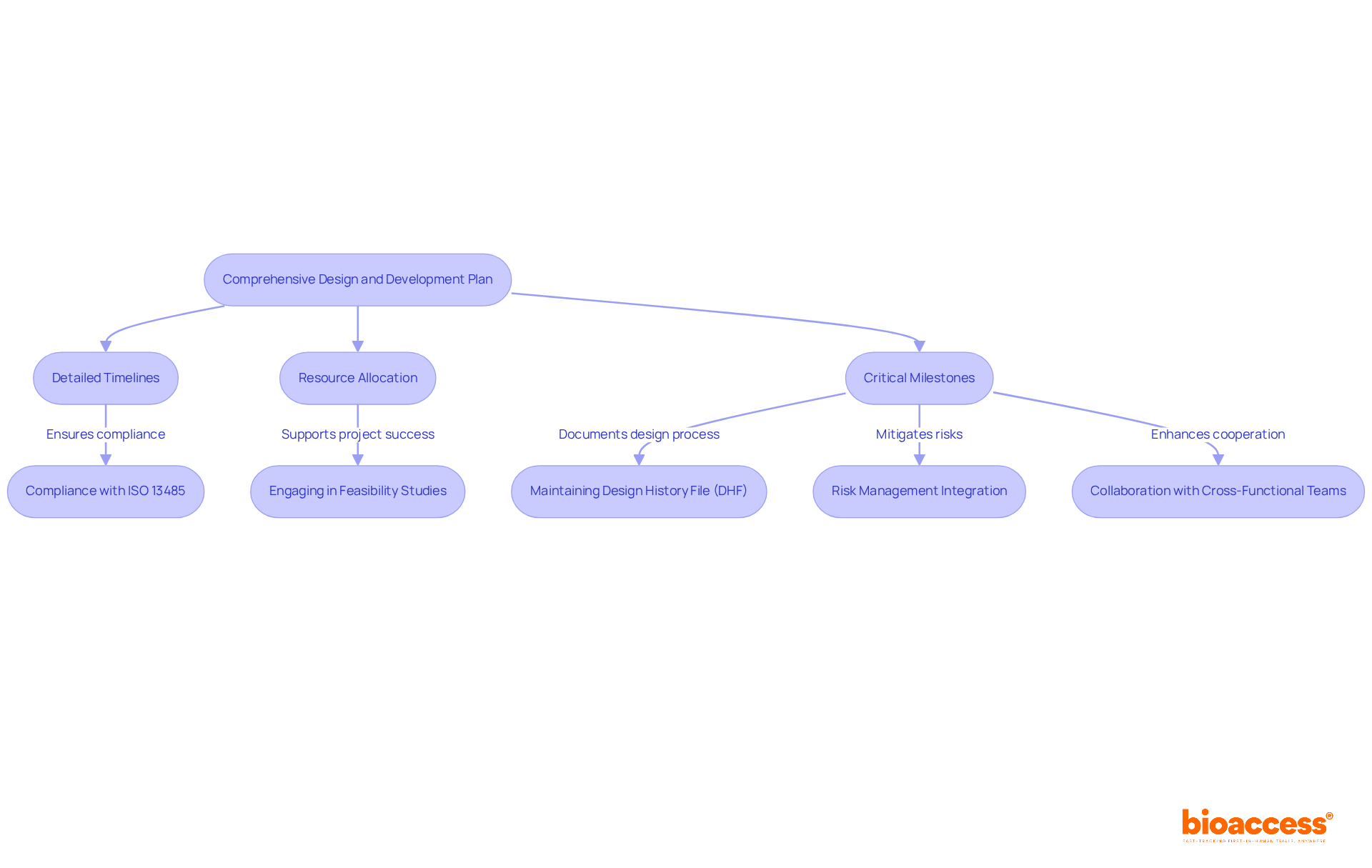
Developing a comprehensive design and device development plan is essential for successful medical device projects. This plan meticulously outlines all phases, from the initial concept to final testing and validation. Key components include:
These components are vital for keeping the project on track and within budget. Efficient project management not only aids in compliance with standards, such as ISO 13485 and FDA guidelines, but also enhances cooperation among cross-functional teams, ensuring that all elements of the design process are considered.
