


The primary objective of this article is to delineate the essential steps necessary for executing a successful randomized control trial (RCT) within the realm of clinical research. It underscores the importance of clearly defining the research question and employing robust methodologies—such as effective randomization, blinding, and ethical considerations—as critical components in guaranteeing valid and reliable study outcomes. These elements are pivotal in enhancing the overall integrity of the clinical research process, thereby reinforcing the significance of meticulous planning and execution in RCTs.
Understanding the intricate landscape of randomized control trials (RCTs) is crucial for advancing clinical research and improving patient outcomes. With the stakes higher than ever, mastering the essential steps of RCTs can unlock opportunities for groundbreaking discoveries and enhance the credibility of findings.
However, the path to success is fraught with challenges, such as:
How can researchers navigate this complex process to ensure their trials yield valid and impactful results?
The first step in the steps of randomised control trial is to define a precise research question. This question must be specific, measurable, and pertinent to the medical field. For instance, instead of asking, 'Does this treatment work?', a more focused question would be, 'How does Treatment A compare to Treatment B in reducing symptoms of Condition X over a 12-week period?' Such clarity is essential for planning the study and for leveraging the comprehensive management services provided by bioaccess®, which encompass:
By clearly outlining your research question, you can effectively navigate the steps of randomised control trial, which include:
ensuring successful study implementation.

Recognizing the suitable target group is essential for efficient randomization in research studies. A thorough comprehension of demographics, medical attributes, and particular patient requirements guarantees that study outcomes are applicable to the wider population. For instance, in studies centered on new diabetes treatments, including individuals diagnosed with diabetes is crucial, while considering factors such as age, gender, and comorbidities.
Recent trends indicate a significant rise in the involvement of older adults in research studies, with individuals over 65 increasing from 10% in 2014 to 39.9% in 2020. However, challenges persist in achieving diverse representation across racial and ethnic groups; Hispanic participation has averaged less than 10 percent during most years, reaching 11.3 percent in 2016.
