


This article outlines the essential steps required for successful Investigational New Drug (IND) approval in clinical trials. It highlights the significance of thorough preparation, which includes:
These practices significantly enhance the likelihood of obtaining IND approval, as they help align research objectives with regulatory expectations and streamline the submission process. By focusing on these critical elements, clinical researchers can navigate the complexities of the IND process more effectively.
Navigating the complex landscape of Investigational New Drug (IND) approval is a critical step for any organization aiming to bring innovative therapies to market. As the demand for efficient clinical trials rises, understanding the essential steps involved in securing IND approval becomes paramount. This article delves into ten vital strategies that not only streamline the approval process but also enhance the chances of success, offering invaluable insights for Medtech, Biopharma, and Radiopharma innovators. With the stakes high, stakeholders must effectively tackle the challenges of IND applications while ensuring compliance and expediting their journey to market.
bioaccess® excels in streamlining the IND approval process by leveraging its profound understanding of regulatory frameworks and clinical research environments. With a strong foothold in Latin America, the Balkans, and Australia, bioaccess® offers customized guidance that empowers Medtech, Biopharma, and Radiopharma innovators to adeptly navigate the intricacies of IND approval. This expert assistance not only speeds up timelines but also greatly enhances the likelihood of successful endorsements, enabling faster market entry for innovative therapies.
Recent advancements in strategic guidance underscore the importance of IND approval. For instance, the FDA's clearance of IND applications for innovative therapies like NTLA-2001 and PM359 exemplifies the potential for expedited pathways when supported by organizations with IND approval. By harnessing its extensive experience, bioaccess® ensures that clients can effectively capitalize on these opportunities. Ultimately, this enhances the efficiency of clinical trials and the delivery of transformative medical solutions.
