


The article titled "10 Essential Strategies for IQ OQ PQ Validation Success" presents effective approaches to achieving validation success in the Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) processes. It asserts that organizations can significantly enhance their validation success by implementing structured methodologies, comprehensive documentation, and continuous improvement practices. These elements are critical for ensuring compliance and maintaining high standards in clinical research. By focusing on these strategies, organizations can navigate the complexities of validation with confidence and authority.
Navigating the intricate landscape of IQ, OQ, and PQ validation is paramount for organizations within the Medtech and Biopharma sectors, where compliance and quality assurance are indispensable. This article reveals ten essential strategies designed to empower companies to streamline their validation processes. These strategies not only ensure adherence to regulatory standards but also enhance operational efficiency. Yet, as regulations evolve and the risk of costly delays looms, how can organizations effectively surmount the challenges inherent in these validation phases and secure enduring success?
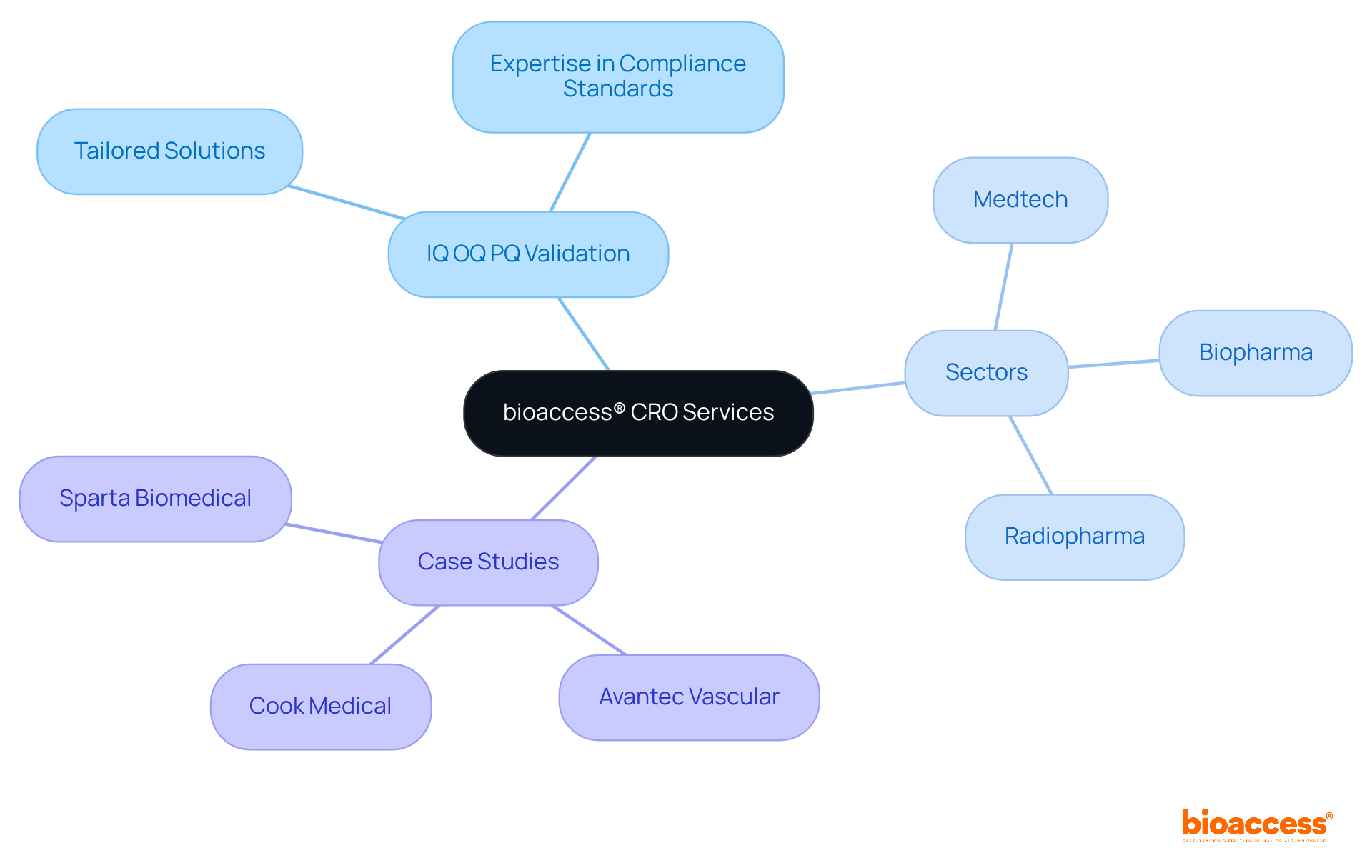
bioaccess® specializes in delivering tailored contract research organization (CRO) services that significantly streamline the IQ OQ PQ validation processes for medical devices. With over 20 years of expertise in clinical trials, bioaccess® leverages its extensive knowledge of compliance standards to ensure effective IQ OQ PQ validation. The organization possesses a deep understanding of the unique challenges faced in the Medtech, Biopharma, and Radiopharma sectors, enabling it to provide solutions that not only accelerate timelines but also enhance the quality of assessment results.
