


This article delineates essential strategies for designing effective Case Report Forms (CRFs) in clinical studies, underscoring the significance of user-centered design, standardization, and technological integration.
By detailing the advantages of electronic CRFs, it illustrates how these tools enhance data collection efficiency, mitigate errors, and bolster stakeholder engagement.
Such improvements ultimately lead to superior study outcomes and ensure compliance with regulatory standards, establishing a robust framework for success in clinical research.
The design of Case Report Forms (CRFs) stands as a critical component in the success of clinical trials, significantly impacting both data quality and regulatory compliance. As technology rapidly evolves and clinical studies grow increasingly complex, researchers encounter the challenge of crafting CRFs that are not only user-friendly but also efficient. This article delves into ten essential strategies that streamline the CRF design process, enhance data integrity, and improve collection methods, ultimately paving the way for more reliable clinical outcomes.
How can researchers effectively balance innovation with usability to meet the ever-changing demands of clinical research?
At bioaccess®, we leverage over 15 years of experience in early-stage research to accelerate the creation of Case Report Forms (CRFs). Our extensive knowledge of regulatory standards and patient demographics empowers us to design clinical study CRFs that enhance data collection and improve the quality of clinical studies.
By integrating local insights from diverse patient groups across Latin America, the Balkans, and Australia, we ensure our CRF frameworks are not only compliant but also contextually relevant. This tailored approach significantly boosts trial efficiency, as demonstrated by the shift from paper-based to electronic CRFs, which has effectively reduced entry errors to zero, compared to a 5% error rate associated with traditional forms.
Completing a paper-based CRF typically takes about 10.54 minutes, while an electronic CRF averages just 8.29 minutes, underscoring the efficiency of eCRFs. Furthermore, engaging stakeholders early in the CRF development process fosters iterative enhancements, resulting in user-friendly forms that facilitate precise data collection.
As illustrated in our case study on stakeholder involvement in the clinical study CRF development, this user-centered approach is crucial for effective information gathering. As we look towards 2025, embracing technological innovations, including AI-driven techniques that can shorten the development timelines of clinical study CRF and user-focused principles is essential for enhancing CRF effectiveness, ultimately supporting the success of research studies.
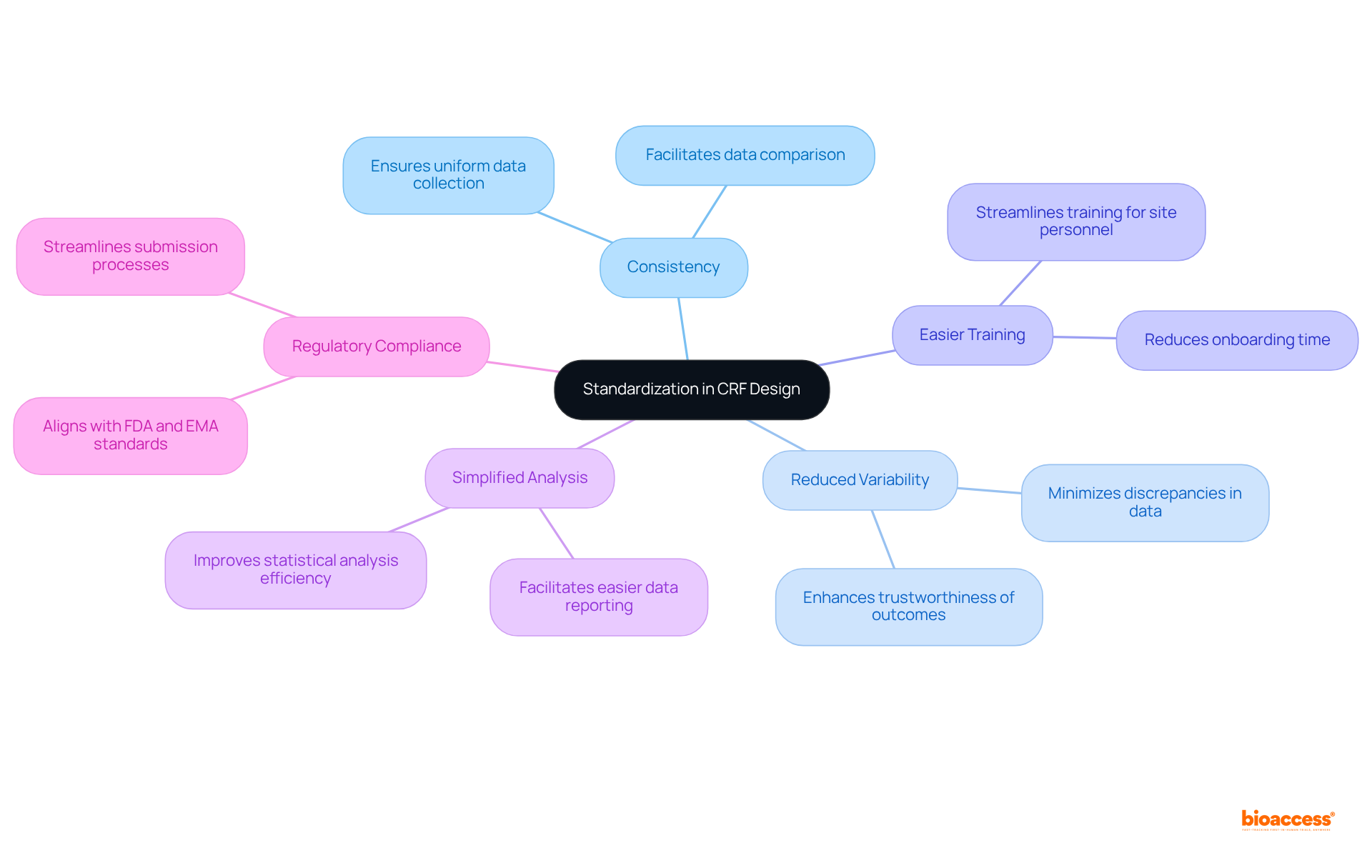
Standardization in the clinical study CRF design is essential for ensuring consistency across clinical trials. By utilizing standardized templates and formats in the clinical study CRF, researchers significantly reduce variability in information collection, thereby enhancing the trustworthiness of the outcomes. This approach streamlines the training process for site personnel, facilitates simpler information analysis and reporting, and ultimately leads to more robust conclusions and compliance with regulations.