The IND application procedure is a critical process encompassing several essential components, including preclinical data, manufacturing information, and clinical protocols. A comprehensive understanding of the requirements for each element is crucial for a successful submission. Companies must familiarize themselves with the FDA's guidelines, which have evolved to emphasize clarity and organization in applications. Recent updates stress the importance of presenting data concisely, avoiding unnecessary information that could lead to confusion or delays.
Thorough preparation of all essential documentation not only facilitates a smoother application experience but also significantly reduces the likelihood of setbacks caused by incomplete or incorrect submissions. Interacting with FDA officials early in the procedure can provide valuable insights into the expectations for IND applications, thereby increasing the chances of IND approval. In this evolving landscape, understanding the nuances of the IND process is paramount for stakeholders aiming to navigate clinical research successfully.

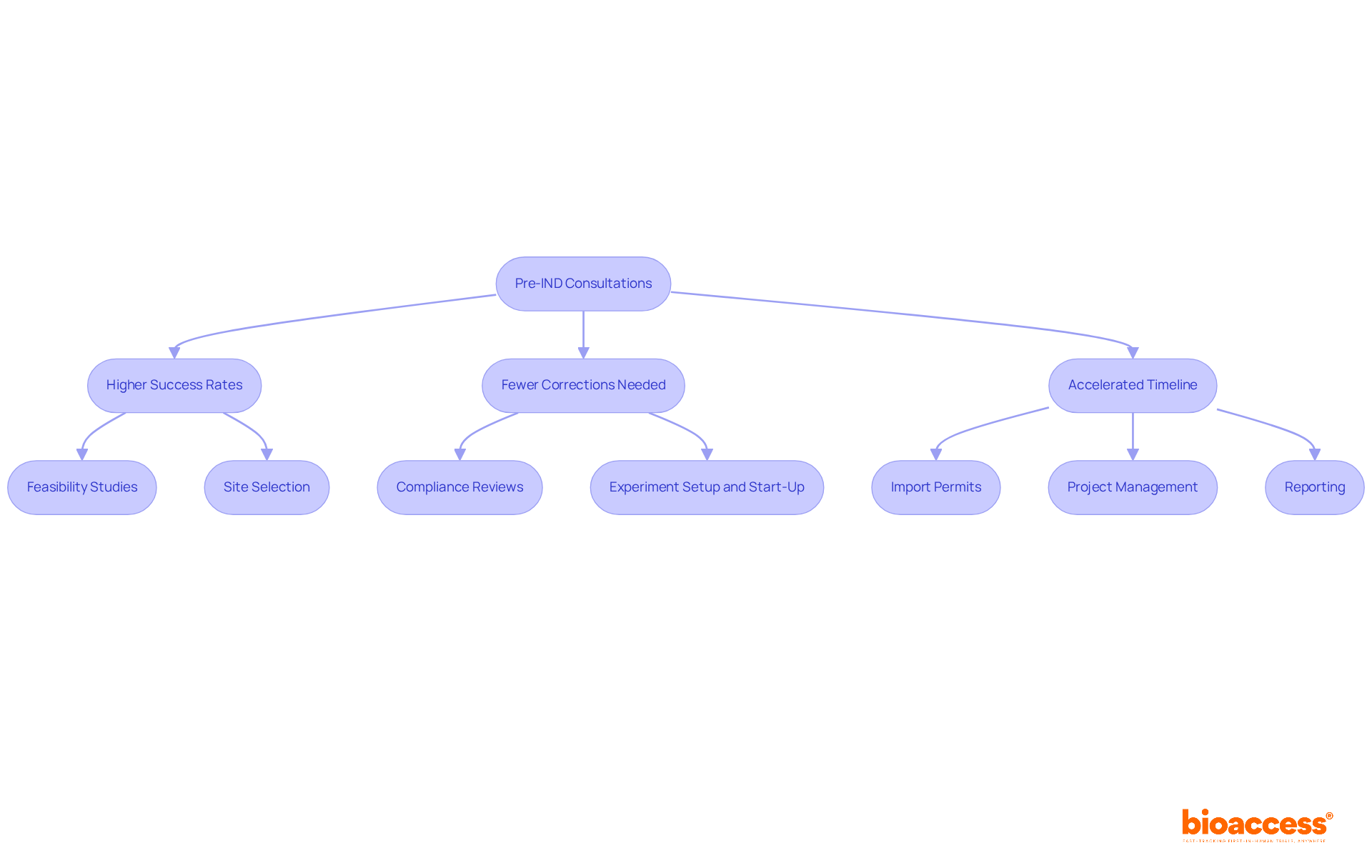
Participating in pre-IND consultations with the FDA or appropriate regulatory bodies is a crucial strategy that enhances transparency in the submission procedure. These meetings provide sponsors with the opportunity to discuss their proposed studies, receive critical feedback, and address potential concerns early on. Research indicates that companies utilizing pre-IND consultations experience significantly higher success rates in obtaining IND approval for their applications, as these interactions help align research objectives with regulatory expectations. For instance, organizations that actively engage in these consultations often report a decrease in the number of corrective responses needed during the IND submission phase, with approximately 9% of submissions requiring multiple corrections. Additionally, successful pre-IND meetings have been demonstrated to accelerate the overall timeline for authorization, creating a more efficient route to clinical trials. Industry leaders stress that these consultations not only clarify regulatory requirements but also promote a cooperative relationship with the FDA, ultimately resulting in a more seamless IND approval process. By proactively seeking guidance, companies can significantly simplify their IND authorization journey.
To enhance this process, bioaccess offers a range of comprehensive clinical trial management services that directly support pre-IND consultations, including:
These services together enhance a more efficient IND approval submission system, ultimately increasing the likelihood of successful endorsement.