Engaging in feasibility studies and selecting appropriate research sites and principal investigators (PIs) are crucial steps that can significantly impact the success of clinical trials. Furthermore, compliance evaluations and trial preparation—including acquiring essential import permits and nationalizing investigational equipment—are crucial for maneuvering through the oversight framework. Maintaining a Design History File (DHF) is vital for demonstrating compliance with design control requirements.
As we move into 2025, the emphasis on comprehensive design plans for device development will only grow, underscoring their role in aligning project goals with market needs and regulatory expectations. This proactive approach not only mitigates risks but also positions organizations to thrive in an increasingly competitive landscape.
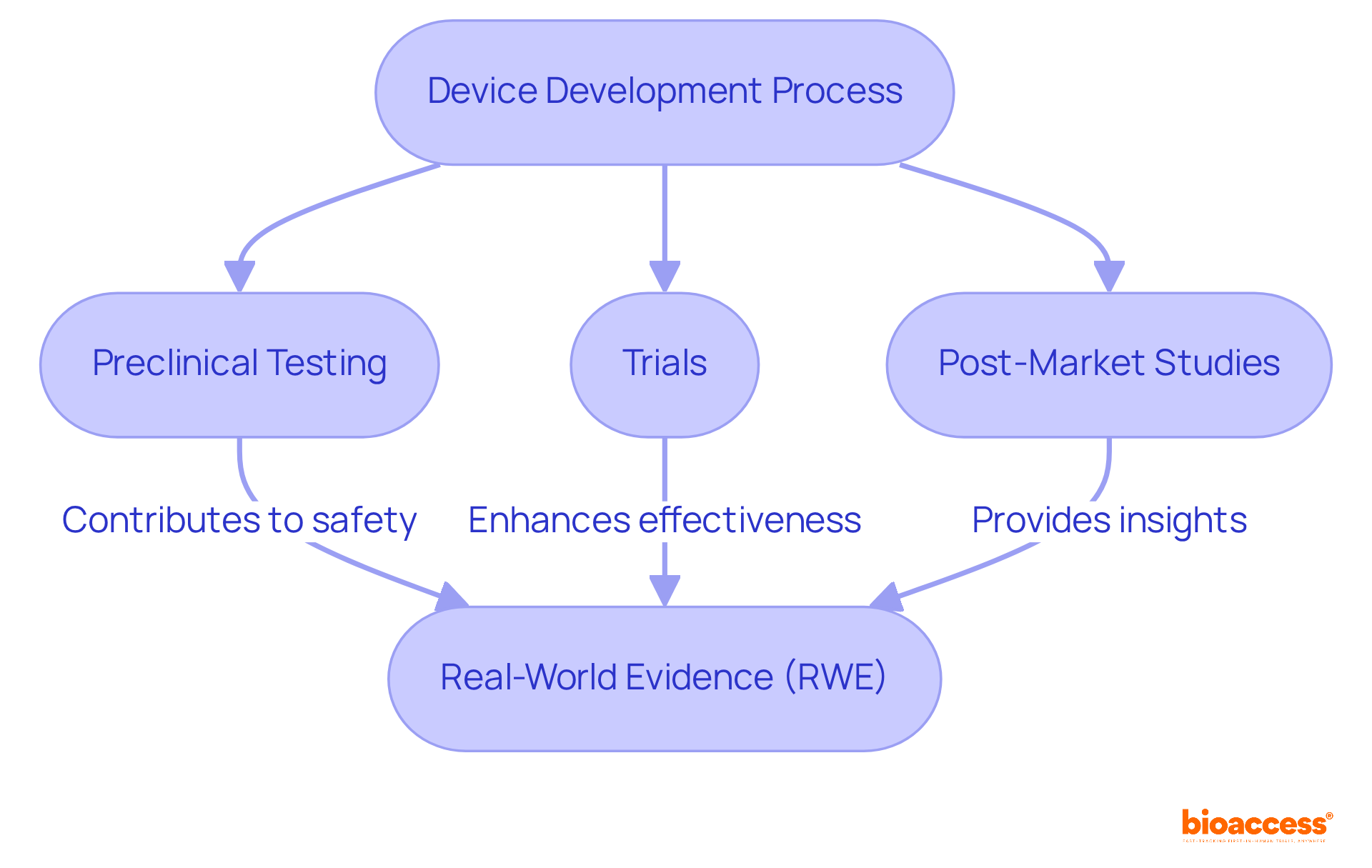
Implementing rigorous testing and validation protocols is crucial for confirming that device development ensures medical devices perform as intended. This process of device development encompasses preclinical testing, trials, and post-market studies, each contributing valuable data on safety and effectiveness. For instance, successful medical trials often leverage advanced statistical techniques, such as Bayesian hierarchical modeling, to enhance the credibility of their findings. Furthermore, real-world evidence (RWE) plays a vital role in analyzing registry data, providing insights that complement clinical trial outcomes.