Electronic Case Report Forms (eCRFs) have fundamentally transformed the information collection process in the clinical study CRF for clinical trials. By enabling real-time information entry and oversight, eCRFs significantly enhance both the precision and thoroughness of the data gathered.
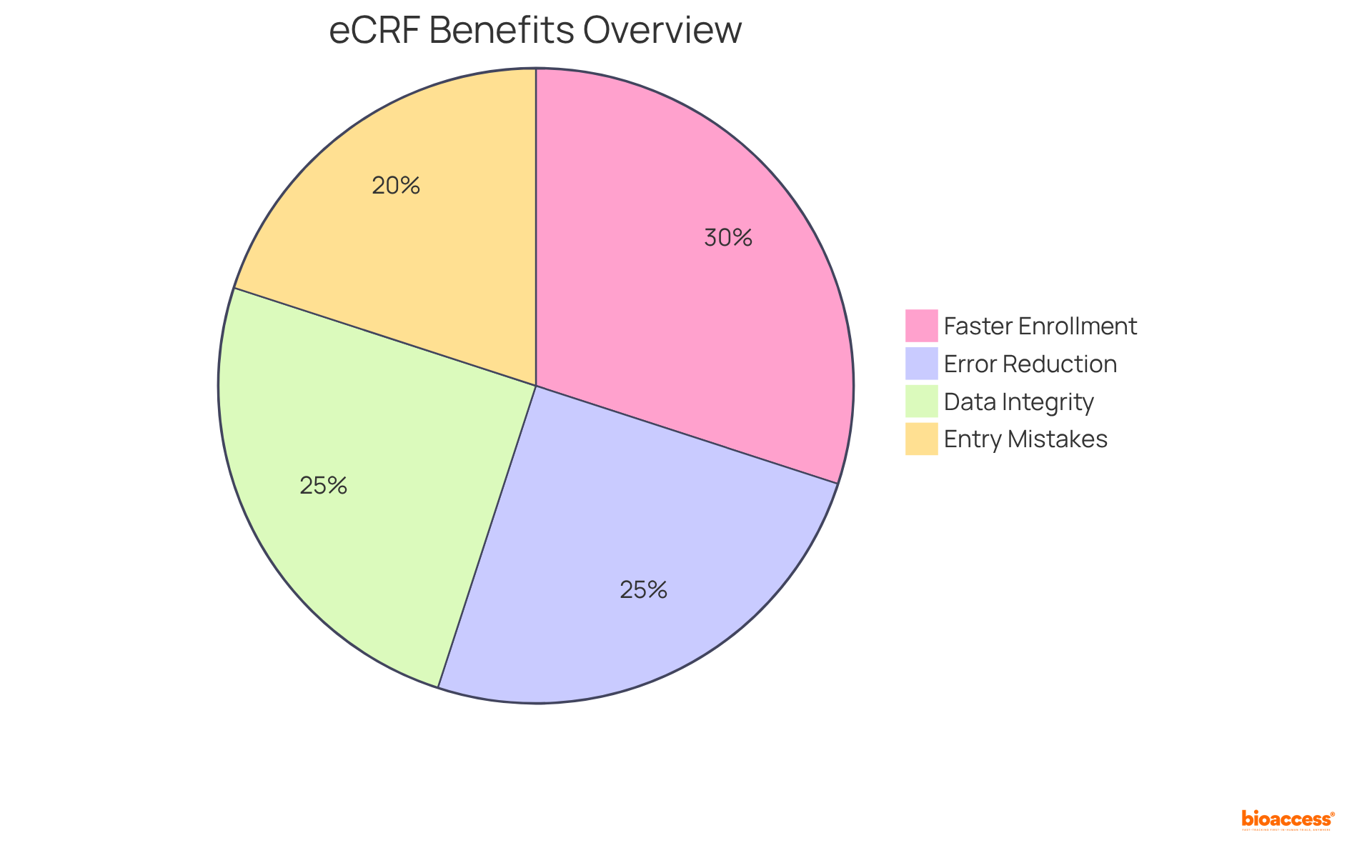
Research indicates that eCRFs reduce entry mistakes to below 1%, with no errors occurring in the eCRF environment, underscoring their advantage in maintaining information integrity. Moreover, eCRFs provide remote access for researchers and sponsors, expediting decision-making and streamlining study management.
The integration of eCRFs with additional digital tools not only fosters collaboration among research teams but also mitigates the risk of transcription errors, achieving a 40% reduction in entry mistakes noted in a Phase III oncology study. Success stories from the Medtech sector illustrate that trials employing integrated, automated data management workflows can complete patient enrollment 30% faster, highlighting the critical role of eCRFs in enhancing trial efficiency.