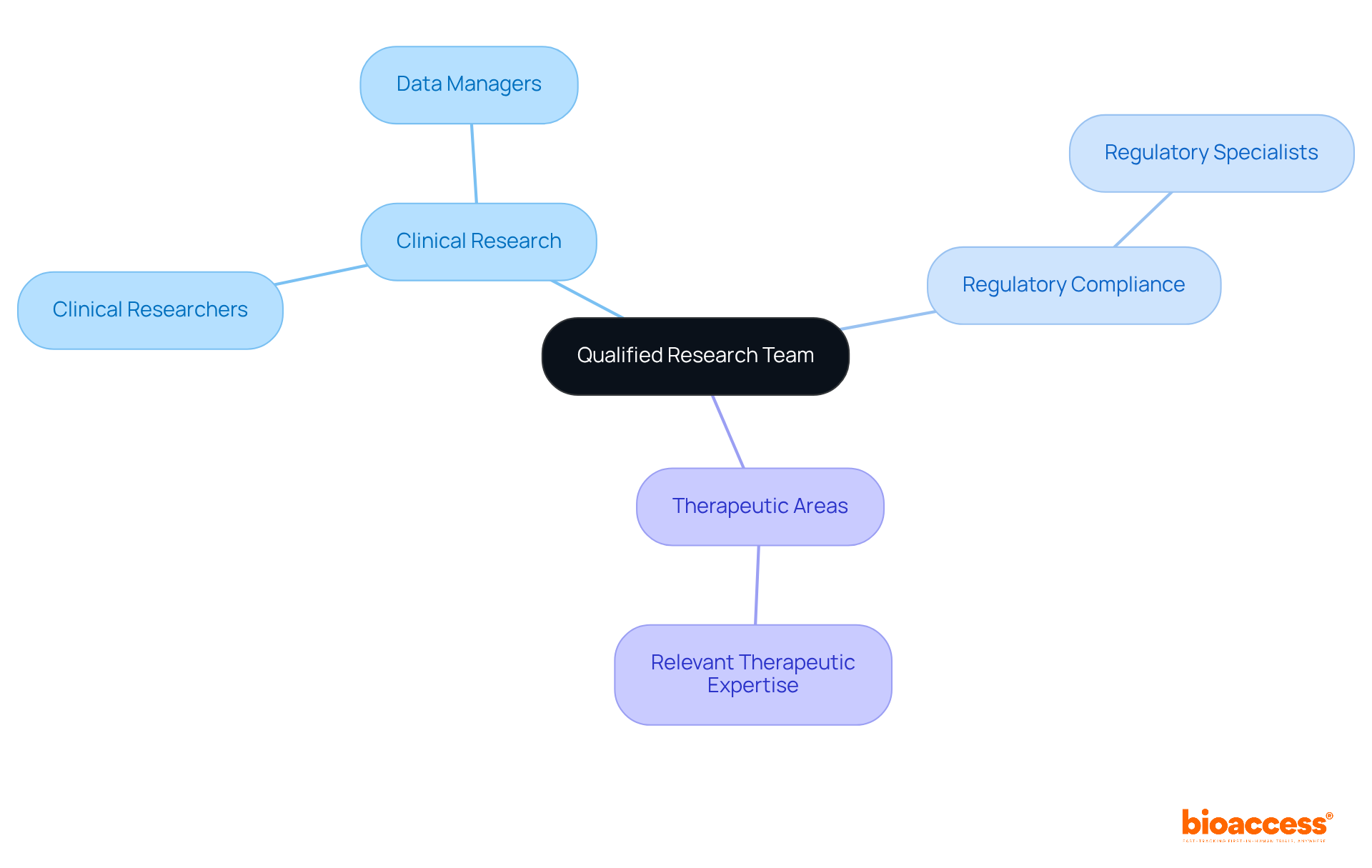
Assembling a skilled research team is crucial for navigating the complexities of the IND application process. This team should include individuals with specialized expertise in:
Essential roles encompass:
Effective collaboration among these specialists not only enhances the quality of the research but also significantly increases the likelihood of receiving IND approval. The composition of the team can directly influence the speed and efficiency of the validation process, underscoring the importance of selecting individuals who bring both expertise and a commitment to excellence in their respective fields.