For instance, successful case studies, such as Avantec Vascular's first-in-human study in Latin America, demonstrate how bioaccess® adeptly navigates the complexities of compliance submissions and site selection, leading to expedited ethical approvals and patient enrollment. By prioritizing ethical adherence and rapid enrollment, bioaccess® positions itself as a strategic partner for innovators striving for success in a competitive landscape.
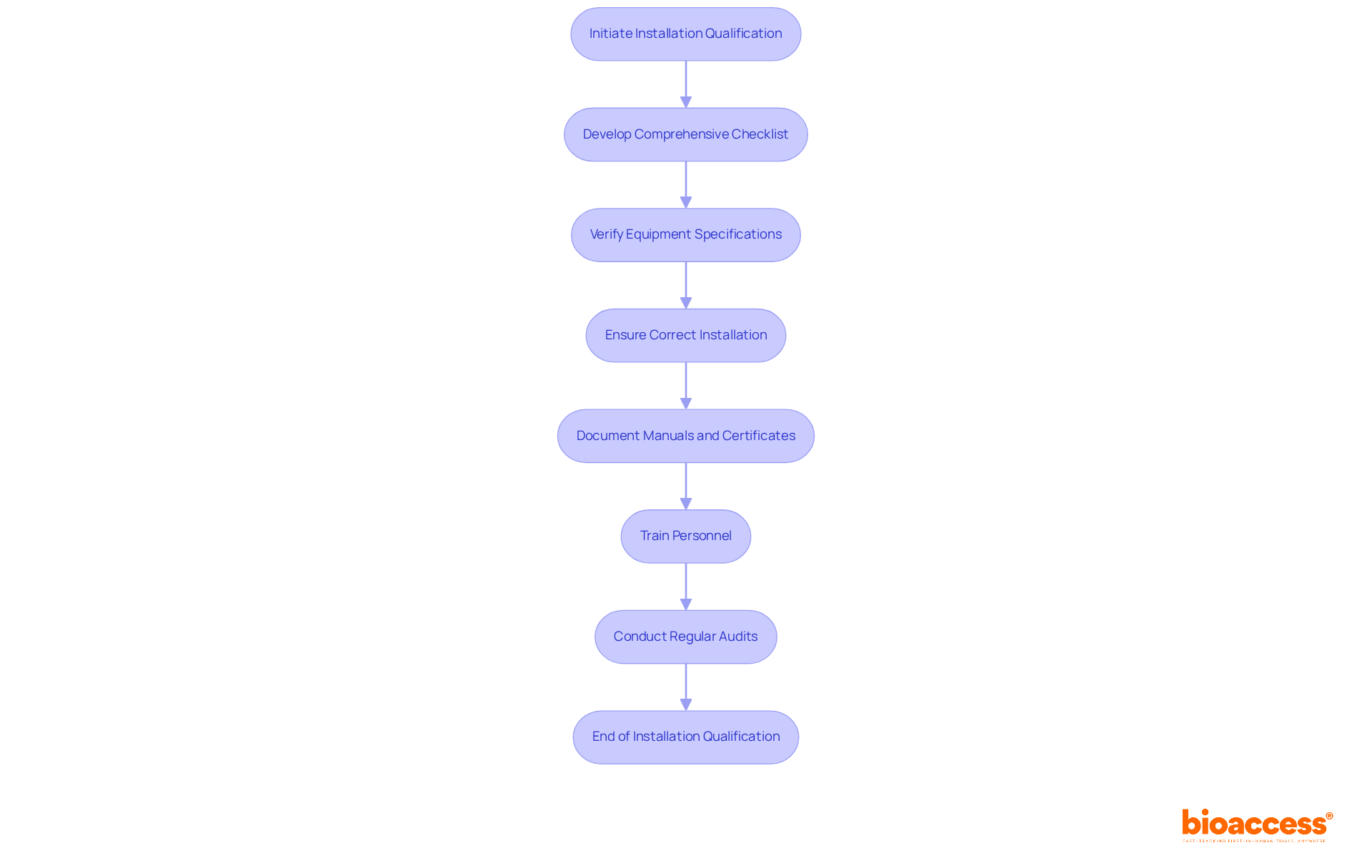
Installation Qualification (IQ) represents a pivotal phase in confirming that equipment and systems are established in alignment with compliance standards, serving as the foundation for effective validation activities. To excel in IQ, organizations must develop a comprehensive checklist that includes verifying equipment specifications, installation procedures, and environmental conditions. This checklist should encompass critical activities, such as:
Furthermore, it is essential to gather and document manuals, drawings, and calibration certificates for instruments to guarantee thorough documentation.
Training personnel involved in the installation process is equally crucial, as documented instructions ensure that all team members understand their roles and responsibilities. Regular audits and assessments of the installation methods can identify potential issues at an early stage, thereby enhancing compliance with legal standards and minimizing the risks of non-conformance. As Rebecca Beausang, a Regulatory Affairs Specialist, asserts, "Installation Qualification (IQ) is the initial documented check, confirming that equipment has been installed correctly and matches all design specifications and purchase orders."
Successful instances of Installation Qualification in clinical research underscore the necessity of a structured approach. For instance, developing a detailed IQ protocol that outlines testing procedures and assigns responsibilities can streamline the installation workflow and bolster accountability. Organizations should also remain vigilant regarding common pitfalls, such as:
These pitfalls can result in costly delays and regulatory scrutiny. In fact, inadequate process verification is a leading citation for medical device firms, underscoring the critical function of IQ in ensuring compliance.