As the industry increasingly embraces eCRFs, the potential for accelerated product approvals and expedited market entry materializes, reinforcing the strategic significance of adopting this technology in alignment with clinical study CRF standards such as ISO 14155:2020 and Good Clinical Practices (GCP).
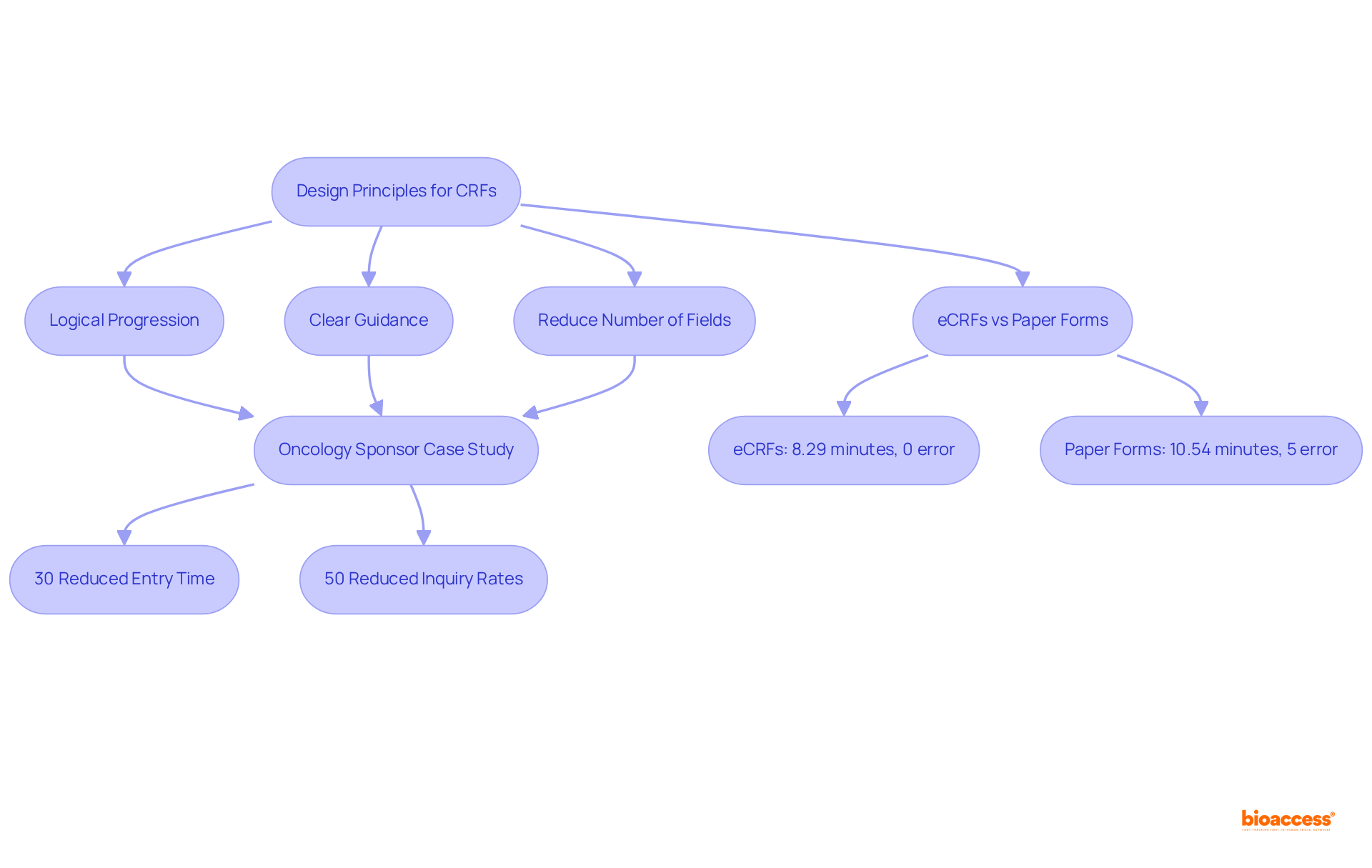
Developing user-friendly clinical study CRFs is crucial for enhancing clarity and usability in clinical research. A logical progression, clear guidance, and a reduction in the number of fields are key factors that ease the cognitive burden on information gatherers.
For instance, an oncology sponsor successfully decreased information entry time by 30% and inquiry rates by 50% after reorganizing their CRF based on site feedback. This case exemplifies the substantial influence of meticulous planning on study efficiency.
Furthermore, studies reveal that electronic CRFs (eCRFs) average just 8.29 minutes to complete, compared to 10.54 minutes for paper forms. Notably, eCRFs report no mistakes in eCRF conditions, whereas paper forms exhibit a 5% error rate. This streamlined approach significantly enhances the efficiency of clinical trials by utilizing clinical study CRFs, facilitating quicker regulatory submissions and faster access to innovative therapies.
Engaging stakeholders early in the CRF design process is essential for ensuring usability and improving the overall quality of information collection.