Effective randomization is one of the important steps of randomized control trial that not only enhances the validity of study outcomes but also fulfills the essential requirement for inclusivity in medical research. As highlighted by Jakub P. Hlávka, insufficient demographic diversity can hinder innovation and access to therapies, underscoring the importance of targeted recruitment strategies that reflect the demographics of the disease population.
Furthermore, the FDA Reauthorization Act of 2017 promotes the participation of more varied patient groups in research studies, thereby strengthening the regulatory backing for these initiatives. To effectively implement these strategies, clinical research professionals must prioritize outreach to underrepresented groups and utilize inclusive recruitment practices throughout the steps of randomized control trial.

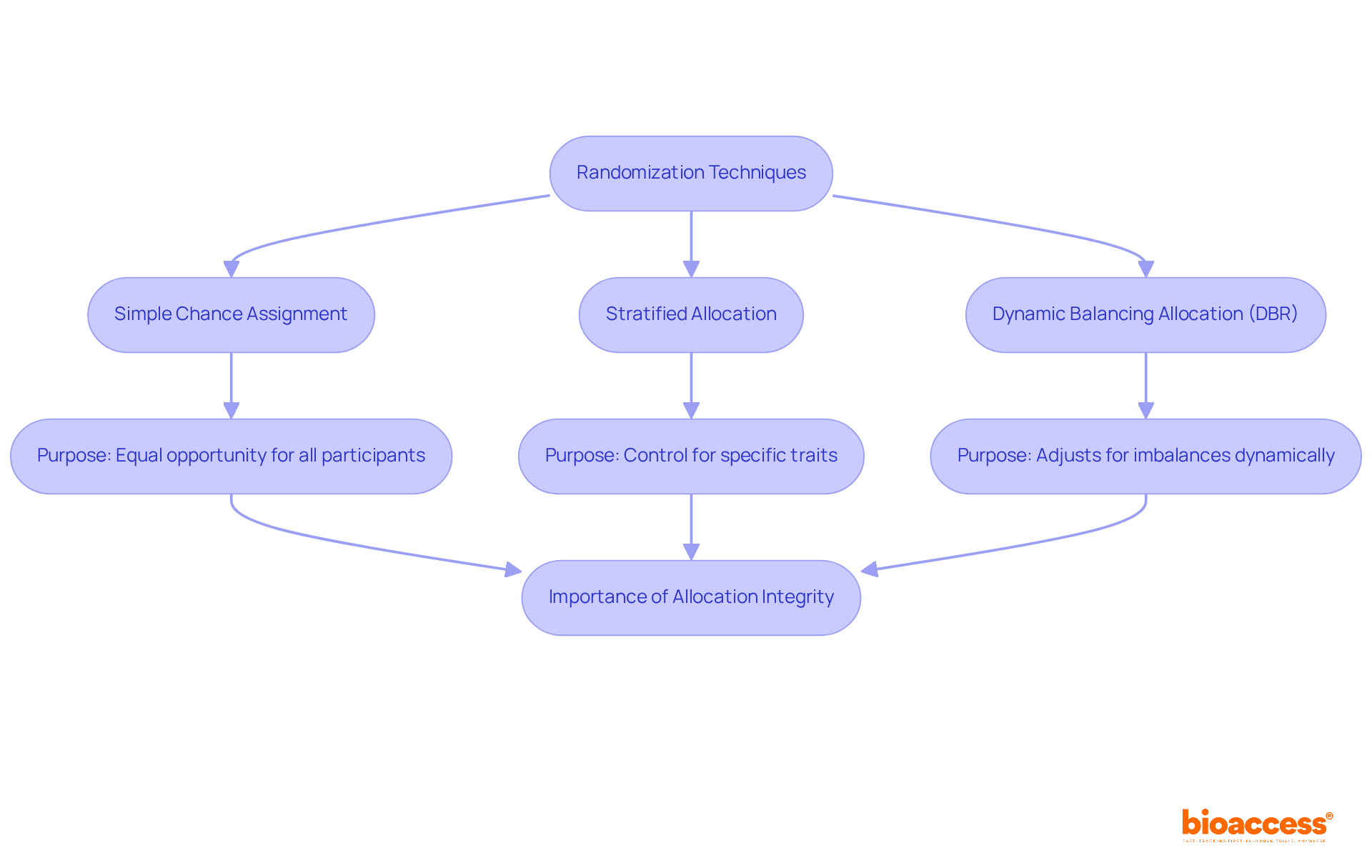
To effectively reduce bias in the steps of randomized control trial (RCTs), it is essential to implement strong random selection methods. Simple chance assignment allocates participants to treatment groups solely by luck, ensuring that each individual has an equal opportunity of being placed in any group. However, for experiments where specific traits—such as age or gender—are crucial, stratified allocation is advised. This method guarantees that these characteristics are evenly distributed across treatment groups, enhancing the comparability of results. A case study on stratified sampling illustrates its effectiveness in controlling treatment imbalances within specific subgroups, leading to more balanced treatment assignments across different centers or regions.
The influence of random assignment on clinical study results cannot be overstated. Correctly implemented random assignment methods yield more dependable assessments of treatment effects, accounting for both recognized and unrecognized factors that could affect outcomes. For instance, research has shown that experiments with insufficient allocation concealment frequently produce effect estimates that are 37% greater than those with proper concealment, underscoring the significance of upholding strict allocation practices.
Currently, the optimal methods for assigning treatments in the steps of randomized control trial (RCTs) include employing dynamic balancing allocation (DBR), which adjusts treatment assignments based on observed discrepancies at the center, region, and study levels. DBR has been demonstrated to maintain low chances of imbalance at all levels, thereby improving the overall efficiency of the study while preserving the integrity of allocation.
In summary, applying effective randomness methods is vital for maintaining the integrity of clinical studies and achieving valid, dependable outcomes. As Jeehyoung Kim noted, allocation concealment is crucial for preventing selection bias, further emphasizing the need for meticulous randomization practices.