Fostering a culture of quality and continuous improvement within the laboratory environment is imperative. A meticulously executed IQ sequence offers documented proof that equipment is suitable for its intended use, ultimately safeguarding patient safety and preserving data integrity. By adhering to these principles, organizations can lay a robust foundation for subsequent validation phases, ensuring operational efficiency and compliance throughout the equipment lifecycle.
Operational Qualification (OQ) serves as a critical phase that assesses a system's performance under standard operating conditions, ensuring compliance with regulatory standards. To succeed in OQ, organizations must establish precise acceptance criteria that delineate the expected performance and functionality of their equipment. Implementing rigorous testing protocols is essential, including standard evaluations such as verifying temperature uniformity, pressure, and flow rates, which are vital for confirming that systems operate within designated limits.
Involving cross-functional teams in the OQ process is crucial, as it guarantees that all operational facets are taken into account, resulting in a more thorough evaluation. Regular reviews and updates of OQ procedures, driven by feedback and advancements in technology, can significantly bolster the effectiveness of this qualification stage. For example, organizations that embrace a systematic approach to OQ, complete with meticulous documentation of results and deviations, have reported enhanced success rates in fulfilling compliance requirements.
Successful case studies from the Biopharma sector underscore the significance of operational qualification. For instance, the OQ of critical equipment, such as autoclaves and tablet compression machines, has shown that adherence to best practices not only secures product quality but also improves patient safety. By adopting these strategies, companies can mitigate risks, avert costly mistakes, and maintain the integrity of their operations, ultimately elevating the overall quality and safety of pharmaceutical products.