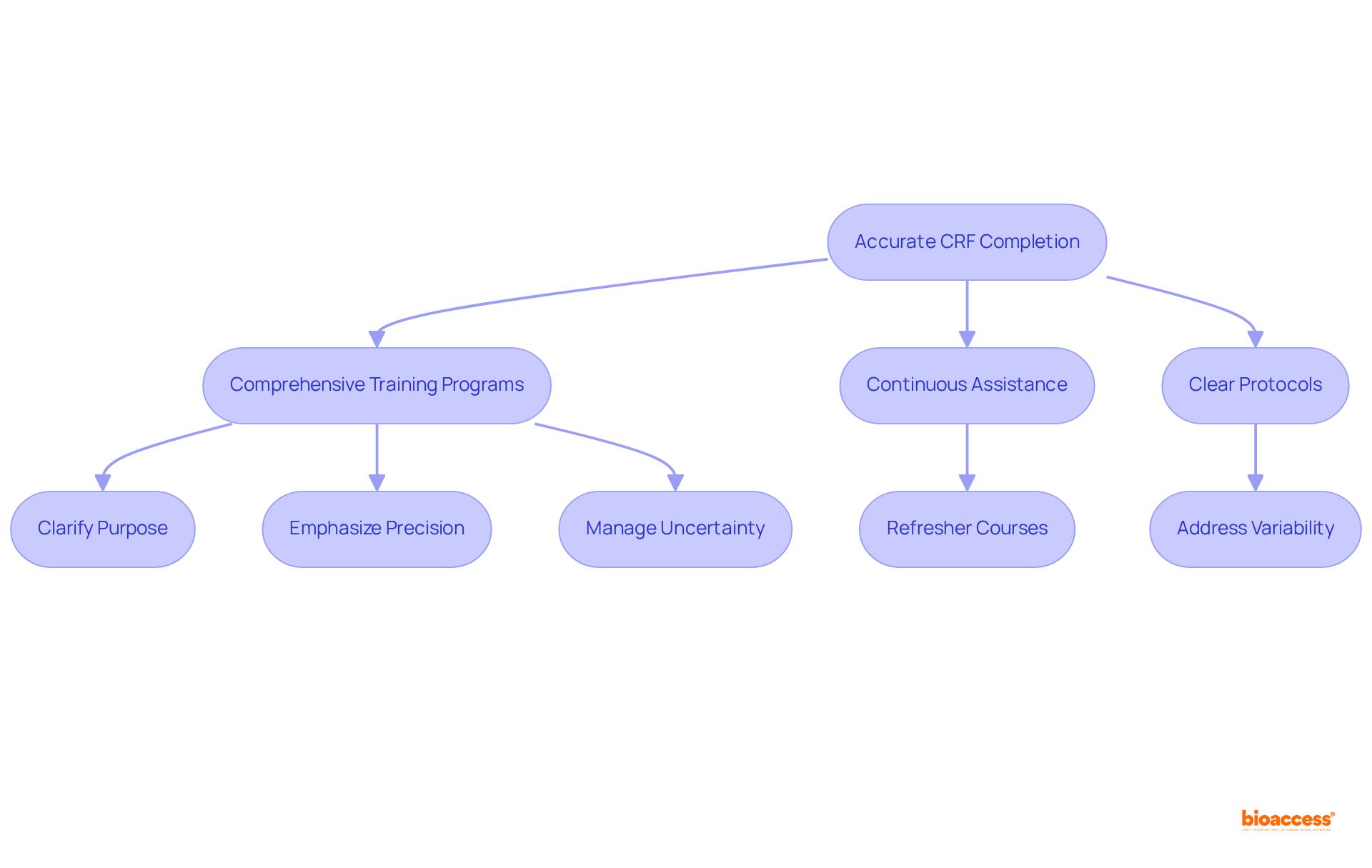
The accurate completion of clinical study CRFs is fundamentally reliant on comprehensive training and well-defined guidelines. Training programs must clarify the purpose of each piece of information, emphasize the critical importance of precision, and outline the procedures for information entry. Continuous assistance, including refresher courses, is essential for maintaining high-quality standards throughout the study. By prioritizing training, organizations empower their research teams to collect trustworthy information necessary for the clinical study CRF that meets regulatory expectations.
Furthermore, clear protocols enhance information integrity by addressing common variability in healthcare environments, ensuring that all staff are equipped to manage absent or uncertain information efficiently. This organized approach not only promotes uniformity but also mitigates the risks associated with discrepancies, ultimately leading to more reliable research outcomes.
Cloud-based storage for clinical study CRF offers significant advantages for clinical trials, particularly in terms of information security and accessibility. By leveraging secure cloud platforms, researchers can protect sensitive patient data while enabling authorized personnel to access information from any location. This flexibility is crucial for remote monitoring and collaboration, which are increasingly essential in today's global research environment.
Furthermore, cloud solutions typically provide robust backup and recovery options, enhancing information integrity and minimizing the risk of loss. A case study on automated backup and disaster recovery solutions underscores that these features are vital for preventing data loss and ensuring business continuity.