Adhering to these protocols not only ensures compliance with regulatory standards but also fosters trust among stakeholders and end-users. Firms that emphasize robust verification and validation (V&V) practices are more likely to produce high-quality products with fewer recalls, ultimately boosting user satisfaction. As the landscape of medical instrument development evolves, staying informed on best practices and emerging trends in device development is essential for achieving successful outcomes.
In the ever-changing Medtech landscape, collaboration is key. By addressing key challenges together, stakeholders can enhance the effectiveness of clinical research. What steps are you taking to ensure your organization remains at the forefront of these developments? The importance of rigorous testing and validation cannot be overstated; it is the foundation upon which trust and quality are built.
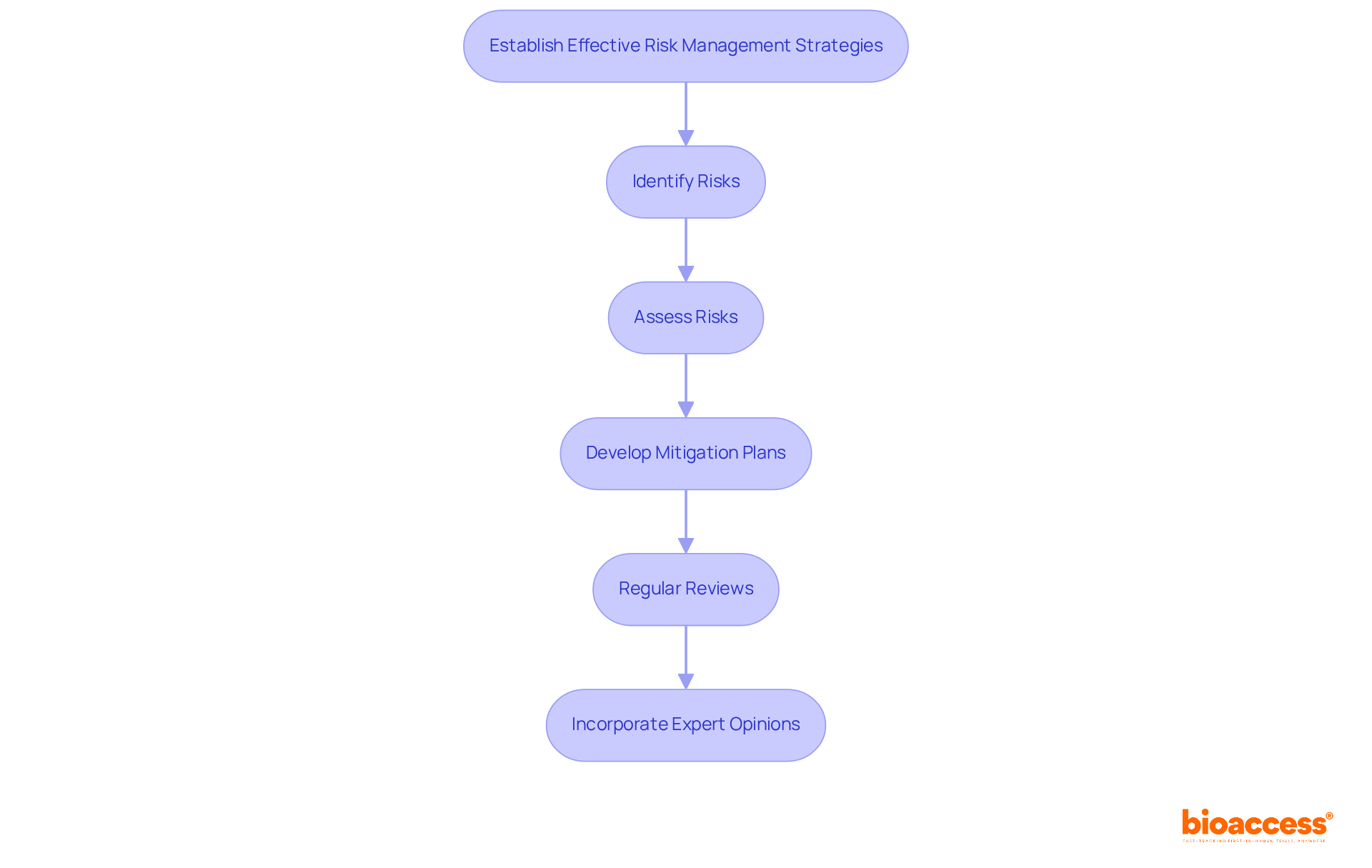
Effective risk management strategies are essential for navigating the complexities of device development. This process begins with a thorough identification of potential risks, including technical and compliance challenges. Consider this: over 700 hospitals are currently facing the threat of closure, which significantly impacts access and patient recruitment for clinical trials. Moreover, the healthcare sector grapples with substantial underpayments from Medicare and Medicaid, exceeding $100 billion, complicating the financial landscape for Medtech projects.