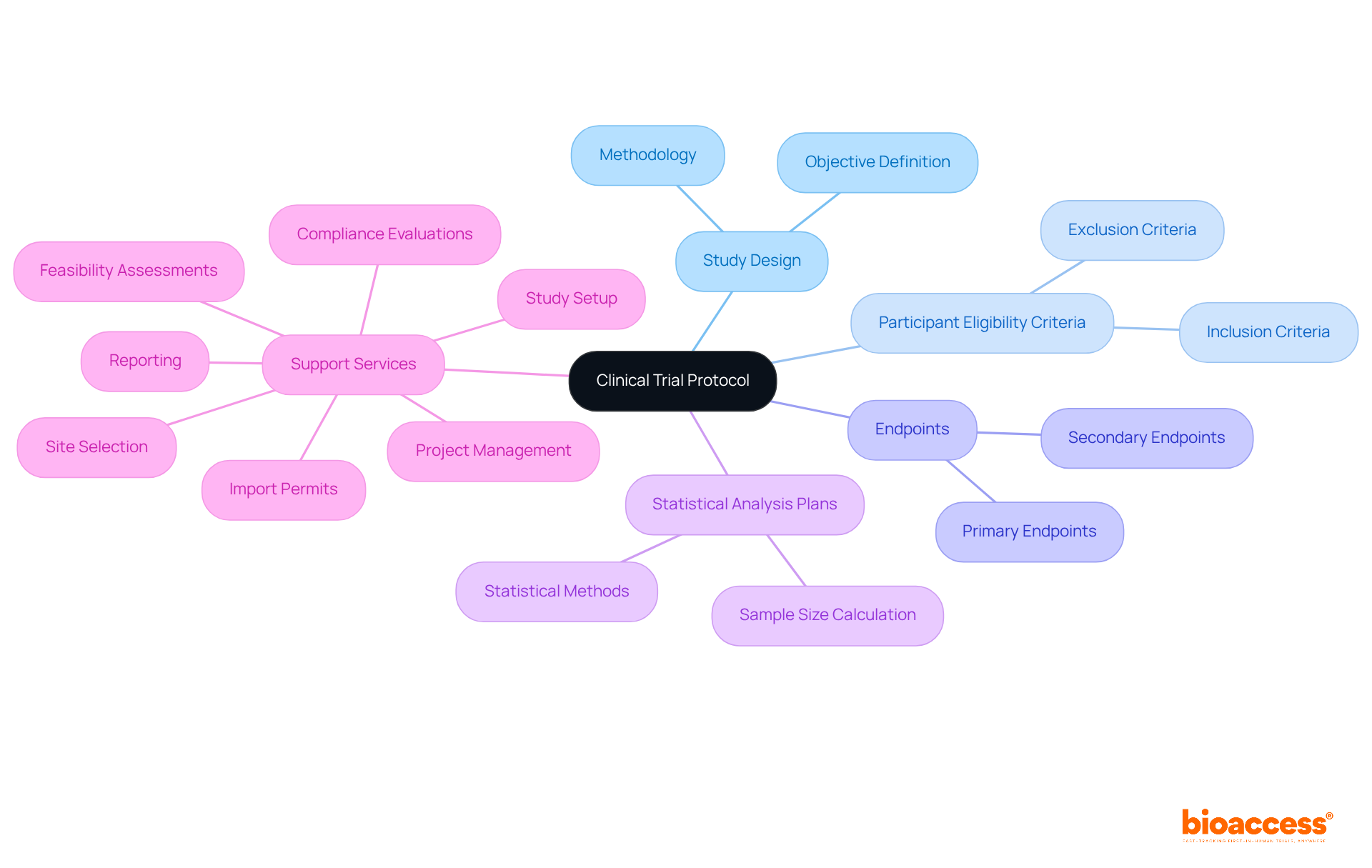
A meticulously crafted clinical study protocol is essential for successful IND approval. This document must encompass all necessary details, including:
It serves not only as a roadmap for the research team but also as a vital component of the IND submission. Regulatory authorities require a comprehensive protocol to assess the study's scientific rigor and planning. A well-structured protocol can significantly enhance the likelihood of approval by demonstrating that the study is methodologically sound and ethically designed.
As emphasized by the International Conference on Harmonization, a precise definition of the study's objectives and design is crucial for efficiency and consistency in protocol writing. Moreover, the protocol serves as an essential quality control instrument, ensuring the health and safety of all participants engaged in the study. Bioaccess provides extensive clinical study management services, including:
These services are essential for developing a robust protocol.
Successful examples of clinical study protocols in IND applications demonstrate the significance of meticulous preparation and compliance with regulatory standards, ultimately resulting in more effective and prompt approvals.
Implementing rigorous documentation practices is essential for maintaining the integrity of clinical research data. Establishing standard operating procedures (SOPs) for data collection, storage, and reporting is crucial. A systematic organization of all trial-related documents facilitates easy access during audits and inspections, ensuring compliance with regulatory requirements. Effective data management not only enhances the quality of research but also significantly impacts the integrity of clinical trials.
For instance, routine data quality audits and interim evaluations can identify potential issues early, reducing complications during the database lock phase. Furthermore, the use of electronic data capture (EDC) systems simplifies data entry, minimizing errors and accelerating the overall data management workflow.
As noted by experts in the field, maintaining data integrity is vital for producing reliable and statistically sound results, ultimately supporting successful IND approval and advancing medical innovations.