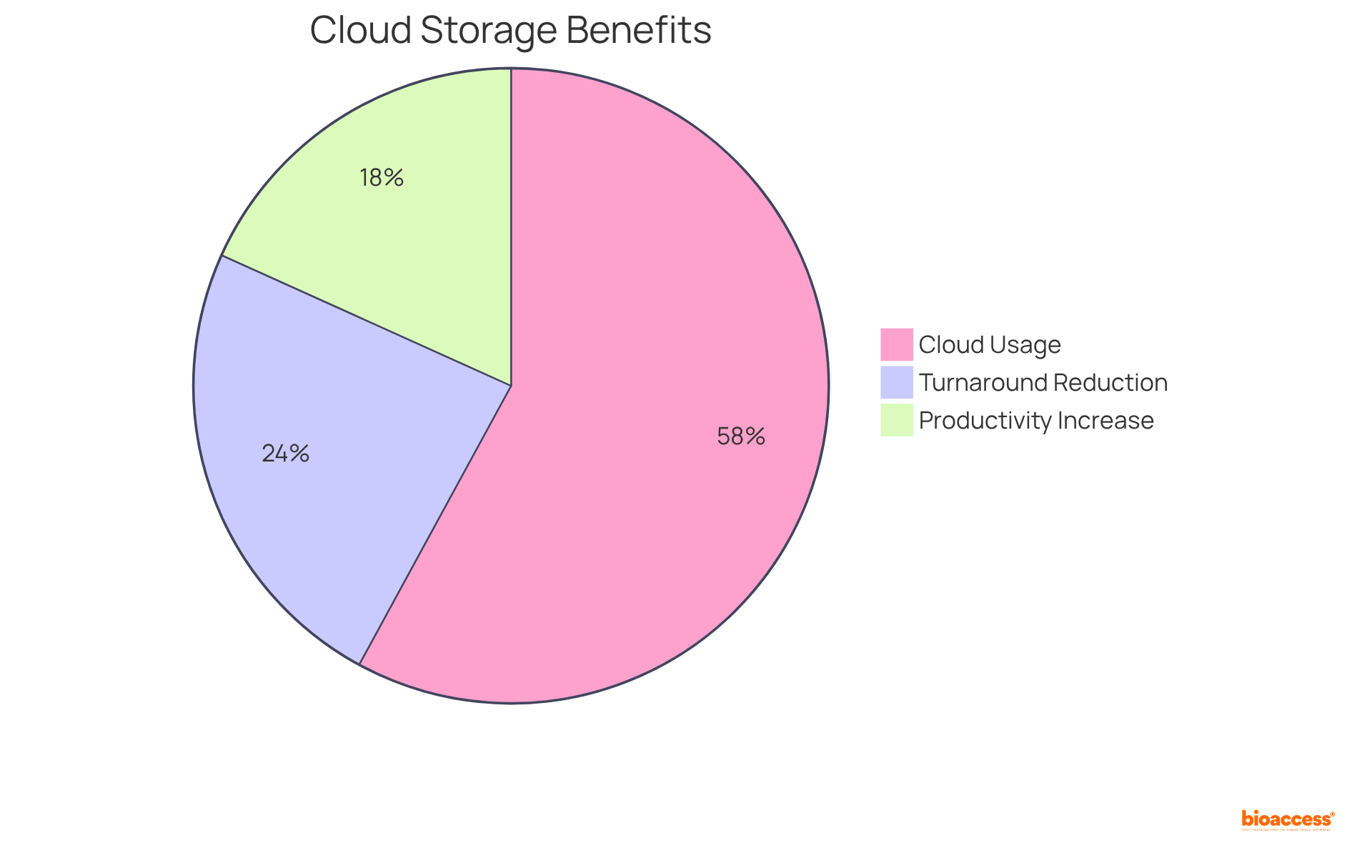
With 73% of pharmaceutical firms currently utilizing cloud-based research management systems, the shift to these technologies highlights their importance in contemporary clinical study CRF. Additionally, organizations that implement cloud storage solutions report a 23% increase in productivity and a 30% reduction in project turnaround time, showcasing the operational advantages alongside improved security measures.
Compliance with regulations such as GDPR and HIPAA is also critical, ensuring that security standards are upheld in clinical trials.
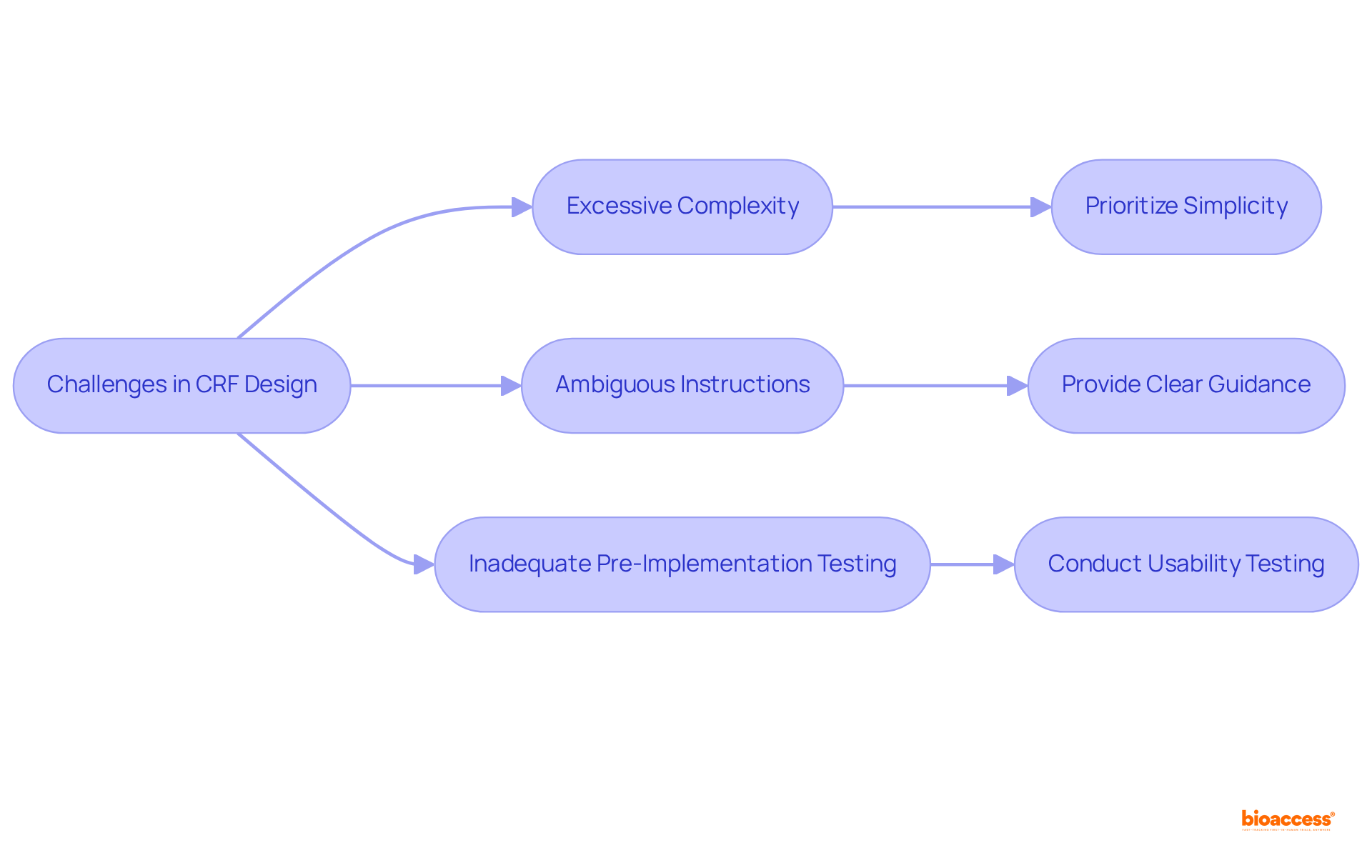
Designing effective clinical study CRFs poses significant challenges, such as excessive complexity, ambiguous instructions, and inadequate pre-implementation testing. To address these issues, researchers must prioritize simplicity in form creation, ensuring that each element is intuitive and user-friendly. Clear guidance for data entry is essential; this can be accomplished by providing detailed instructions and examples that facilitate accurate data collection.