Applying blinding techniques—such as single-blind, where participants are unaware of their group assignment, and double-blind, where both participants and researchers lack knowledge of group allocations—is essential for enhancing objectivity in the steps of randomised control trial. This practice significantly mitigates the risk of bias in reporting and evaluating outcomes, ultimately leading to more reliable results. Research indicates that non-blinded outcome evaluators can inflate odds ratios by an average of 36% in binary outcome analyses, while a combined effect size in studies with measurement scale results can be overstated by as much as 68%. Such statistics underscore the critical necessity of blinding in the steps of randomised control trial.
Moreover, an examination of the steps of randomised control trial has revealed that over half of 'double-blind' studies did not adequately outline the blinding status of involved personnel, highlighting the urgent need for more precise reporting standards. Current trends in blinding techniques emphasize the adoption of straightforward solutions, such as over-encapsulation, to preserve the integrity of trial results. However, it is also crucial to recognize that blinding can adversely affect study recruitment, presenting a challenge for researchers.
As Roger R. Dmochowski asserts, understanding blinding is vital for evidence-based best practices, emphasizing its role in minimizing bias and enhancing the credibility of clinical research. By prioritizing effective blinding strategies, researchers can ensure that their findings are both valid and applicable to real-world scenarios.

Establishing clear and specific outcome measures is paramount in aligning with the research question. For instance, when evaluating a new medication for hypertension, relevant outcomes might encompass:
These measures must be defined prior to the experiment to ensure consistency and reliability in data collection. Moreover, leveraging comprehensive clinical study management services, such as feasibility assessments and site selection, enhances the accuracy of these outcome measures. By ensuring adherence through thorough evaluations and efficient experiment setup, researchers can adeptly manage the complexities of data collection and reporting, ultimately leading to more precise assessments.
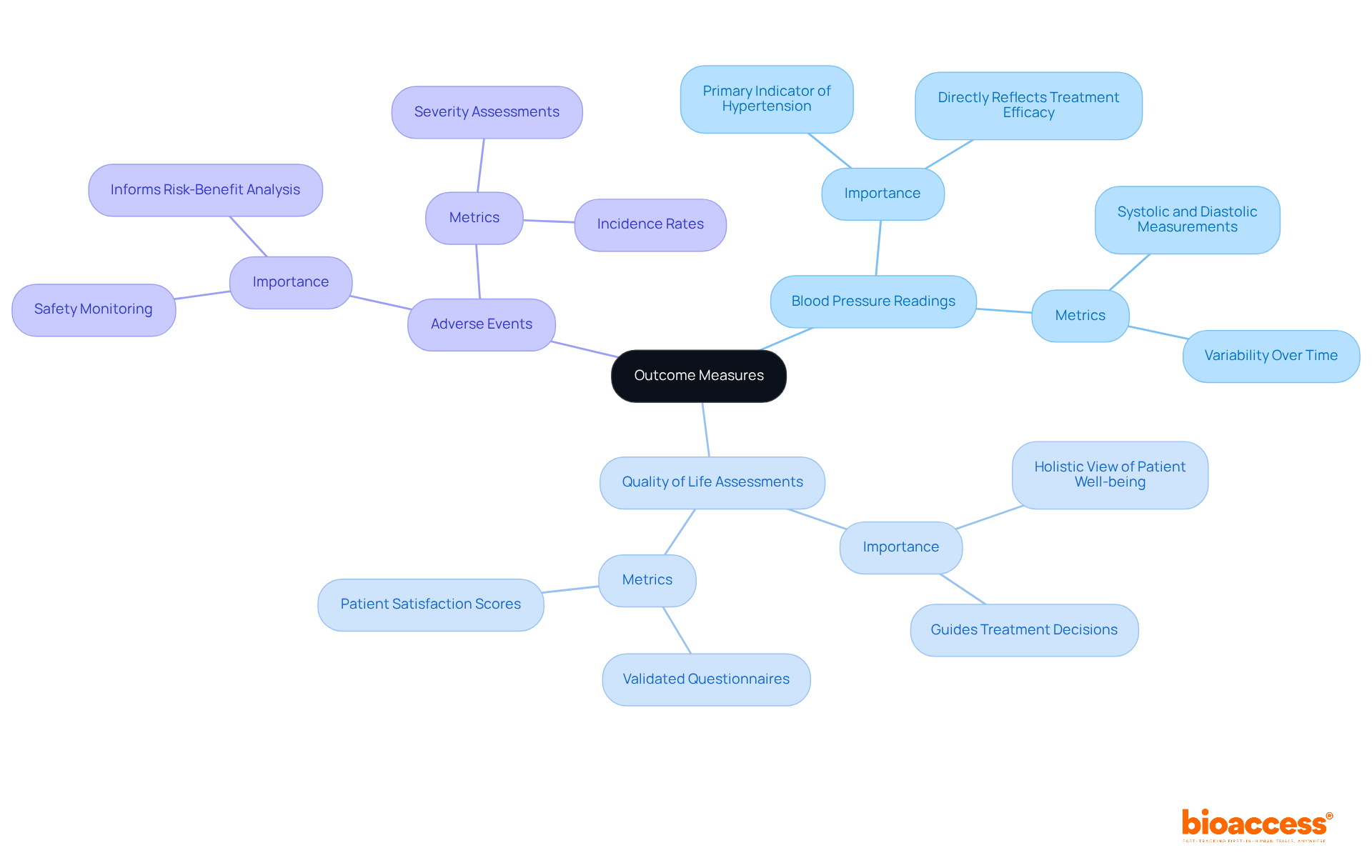
Addressing ethical considerations is paramount in clinical research; thus, obtaining informed consent from all participants is essential. Participants must fully understand the study's purpose, procedures, risks, and benefits. Additionally, establishing an independent ethics committee is crucial to evaluate the research protocol and ensure adherence to ethical standards throughout the experiment.
In Colombia, this process involves:
This unwavering commitment to ethics not only safeguards participants but also enhances the credibility of the research, effectively addressing the regulatory challenges faced by medical device startups in clinical studies.
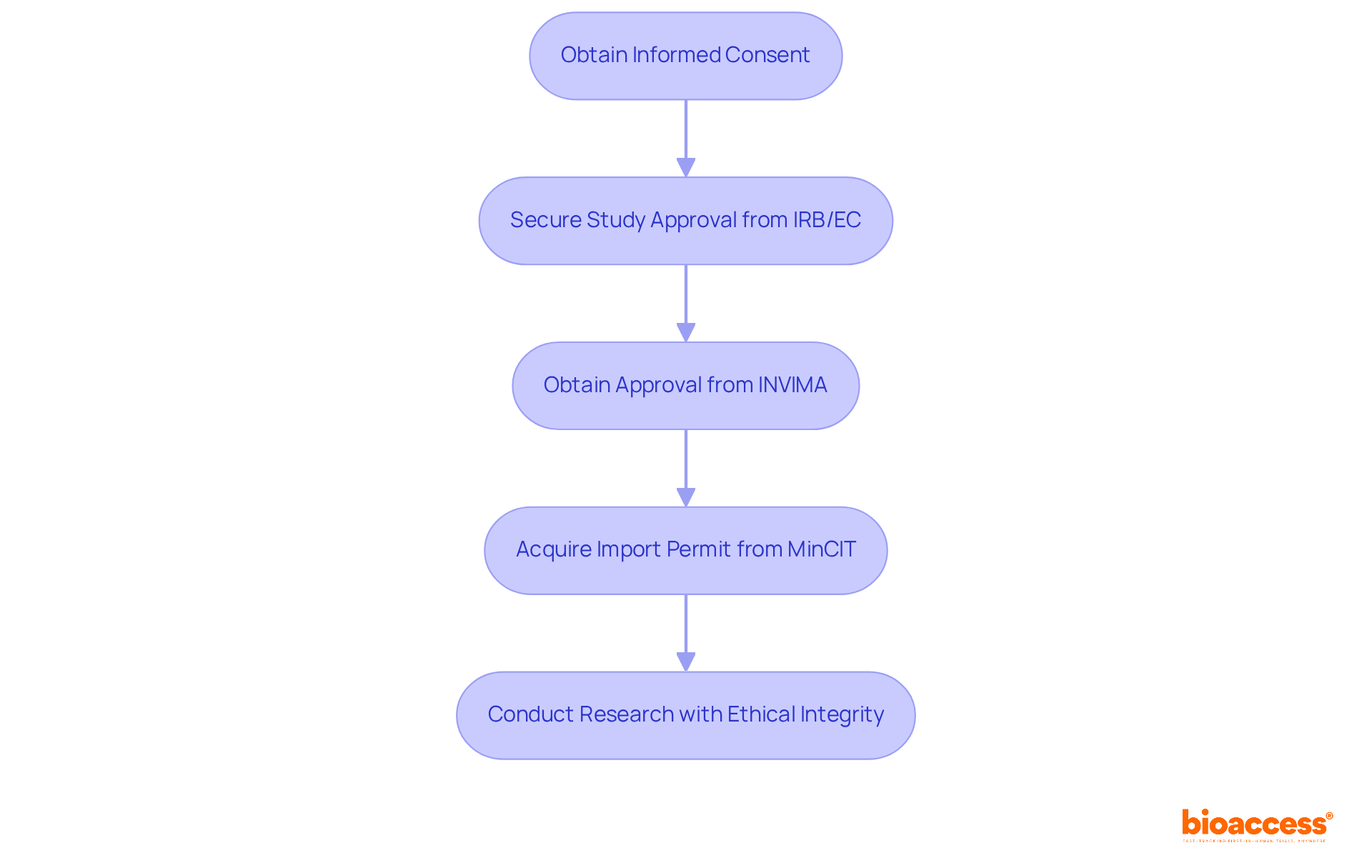
Establishing a comprehensive data analysis approach is vital for the success of randomized controlled studies (RCTs). This strategy must explicitly delineate the statistical methods employed to analyze study data, including the precise definitions of primary and secondary endpoints.
The selection of appropriate statistical tests is crucial, as it directly influences the validity of the conclusions drawn from the experimental results. Moreover, addressing the management of missing data is essential, given its potential to significantly impact the integrity of the findings.
A meticulously structured analysis plan not only bolsters the reliability of the results but also aligns with contemporary best practices in statistical methodology, thereby ensuring that the trial adheres to regulatory standards and provides valuable insights to the field.