To proactively tackle these challenges, developing comprehensive mitigation plans is crucial. Regular reviews and updates of risk management strategies are necessary to adapt to the evolving project landscape and emerging threats, such as cybersecurity risks, which accounted for over half of all healthcare cyber incidents in recent years. By incorporating expert opinions and case studies, organizations can better assess technical and regulatory risks, ensuring their strategies remain robust and effective.
For instance, the decline of obstetrical services in rural hospitals, where 238 facilities closed their units between 2010 and 2022, illustrates the impact of economic realities on healthcare access. This situation underscores the importance of recognizing and addressing risks early in the device development process to safeguard against potential industry disruptions. By prioritizing risk management, Medtech innovators can significantly enhance their chances of successful device development and commercialization.
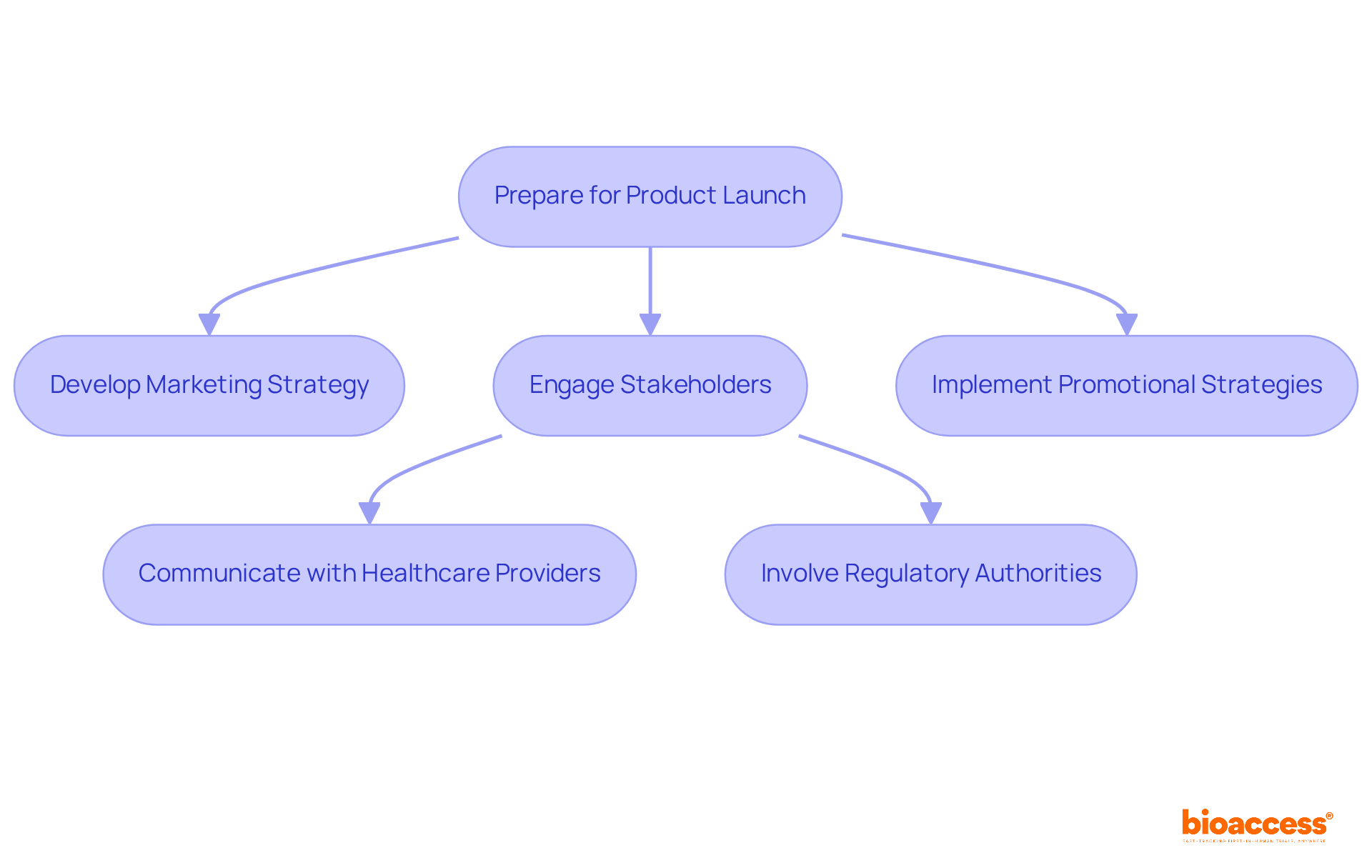
Launching a product in the medical equipment sector demands a robust marketing strategy that covers pricing, distribution, and promotional activities. Engaging essential stakeholders, including healthcare providers and regulatory authorities, is vital for a smooth entry into the industry. This involvement not only boosts awareness but also fosters acceptance of the new product among potential users.
Promotional strategies play a crucial role in navigating the complexities of the technology sector, particularly as the global MedTech industry is projected to reach $800 billion by 2030, with a compound annual growth rate of 5.6%. Companies must emphasize transparent communication and ethical storytelling, responsibly sharing patient narratives to resonate with healthcare professionals. Leveraging data-driven insights can further refine marketing efforts, ensuring campaigns meet the specific needs and behaviors of target audiences.
By focusing on these elements, organizations can enhance their market visibility and facilitate the successful acceptance of their innovative medical products. Collaboration is key, and the next steps involve aligning strategies with stakeholder expectations to drive impactful results.
Carrying out post-market monitoring is crucial for the ongoing enhancement of medical instruments. It involves systematically gathering and analyzing data on performance in real-world environments. This continuous evaluation not only helps identify potential issues but also provides valuable insights that inform future enhancements in device development. By ensuring that equipment consistently meets user needs and compliance standards, organizations can significantly boost performance and user satisfaction.
In 2025, the emphasis on real-world performance evaluation is more critical than ever. It enables data-driven decision-making that fosters innovation and operational excellence. A robust post-market surveillance strategy is essential for device development, allowing for adaptation to changing market conditions and achieving sustainable growth in the Medtech sector. bioaccess® specializes in conducting post-market clinical follow-up studies (PMCF) in Latin America, leveraging over 20 years of Medtech expertise to ensure that your products not only meet regulatory standards but also excel in practical applications.