Performance Qualification (PQ) serves as the crucial concluding phase in the confirmation of systems, ensuring their effective operation in real-world conditions. To excel in PQ, organizations must establish precise performance metrics that reflect operational expectations. A compelling case study in clinical research illustrates this: implementing a performance metric of 95% system uptime significantly improved project outcomes. Comprehensive testing across diverse scenarios is essential to validate these metrics. Engaging end-users throughout the PQ process not only enhances system usability but also yields critical insights into performance. As Doug Conant asserts, "Intentions are important, but that’s not what you will be measured on. Everything boils down to results, results, results." Furthermore, establishing ongoing monitoring and feedback systems after assessment is vital for maintaining performance standards and identifying opportunities for enhancement. By adopting a proactive approach to PQ, organizations can significantly enhance overall project outcomes.

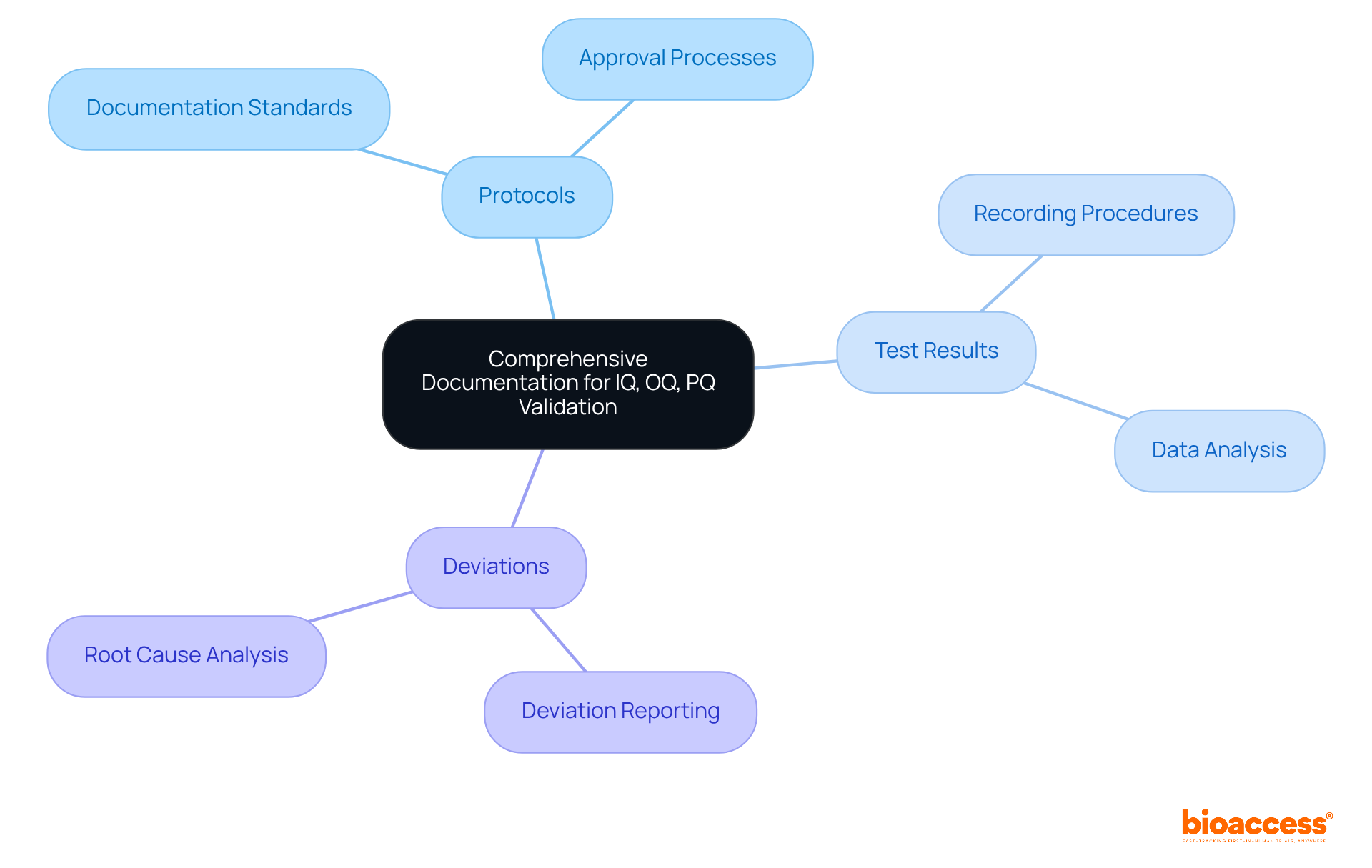
Thorough documentation is paramount for the confirmation of IQ, OQ, and PQ. Organizations must maintain comprehensive records of all verification activities, including:
A centralized documentation system not only streamlines access to information but also enhances traceability. Regularly reviewing and updating documentation practices is essential for compliance with evolving standards and facilitates smoother audits. Furthermore, training staff on the significance of documentation cultivates a culture of accountability and precision.