Usability testing is crucial in enhancing CRF effectiveness. Engaging end-users in the testing process allows for the identification of potential issues before full-scale implementation. For instance, iterative usability testing can reveal usability issues and highlight areas for enhancement, ensuring that the final product meets user needs and expectations. Key methodological considerations during usability testing include:
Real-world examples illustrate the impact of addressing common pitfalls in CRF creation. One case study emphasized the importance of involving stakeholders early in the design process, resulting in the development of more functional forms that align with operational realities. Another example showcased how implementing version-controlled templates and maintaining comprehensive change logs mitigated regulatory risks associated with untracked updates.
By focusing on these strategies, researchers can significantly enhance the quality and efficiency of information gathering in health studies, ultimately leading to more reliable results and streamlined procedures. As Gilbert Hunter notes, the clinical study CRF is a document designed to capture all patient information that must be gathered during a research study, underscoring its vital role in the research process.
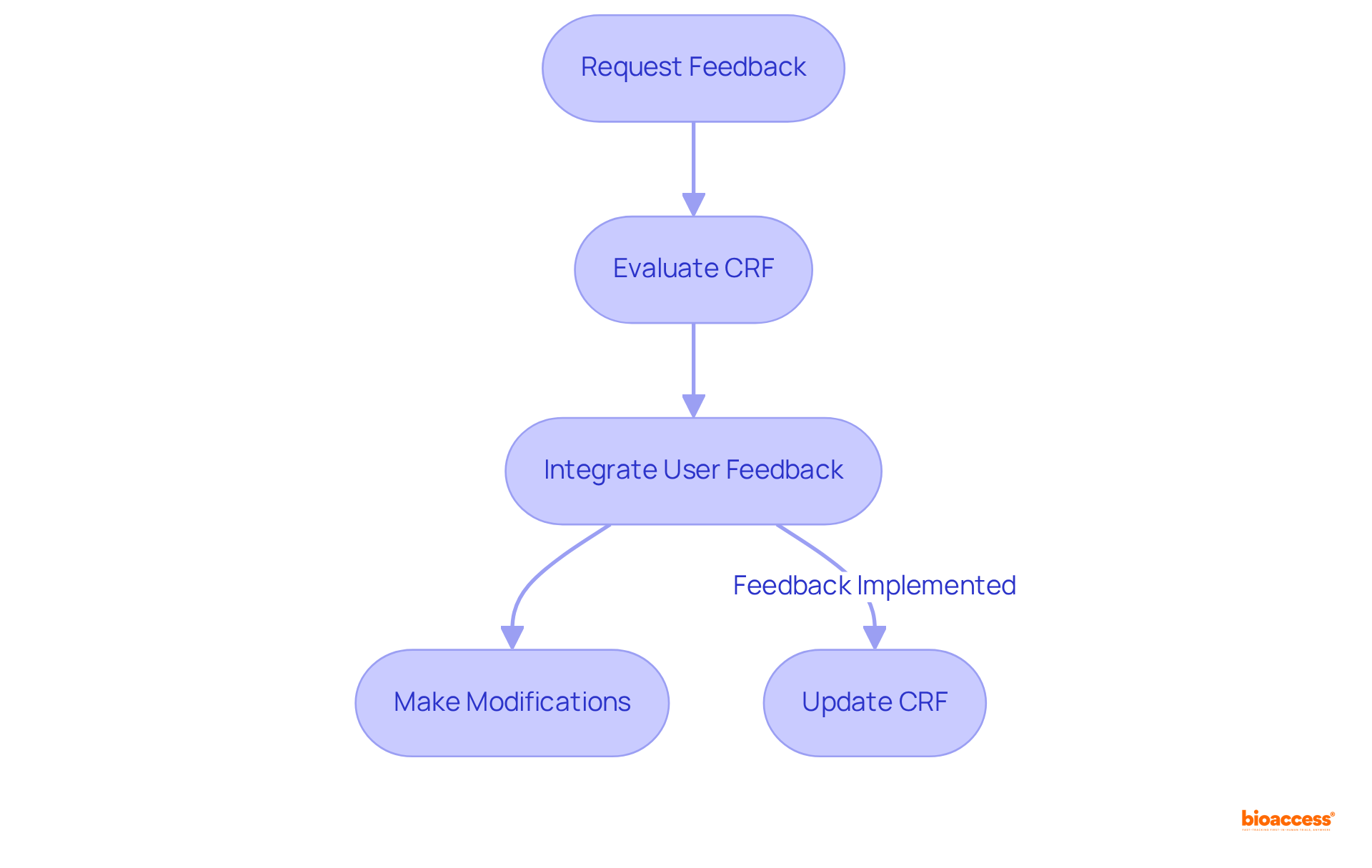
Implementing robust feedback mechanisms is essential for elevating the quality of Case Report Forms (CRFs). Proactively requesting feedback from information gatherers and study coordinators enables researchers to identify specific areas for improvement and make informed modifications. Consistent evaluations of the clinical study CRF, along with the integration of user feedback into development iterations, can yield forms that are more effective and aligned with the practical requirements of clinical studies. This iterative approach not only fosters a culture of continuous enhancement but also significantly improves overall information quality.