Consistent communication with regulatory authorities during the IND application stage is essential for success. This engagement must encompass:
By cultivating a collaborative relationship with regulators, sponsors can proactively address concerns, thereby demonstrating their commitment to compliance and patient safety. Efficient communication not only aids in navigating the complexities of the approval system but also significantly influences IND approval, ensuring a more seamless pathway to clinical studies.
Creating a clear communication plan is crucial for keeping all stakeholders informed throughout the clinical study process. This includes regular updates to team members, sponsors, and regulatory authorities. Utilizing project management tools and communication platforms facilitates transparency and ensures that everyone is aligned on objectives and timelines. A well-structured communication plan enhances collaboration and helps mitigate potential issues before they escalate.
Clinical studies frequently face unexpected challenges and setbacks, ranging from regulatory hurdles to recruitment issues. To effectively navigate these complexities, it is essential to develop robust contingency plans and identify alternative strategies. By proactively anticipating potential challenges, research teams can respond swiftly and efficiently, thereby minimizing disruptions to the study timeline and sustaining progress toward achieving IND approval.
Industry leaders underscore the importance of clear communication and training for all stakeholders involved, ensuring preparedness to adapt to changing circumstances. For instance, the implementation of long-acting injectable antiretroviral therapy (LAI ART) illustrates how community engagement and education can facilitate successful adoption, even amid complex regulatory environments.
Companies that have adeptly navigated regulatory hurdles often highlight the significance of maintaining flexibility in their approach, allowing for necessary adjustments to protocols and timelines. Recent discussions in the field emphasize the need to address prevalent issues in clinical research, such as staffing challenges and the demand for clear guidelines, to enhance the overall effectiveness of studies.
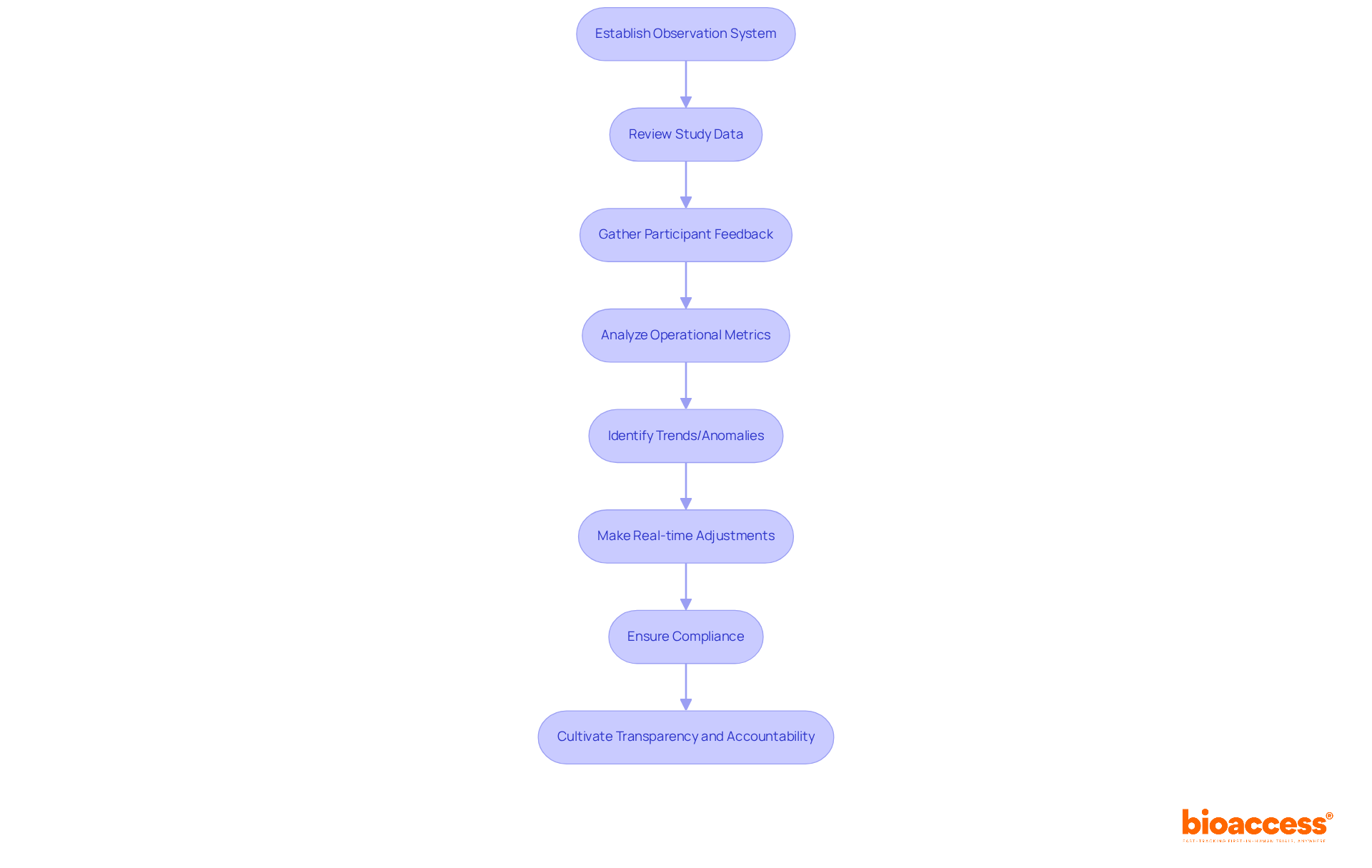
Establishing a robust system for ongoing observation during the research process is crucial for pinpointing areas that require enhancement and ensuring compliance with regulatory standards. By systematically reviewing study data, participant feedback, and operational metrics, research teams can make informed adjustments in real-time. This proactive approach not only elevates the quality of the research but also significantly boosts the likelihood of receiving IND approval.
For instance, the integration of advanced analytics and machine learning empowers researchers to identify trends and anomalies, enabling timely protocol adaptations that prioritize participant welfare. Experts in the field assert that effective continuous monitoring cultivates a culture of transparency and accountability, which is vital for upholding high standards of patient safety and data integrity. Moreover, adaptive trial methodologies, such as those demonstrated in the PRO-MSACTIVE study, showcase how real-time data evaluation can lead to prompt adjustments and enhance overall trial effectiveness.