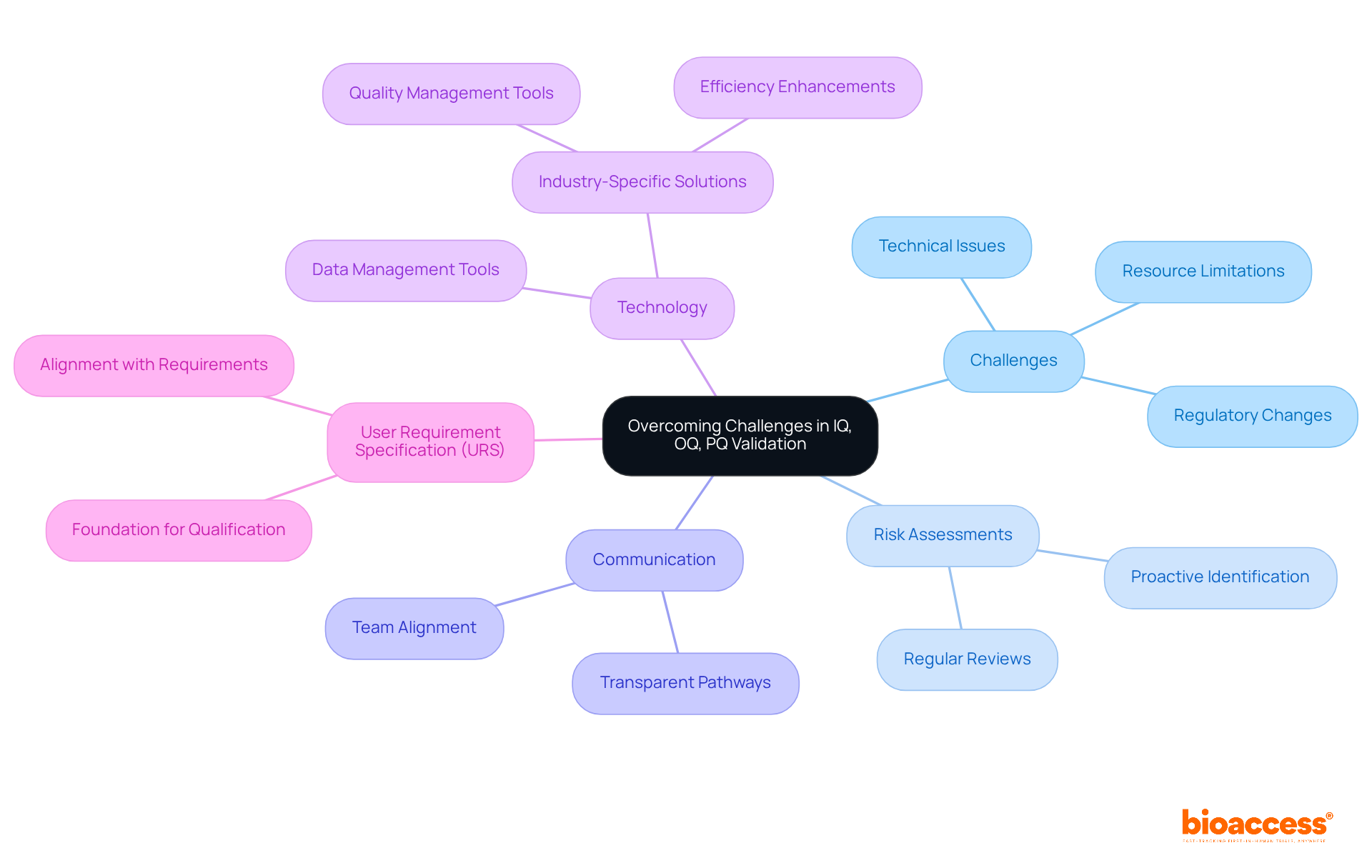
Challenges in the iq oq pq validation process can emerge from various sources, including regulatory changes, resource limitations, and technical issues. To effectively address these challenges, organizations must conduct regular risk assessments to identify potential obstacles early. Notably, eighty-five percent of failures stem from shortcomings in systems and procedures rather than employee performance. This statistic underscores the critical need for proactive risk management in assessment efforts.