Additionally, bioaccess® manages a variety of studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which further support continuous improvement efforts. Involving employees in the continuous improvement process empowers them to contribute to identifying areas for enhancement, fostering a culture of collaboration and accountability. This collaborative approach is vital for addressing key challenges in clinical research and ensuring that innovations are effectively implemented.
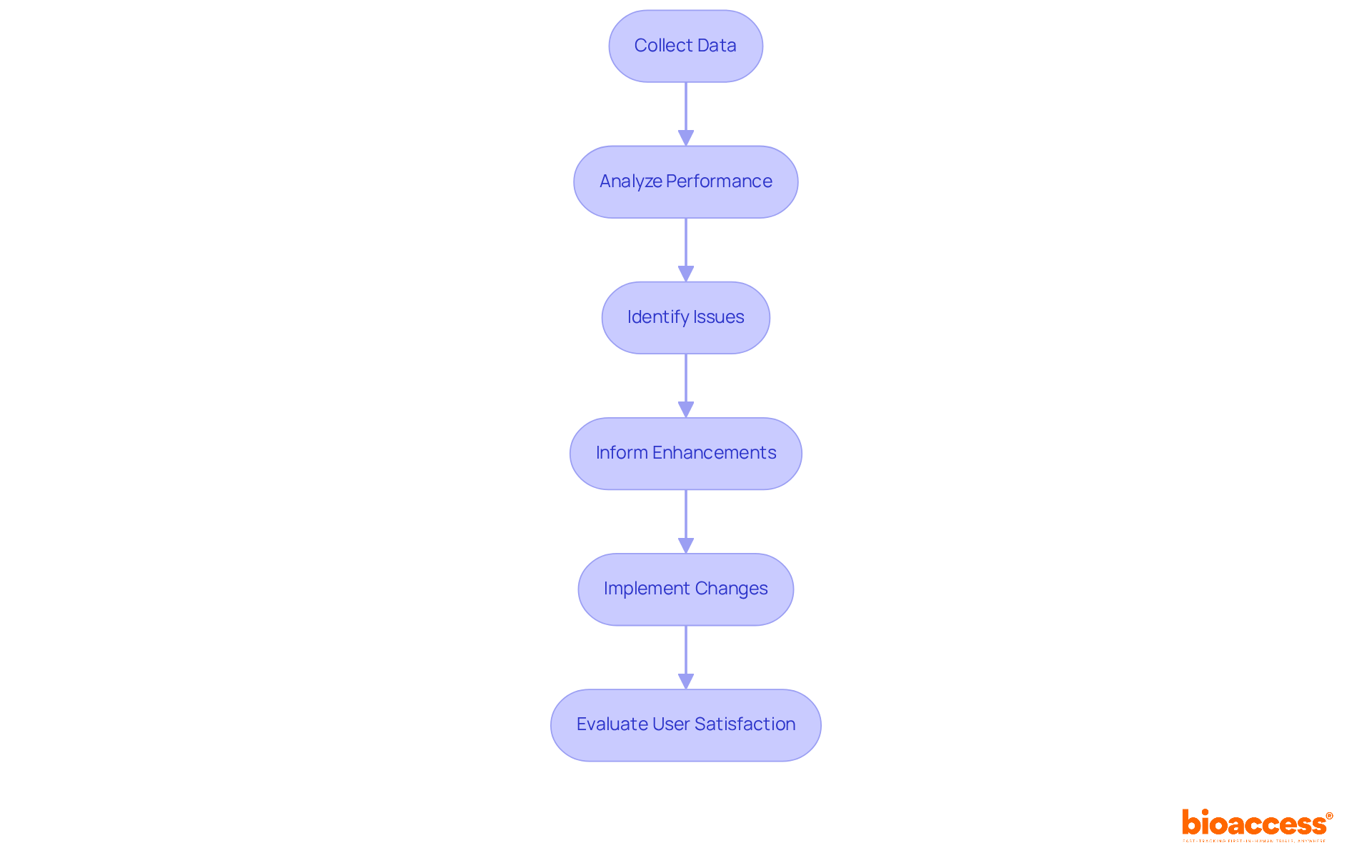
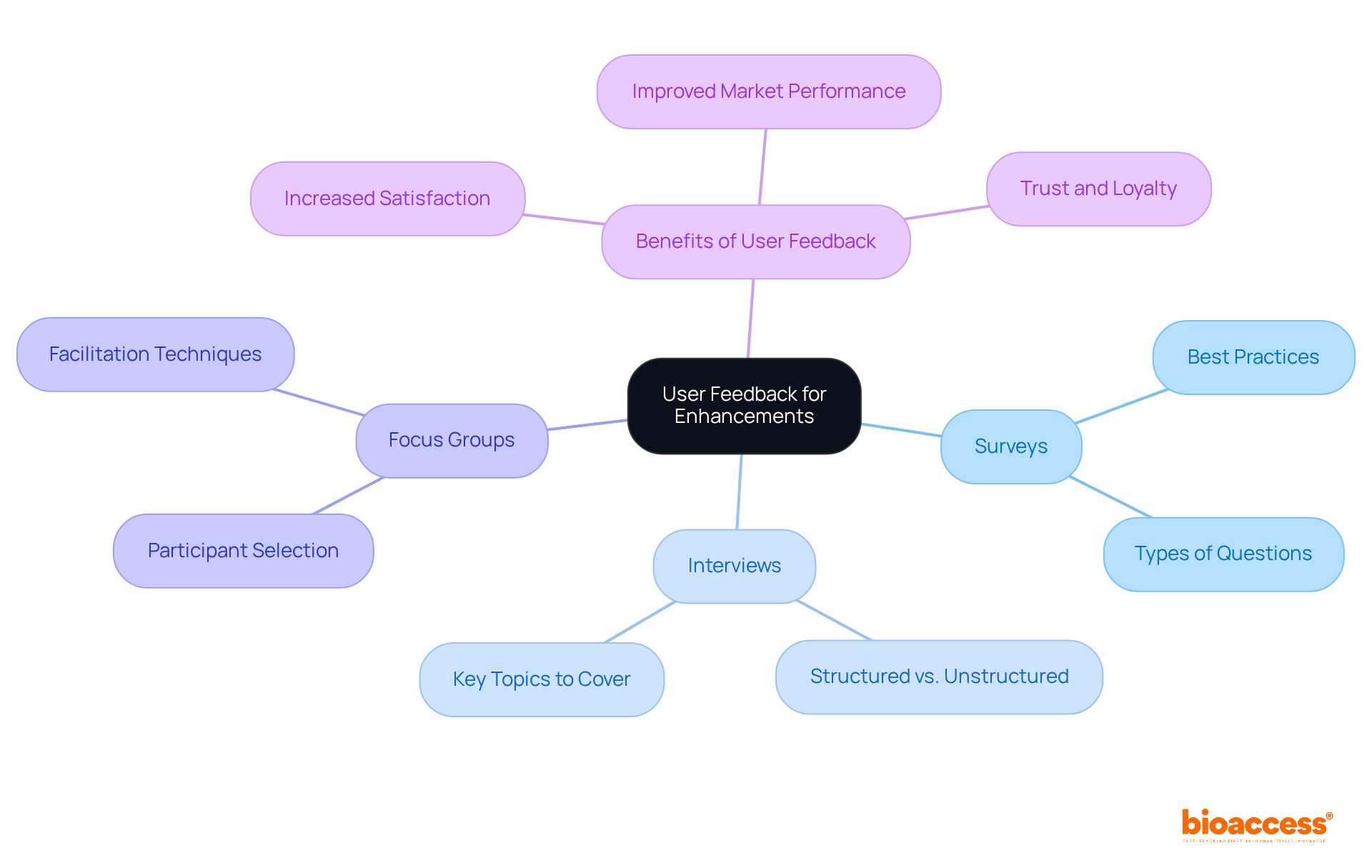
Collecting user input is essential for pinpointing both strengths and weaknesses in medical equipment. Effective methods for gathering this feedback include:
By systematically evaluating the insights obtained, organizations can make informed decisions about future improvements, ensuring that equipment evolves to meet changing needs and expectations.