Report results transparently by adhering to established guidelines such as CONSORT (Consolidated Standards of Reporting Trials). This encompasses offering thorough details on the design of the research, participant flow, baseline traits, and results. Transparency in reporting not only enhances the credibility of the findings but also contributes to the body of knowledge in the field.
By utilizing bioaccess's expertise in overseeing various types of research, including Early-Feasibility Assessments (EFA) and First-In-Human Trials (FIH), researchers can guarantee that their reporting meets the highest standards. Ultimately, this fosters trust in their findings and underscores the importance of collaboration in advancing clinical research.
Identifying potential biases is crucial for maintaining the integrity of the steps of randomised control trial (RCTs). Common biases, including selection bias, performance bias, and detection bias, can significantly distort research outcomes. To mitigate these biases, researchers should follow the steps of randomised control trial, which include implementing robust strategies such as:
By proactively confronting these biases, researchers can greatly enhance the reliability of their investigations, ensuring that the findings are both trustworthy and relevant to wider medical practice.
The CONSORT statement offers a valuable framework, presenting a 22-point checklist that assists authors in thoroughly reporting the steps of randomised control trial (RCT) methodology, further emphasizing the significance of transparency and rigor in research. Moreover, the Jadad scoring system serves as a recognized method for evaluating the methodological quality and internal validity of research studies, providing further context for assessing study quality. Incorporating strategies such as the target experiment framework and quantitative bias analysis can further enhance efforts to minimize bias in RCTs.