Establishing transparent communication pathways among team members facilitates problem-solving and ensures alignment regarding assessment objectives. Moreover, leveraging technology for data management and analysis streamlines workflows and enhances efficiency, thereby reducing the likelihood of errors and delays. Organizations that utilize industry-specific quality management tools are twice as likely to meet their quality objectives compared to those relying on general-purpose solutions. This highlights the significant impact of tailored technology on iq oq pq validation efficiency.
Additionally, the User Requirement Specification (URS) serves as a foundational element for qualification activities, ensuring that subsequent steps align with functional and performance requirements. By implementing these strategies, organizations can bolster their ability to adapt to regulatory changes and maintain compliance in their assessment efforts.
Quality by Design (QbD) serves as a structured framework that underscores the importance of quality throughout the confirmation process. Introduced by the FDA in 2004, QbD promotes the proactive identification of critical quality attributes (CQAs) and the establishment of robust control strategies from the outset of product development. This foresight enables more precise measurement of confirmation success and enhances overall product quality and patient safety.
For example, the application of QbD principles in developing a generic two-API oral solid dosage form has proven effective in ensuring compliance with regulatory standards. Moreover, providing teams with training on QbD methodologies significantly enhances their ability to implement these principles effectively, fostering a strong culture of quality within the organization.
However, cultivating such a culture can be challenging, as resistance to change remains a substantial barrier. The integration of QbD into clinical research verification methods has shown the potential to improve operations and outcomes, establishing iq oq pq validation as an essential approach for achieving verification success.

Establishing a proficient assessment group is essential for the success of the IQ OQ PQ validation processes in clinical research. Organizations must prioritize recruiting individuals with diverse skill sets and relevant experience. As Verna Myers aptly states, "Diversity is being invited to the party; inclusion is being asked to dance," underscoring the importance of not just diversity but also fostering an inclusive environment. At bioaccess®, our advisory board comprises specialists in compliance matters and medical advancements, ensuring our assessment teams are equipped with the latest insights and best practices. This collaborative atmosphere allows team members to share insights, significantly enhancing overall performance.
Moreover, it is crucial to acknowledge that it can take up to 17 years for new evidence to become standard clinical practice. This highlights the necessity for a knowledgeable assessment team that remains acutely aware of industry trends and regulatory changes. Consistent training and development opportunities are vital to ensure that the team is prepared to address verification challenges effectively. Additionally, with over 60% of patients potentially participating in Walgreens clinical trials being female, the significance of varied involvement in clinical trials is clear, strengthening the case for diverse assessment teams.
Integrating these components will not only enhance the team's capabilities but also elevate the results of the assessment procedures, ultimately aiding the success of clinical trials in the Medtech and Biopharma fields.

Encouraging ongoing enhancement in iq oq pq validation processes is vital for upholding high standards in clinical research. Organizations must establish regular review cycles to evaluate verification practices and identify areas for improvement. By fostering an environment where feedback from team members and stakeholders is valued, organizations can gain valuable insights into potential enhancements. Furthermore, employing performance metrics to monitor progress and results will assist organizations in making data-informed decisions that enhance the efficiency of their assessment efforts.