Involving stakeholders early in the planning phase can lead to iterative improvements that enhance adherence and streamline information collection, ultimately contributing to better trial outcomes. As noted by Eli Chachak, 'Effective CRF structure emphasizes not only the information being gathered but also the experience of the individual supplying that information.' By focusing on user-centered principles, researchers can create CRFs that are intuitive and functional, ultimately resulting in superior information collection outcomes.
Regular updates and maintenance of CRFs based on user feedback are also critical to ensure ongoing effectiveness and relevance.
Navigating regulatory considerations is essential for effective CRF creation. Researchers must ensure adherence to guidelines set by regulatory agencies such as the FDA and EMA, which encompass standards for information collection, patient confidentiality, and reporting. By integrating these regulatory requirements into the design process from the outset, organizations can streamline the approval process and significantly reduce the risk of non-compliance.
For instance, organizations that implement comprehensive regulatory analytics programs have achieved a 63% reduction in compliance-related incidents, underscoring the value of proactive compliance strategies. Moreover, adhering to the FDA's guidelines for annotated case report forms (aCRFs) is crucial, as these forms must align information points to datasets and variables precisely.
aCRFs should not contain any blank pages and must be submitted in specific PDF versions, ensuring clarity and compliance. By following these guidelines, researchers can enhance the quality of clinical study information and facilitate smoother regulatory procedures, ultimately leading to more effective study outcomes.
As Dr. Sarah Chen notes, regulatory analytics transforms compliance from a reactive exercise into a dynamic, intelligence-driven function that can anticipate risks before they materialize.
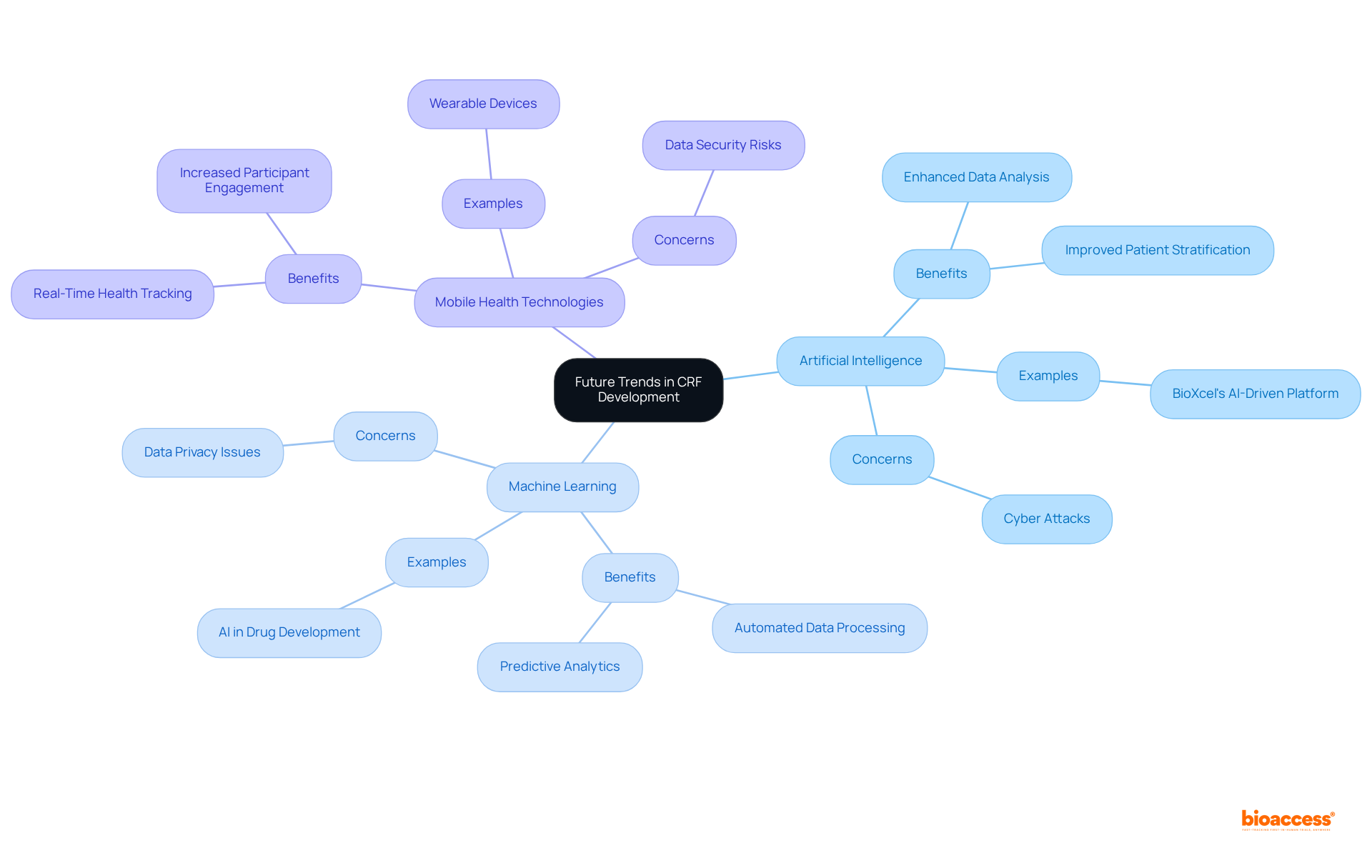
The future of CRF development is poised for a transformation through the integration of artificial intelligence, machine learning, and mobile health technologies. These innovations not only enhance information collection processes but also significantly improve patient involvement and streamline study management.