By embracing these methodologies, clinical research teams can adeptly navigate the complexities of IND approval with increased agility and confidence.
Successfully navigating the IND approval process is essential for bringing innovative therapies to market. This article outlines critical steps that stakeholders must consider, emphasizing the importance of thorough preparation, effective communication, and strategic engagement with regulatory authorities. By understanding these elements, companies can significantly enhance their chances of achieving IND approval and ultimately advancing medical research.
Key insights include:
These steps not only facilitate compliance with regulatory requirements but also promote the successful execution of clinical trials.
The significance of these practices cannot be overstated. By prioritizing a proactive approach, organizations can better position themselves for success in the competitive landscape of clinical research. Embracing these essential steps will not only expedite the IND approval process but also pave the way for the delivery of transformative medical solutions that can improve patient outcomes.
What is bioaccess® and how does it assist in the IND approval process?
bioaccess® specializes in streamlining the IND approval process by leveraging its deep understanding of regulatory frameworks and clinical research environments. It offers customized guidance to Medtech, Biopharma, and Radiopharma innovators, helping them navigate the complexities of IND approval, which accelerates timelines and enhances the chances of successful endorsements for faster market entry.
Why is understanding the IND application process important?
A thorough understanding of the IND application process is crucial as it involves essential components such as preclinical data, manufacturing information, and clinical protocols. Familiarity with FDA guidelines, which emphasize clarity and organization, helps ensure successful submissions and reduces the likelihood of setbacks due to incomplete or incorrect documentation.
What are pre-IND consultations and why are they beneficial?
Pre-IND consultations are meetings with the FDA or relevant regulatory bodies that allow sponsors to discuss their proposed studies and receive feedback. These consultations enhance transparency, align research objectives with regulatory expectations, and have been shown to significantly increase success rates for IND approval, while also accelerating the overall timeline for authorization.
What services does bioaccess® offer to support pre-IND consultations?
bioaccess® offers a range of clinical trial management services to support pre-IND consultations, including feasibility studies, site selection, compliance reviews, experiment setup and start-up, import permits for investigational devices, project management and monitoring, and detailed reporting on study status and compliance.
How do pre-IND consultations impact the likelihood of IND approval?
Companies that engage in pre-IND consultations tend to experience higher success rates in obtaining IND approval. These interactions help clarify regulatory requirements, reduce the number of corrective responses needed during the IND submission phase, and promote a cooperative relationship with the FDA, leading to a more efficient approval process.