It is essential to acknowledge that data inaccuracies in clinical trials, stemming from double entry techniques, can vary significantly—ranging from 2.3% to 26.9%. This statistic underscores the necessity for robust verification measures. The FDA advocates for a team-oriented approach to process assessment, aligning with the goal of promoting a culture of quality within organizations. Regular evaluations, as highlighted in the case study 'Periodic Review and Continuous Adherence,' are crucial for ensuring ongoing compliance with industry standards, particularly in relation to iq oq pq validation as specified in Section 211.180(e). This section mandates periodic assessments of product quality and manufacturing practices, reinforcing the importance of a systematic approach to quality assurance.

Incorporating adherence to regulations into IQ OQ PQ validation is vital for the success of clinical research. Organizations must remain vigilant regarding evolving regulations and guidelines, ensuring that all verification activities strictly align with these standards.
Routine training for team members significantly enhances awareness and compliance with legal requirements, ultimately improving verification outcomes.
Furthermore, establishing a compliance oversight committee can provide essential support in navigating the complexities of regulatory landscapes, ensuring that processes for IQ OQ PQ validation are both robust and defensible against scrutiny.
The strategies for achieving success in IQ OQ PQ validation outlined in this article emphasize the importance of a structured and proactive approach to ensure compliance and operational excellence in clinical research. By focusing on Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), organizations can create a solid foundation that not only meets regulatory standards but also enhances the quality and safety of medical devices.
Key insights include:
Each phase requires meticulous documentation and a commitment to continuous improvement, enabling organizations to address challenges and leverage Quality by Design (QbD) principles effectively. The integration of expert CRO services further amplifies these efforts, providing tailored support that accelerates validation processes and enhances overall outcomes.
In conclusion, prioritizing these validation strategies is vital for organizations aiming to thrive in the competitive landscape of Medtech and Biopharma sectors. By fostering a culture of quality, embracing ongoing training, and adapting to regulatory changes, companies can ensure not only compliance but also the successful delivery of safe and effective medical products. Embracing these best practices will ultimately lead to improved patient safety and trust in the healthcare system.
What services does bioaccess® provide?
bioaccess® specializes in tailored contract research organization (CRO) services that streamline the Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) validation processes for medical devices.
What experience does bioaccess® have in the industry?
bioaccess® has over 20 years of expertise in clinical trials and a deep understanding of compliance standards, particularly in the Medtech, Biopharma, and Radiopharma sectors.
Can you provide an example of bioaccess®'s successful work?
A successful case study includes Avantec Vascular's first-in-human study in Latin America, where bioaccess® effectively navigated compliance submissions and site selection, leading to expedited ethical approvals and patient enrollment.
What is Installation Qualification (IQ)?
Installation Qualification (IQ) is a phase that confirms equipment and systems are installed in accordance with compliance standards, serving as the foundation for effective validation activities.
What are key activities involved in Installation Qualification (IQ)?
Key activities include verifying equipment specifications, installation procedures, and environmental conditions, ensuring correct installation of components, and documenting manuals, drawings, and calibration certificates.
Why is training personnel important during Installation Qualification (IQ)?
Training ensures that all team members understand their roles and responsibilities, which is crucial for effective and compliant installation processes.
What are common pitfalls in Installation Qualification (IQ)?
Common pitfalls include insufficient documentation and failure to meet acceptance criteria, which can lead to costly delays and regulatory scrutiny.
What is Operational Qualification (OQ)?
Operational Qualification (OQ) is a phase that assesses a system's performance under standard operating conditions to ensure compliance with regulatory standards.
What are best practices for implementing Operational Qualification (OQ)?
Best practices include establishing precise acceptance criteria, implementing rigorous testing protocols, involving cross-functional teams, and regularly reviewing and updating OQ procedures.
How can organizations benefit from effective Operational Qualification (OQ)?
Effective OQ practices can secure product quality, improve patient safety, mitigate risks, and maintain operational integrity, ultimately enhancing the quality and safety of pharmaceutical products.