To enhance the influence of findings from randomized controlled studies on medical practice, it is imperative to disseminate results through a variety of channels, including:
Engaging with stakeholders—such as healthcare professionals, patient advocacy groups, and regulatory bodies—is essential in sharing insights and promoting the adoption of new practices based on study results. Media coverage, particularly from reputable sources like Clinical Leader, significantly boosts the visibility of clinical trials in regions such as Latin America and Colombia, thereby influencing local economies through:
For example, the RECAP research underscores the necessity of early planning in sharing results, tailoring communication to participant preferences, and ensuring timely dissemination. This approach not only enhances understanding but also encourages ongoing participation in future research, as participants often express a desire to be informed about the results of studies they contribute to. Current trends indicate a shift towards more interactive and responsive communication modes, with many participants preferring personalized updates over generic summaries. By prioritizing effective dissemination strategies, researchers can make substantial contributions to advancements in clinical practice and ultimately improve patient outcomes.
The journey through the essential steps of a randomized control trial (RCT) is vital for ensuring the integrity and success of clinical research. By meticulously defining the research question, identifying target populations, and implementing robust randomization techniques, researchers lay a strong foundation for producing reliable and applicable results. Each step, from establishing clear outcome measures to addressing ethical considerations, plays a crucial role in enhancing the credibility and impact of the findings.
Key insights from the article emphasize the importance of minimizing biases through effective blinding methods and comprehensive data analysis strategies. The necessity of transparent reporting cannot be overstated, as it fosters trust in the research community and among stakeholders. Furthermore, actively disseminating findings through various channels ensures that the results reach the relevant audiences, ultimately influencing clinical practice and improving patient outcomes.
In conclusion, the meticulous execution of these ten essential steps not only strengthens the validity of randomized control trials but also underscores the critical role of ethical practices and effective communication in advancing medical research. By adhering to these principles, researchers can significantly contribute to the evolution of clinical practices, ensuring that innovations in healthcare are both trustworthy and beneficial to society at large.
What is the first step in conducting a randomized control trial (RCT)?
The first step is to define a precise research question that is specific, measurable, and relevant to the medical field.
Can you provide an example of a well-defined research question for an RCT?
A focused research question could be, "How does Treatment A compare to Treatment B in reducing symptoms of Condition X over a 12-week period?"
What services does bioaccess® provide to support RCTs?
bioaccess® offers comprehensive management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
What are the steps involved in a randomized control trial?
The steps include Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
Why is identifying target populations important for effective randomization?
Recognizing the suitable target group ensures that study outcomes are applicable to the wider population and enhances the validity of the results.
What trends have been observed regarding the participation of older adults in research studies?
The involvement of individuals over 65 in research studies increased from 10% in 2014 to 39.9% in 2020.
What challenges exist in achieving diverse representation in research studies?
There are challenges in achieving diverse representation across racial and ethnic groups, with Hispanic participation averaging less than 10% in most years.
How does the FDA Reauthorization Act of 2017 contribute to diversity in research studies?
The act promotes the participation of more varied patient groups in research studies, strengthening regulatory support for these initiatives.
What randomization techniques can be implemented to minimize bias in RCTs?
Strong random selection methods such as simple chance assignment and stratified allocation can be used to minimize bias.
What is stratified allocation and why is it important?
Stratified allocation ensures that specific traits, such as age or gender, are evenly distributed across treatment groups, enhancing the comparability of results.
What are the consequences of insufficient allocation concealment in clinical studies?
Studies with insufficient allocation concealment can produce effect estimates that are significantly greater than those with proper concealment.
What is dynamic balancing allocation (DBR) and its benefits?
DBR is a method that adjusts treatment assignments based on observed discrepancies and helps maintain low chances of imbalance, improving study efficiency and integrity.
Why is allocation concealment crucial in RCTs?
Allocation concealment is vital for preventing selection bias, which emphasizes the need for meticulous randomization practices in clinical studies.