For instance, AI-powered platforms can analyze extensive datasets to identify patterns, facilitating more accurate patient classification and enhancing study outcomes. A notable example is BioXcel's AI-driven platform, which effectively analyzed research data for neuroscience drug candidates, identifying patterns that improved patient stratification.
Furthermore, mobile health technologies enable real-time tracking of patient health metrics, allowing for more tailored treatment plans and fostering increased participant engagement in studies. By embracing these cutting-edge technologies, researchers can develop more flexible and responsive clinical study CRFs that meet the evolving demands of medical studies.
The AI in medical studies market reached USD 1.3 billion in 2023 and is projected to expand at a CAGR of 14% from 2024 to 2032, underscoring the growing recognition of AI by regulatory bodies. Maintaining a competitive edge in these trends is crucial for organizations aiming to excel in the dynamic landscape of medical research, as the demand for efficient and effective study methodologies continues to rise.
However, it is essential to acknowledge the concerns surrounding AI, with 80% of consumers expressing worries about potential cyber attacks. A balanced perspective that considers both the advantages and challenges will provide a comprehensive understanding of the integration of AI in clinical study CRF during clinical trials.
The design of Case Report Forms (CRFs) is a pivotal aspect of clinical trials that directly impacts data quality and research outcomes. By employing essential strategies such as standardization, user-centered design, and the integration of electronic formats, researchers can significantly enhance the efficiency and accuracy of data collection. Embracing innovations like cloud storage and artificial intelligence further positions organizations to meet the evolving demands of clinical research.
Key insights from this discussion highlight the benefits of transitioning from traditional paper forms to electronic CRFs, which not only reduce errors but also streamline the data entry process. The importance of thorough training, adherence to regulatory standards, and the implementation of robust feedback mechanisms cannot be overstated, as these elements collectively contribute to the development of effective and user-friendly CRFs. Engaging stakeholders early in the design process and continuously iterating based on user feedback ensures that CRFs are tailored to meet the practical needs of clinical studies.
Looking ahead, the integration of advanced technologies will be crucial in shaping the future of CRF development. By adopting innovative approaches and remaining responsive to industry trends, researchers can enhance the quality and efficiency of clinical trials. As the landscape of medical research continues to evolve, it is imperative for organizations to prioritize effective CRF design strategies that will ultimately lead to more reliable data and successful study outcomes. Embracing these practices not only facilitates regulatory compliance but also fosters a culture of continuous improvement in clinical research methodologies.
What is bioaccess® and how does it contribute to clinical trial design?
bioaccess® leverages over 15 years of experience in early-stage research to accelerate the creation of Case Report Forms (CRFs), enhancing data collection and improving the quality of clinical studies through knowledge of regulatory standards and patient demographics.
How does bioaccess® ensure CRF frameworks are relevant?
By integrating local insights from diverse patient groups across Latin America, the Balkans, and Australia, bioaccess® designs CRF frameworks that are compliant and contextually relevant, significantly boosting trial efficiency.
What are the advantages of electronic CRFs (eCRFs) over paper-based CRFs?
eCRFs reduce entry errors to below 1%, whereas traditional paper-based CRFs have a 5% error rate. Additionally, completing an eCRF takes an average of 8.29 minutes compared to 10.54 minutes for a paper-based CRF, highlighting their efficiency.
Why is stakeholder engagement important in CRF development?
Engaging stakeholders early in the CRF development process fosters iterative enhancements, resulting in user-friendly forms that facilitate precise data collection, as illustrated in bioaccess®'s case study on stakeholder involvement.
What role does standardization play in CRF design?
Standardization ensures consistency across clinical trials, reduces variability in information collection, streamlines training for site personnel, and leads to more robust conclusions and compliance with regulations.
How do eCRFs enhance data collection efficiency?
eCRFs allow for real-time information entry and oversight, significantly enhancing the precision and thoroughness of data gathered, and provide remote access for researchers and sponsors, expediting decision-making.
What are the benefits of integrating eCRFs with digital tools?
Integration with additional digital tools fosters collaboration among research teams, mitigates transcription errors, and has been shown to achieve a 40% reduction in entry mistakes, enhancing overall trial efficiency.
How do eCRFs impact trial timelines and product approvals?
Trials employing integrated, automated data management workflows can complete patient enrollment 30% faster, potentially leading to accelerated product approvals and expedited market entry, reinforcing the importance of adopting eCRF technology.