In 2025, the emphasis on user-centered design is more pronounced than ever. Medtech companies increasingly recognize that user feedback is not merely advantageous but vital for product enhancement. For example, companies that actively involve users in the design process often experience higher satisfaction rates and improved market performance. As Arda Ural, PhD, points out, "Organizations that prioritize differentiated innovation will be best positioned to deliver long-term growth and shareholder value."
Expert opinions highlight the necessity of integrating user insights into the device development cycle. Industry leaders note that prioritizing user feedback can lead to innovations in device development that resonate with healthcare providers and patients alike, ultimately driving the success of new devices in a competitive landscape. This approach not only enhances product functionality but also builds trust and loyalty among users, paving the way for sustained success in the Medtech sector.
Successful device development relies on a structured approach that encompasses several critical steps. It all starts with a solid understanding of the regulatory landscape and market opportunities, ensuring compliance while aligning with consumer needs. Innovators can effectively navigate the complexities of clinical research by leveraging platforms like bioaccess®, which accelerates timelines and enhances collaboration.
Key insights from the article underscore the importance of comprehensive planning, rigorous testing, and effective risk management. Each phase, from conceptualization to post-market surveillance, plays a vital role in ensuring that medical devices not only meet regulatory requirements but also tackle real-world challenges. By emphasizing user feedback throughout the development cycle, the connection between product functionality and user satisfaction is further strengthened, ultimately driving success in the competitive Medtech landscape.
As the medical device industry continues to evolve, embracing these essential steps will be crucial for organizations aiming to innovate and thrive. By prioritizing collaboration, compliance, and continuous improvement, stakeholders can significantly enhance their impact on healthcare, paving the way for groundbreaking solutions that improve patient outcomes. The call to action is clear: engage with these best practices and leverage available resources to ensure that the next generation of medical devices meets the highest standards of safety and effectiveness.
What is bioaccess® and what advantages does it offer for clinical research?
bioaccess® is a platform that leverages Colombia's competitive advantages to provide significant cost savings of over 30% compared to North America and Western Europe. It facilitates regulatory speed, allowing device development alongside IRB/EC and MoH (INVIMA) reviews in just 90-120 days, enabling Medtech, Biopharma, and Radiopharma innovators to expedite their breakthroughs.
How is the Latin America trials market projected to grow?
The Latin America trials market is projected to reach approximately USD 7.94 billion by 2034, with a compound annual growth rate (CAGR) of 6.20% from 2024 to 2034, indicating the region's increasing recognition as a key site for research.
What is the significance of ethical approvals in clinical studies through bioaccess®?
bioaccess® achieves ethical approvals in as little as 4-6 weeks, with 90.9% of studies receiving ethical approval in first-in-human trials. This significantly reduces timelines for clinical studies and enables quicker patient access to innovative medical solutions.
What are the key regulatory requirements for medical device development?
Understanding compliance with regulatory bodies like the FDA in the U.S. and the EMA in Europe is crucial. Adhering to these regulations ensures the safety and effectiveness of medical devices and streamlines the approval process, reducing time to market.
What challenges do companies face in the regulatory submission process?
Nearly 32% of submissions failed the initial acceptance for review check in the year leading up to September 2022, highlighting the need for thorough preparation and compliance knowledge to achieve timely approvals.
How can industry leaders adapt to changing compliance requirements?
Staying informed about recent updates in regulations, such as those regarding companion diagnostics set for December 17, 2024, is essential. Viewing compliance as an ongoing commitment to patient safety and product quality can help transform challenges into opportunities.
What role does market research play in device development?
Conducting thorough market research helps innovators identify unmet needs and evaluate potential demand for device development. This involves analyzing current products, gathering customer insights, and monitoring industry trends to ensure effective solutions.
How can AI tools enhance market research in the Medtech sector?
AI tools can improve data precision and segmentation, allowing companies to identify specific gaps within the industry. This strategic analysis helps align device development with consumer demands and real-world challenges.
Who can provide support in navigating regulatory complexities in medical device development?
Professionals with extensive experience in compliance and biomedical engineering, such as Ana Criado, Director of Compliance at bioaccess, can offer invaluable support in navigating the complexities of regulatory requirements.