


The article '10 Essential Strategies for Medical Device Quality Control' delineates critical approaches that organizations must embrace to bolster quality control within the medical device sector. It underscores the necessity of implementing a Quality Management System (QMS), conducting thorough testing and validation, ensuring adherence to regulatory standards, and promoting continuous improvement. These strategies are vital for upholding high standards and safeguarding patient safety throughout the medical device development process.
The landscape of medical device quality control is evolving rapidly, propelled by the urgent need for innovation and compliance within an increasingly complex regulatory environment. As the Medtech sector anticipates substantial growth, organizations must understand and implement effective quality control strategies to ensure patient safety and product efficacy. However, with numerous standards and practices to navigate, how can companies effectively balance compliance with the demands of a competitive market? This article explores ten essential strategies that enhance quality management systems and foster a culture of continuous improvement, ultimately paving the way for successful medical device development and deployment.
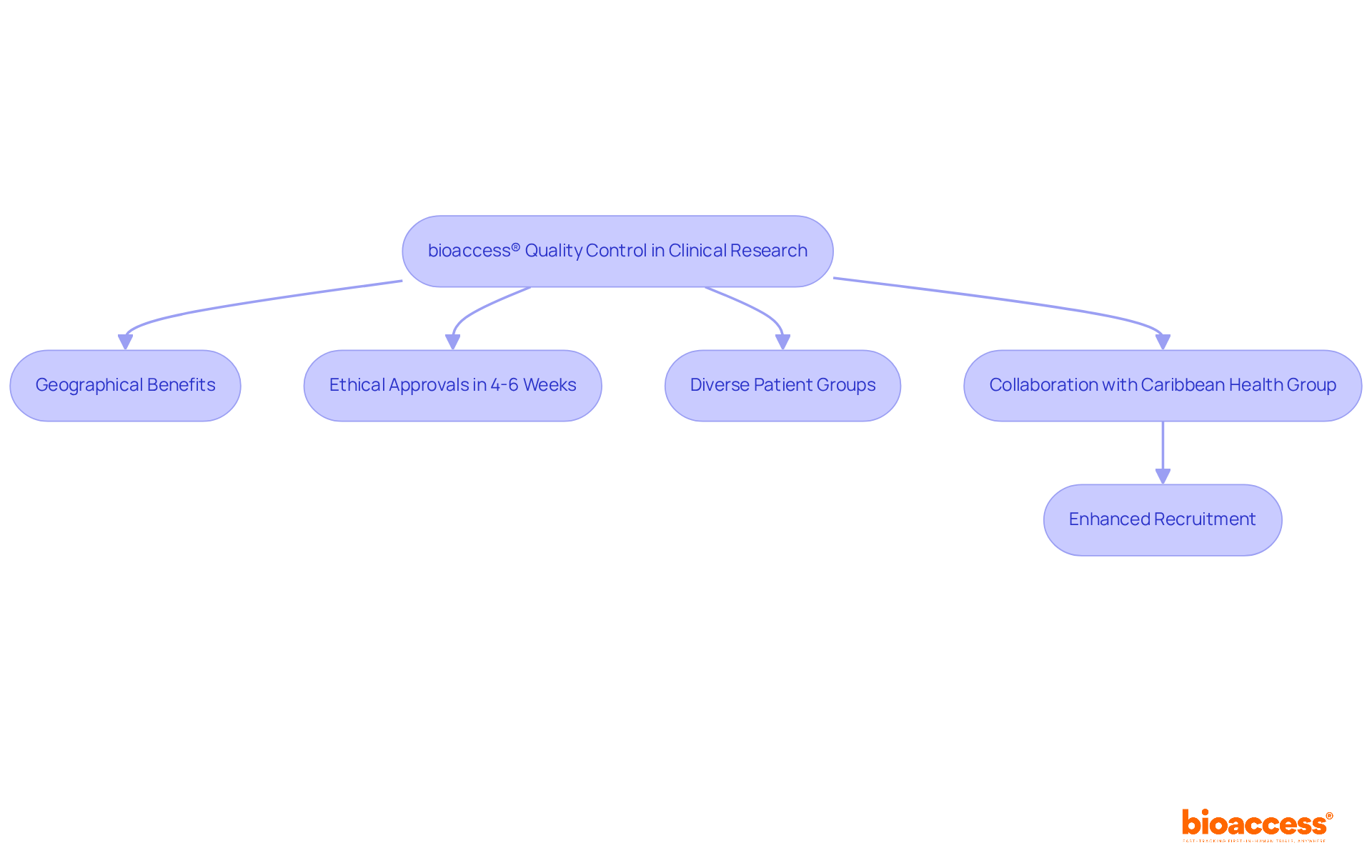
bioaccess® pioneers medical device quality control standards for clinical research by leveraging its strategic geographical benefits in Latin America, the Balkans, and Australia. This unique positioning enables the organization to secure ethical approvals in an impressive timeframe of just 4-6 weeks, supported by its pre-qualified networks of over 50 activated sites. Such rapid processing not only enhances the standard of clinical data but also facilitates faster patient enrollment, which is crucial for maintaining the integrity of research outcomes. By utilizing local regulatory knowledge and engaging diverse patient groups, bioaccess® consistently meets and often exceeds control standards, fostering innovation within the Medtech sector.
The collaboration with Caribbean Health Group aims to position Barranquilla as the premier destination for clinical research in Latin America, significantly enhancing recruitment potential. This initiative has garnered support from Colombia's Minister of Health, underscoring the commitment to transforming the region into a clinical trial hub. As the clinical trials sector in Latin America is projected to reach USD 2,654.0 million by 2030, with a compound annual growth rate (CAGR) of 6.7% from 2024 to 2030, the focus on medical device quality control becomes increasingly vital in ensuring successful trial results and promoting healthcare technologies.
Julio Martinez-Clark, CEO of bioaccess, highlights the advantages of conducting clinical trials in Latin America, particularly in addressing recruitment challenges, where 35% of delays in clinical trials stem from insufficient subject recruitment. By leveraging these advantages, bioaccess® is poised to enhance the efficiency and effectiveness of clinical trials in the region.
Implementing a Quality Management System (QMS) is essential for efficient medical device quality control during the development process. A well-organized QMS specifies the essential processes, responsibilities, and procedures necessary to achieve excellence objectives, ensuring that all production and research elements are meticulously recorded and overseen. This systematic approach not only aids in adhering to regulatory standards but also enhances operational efficiency and medical device quality control, contributing to product excellence.
By adopting ISO 13485 standards, organizations can significantly elevate their management practices, leading to improved patient outcomes. As the FDA's QMSR final rule, effective February 2, 2026, aligns US regulations with ISO 13485, manufacturers are encouraged to adopt these standards for medical device quality control to streamline their quality processes and enhance global market integration.
Success stories from Medtech companies demonstrate that implementing ISO 13485 not only fulfills compliance requirements but also fosters a culture of continuous improvement, ultimately promoting innovation and excellence in patient care.
In Colombia, navigating the regulatory landscape is supported by INVIMA, the National Food and Drug Surveillance Institute, which oversees the compliance of healthcare products and ensures adherence to safety and efficacy standards. By leveraging comprehensive clinical trial management services—including feasibility studies, site selection, trial setup, project management, compliance reviews, and reporting—organizations can effectively manage their clinical research projects while aligning with INVIMA's regulations. This alignment contributes to local economic growth and healthcare improvement.

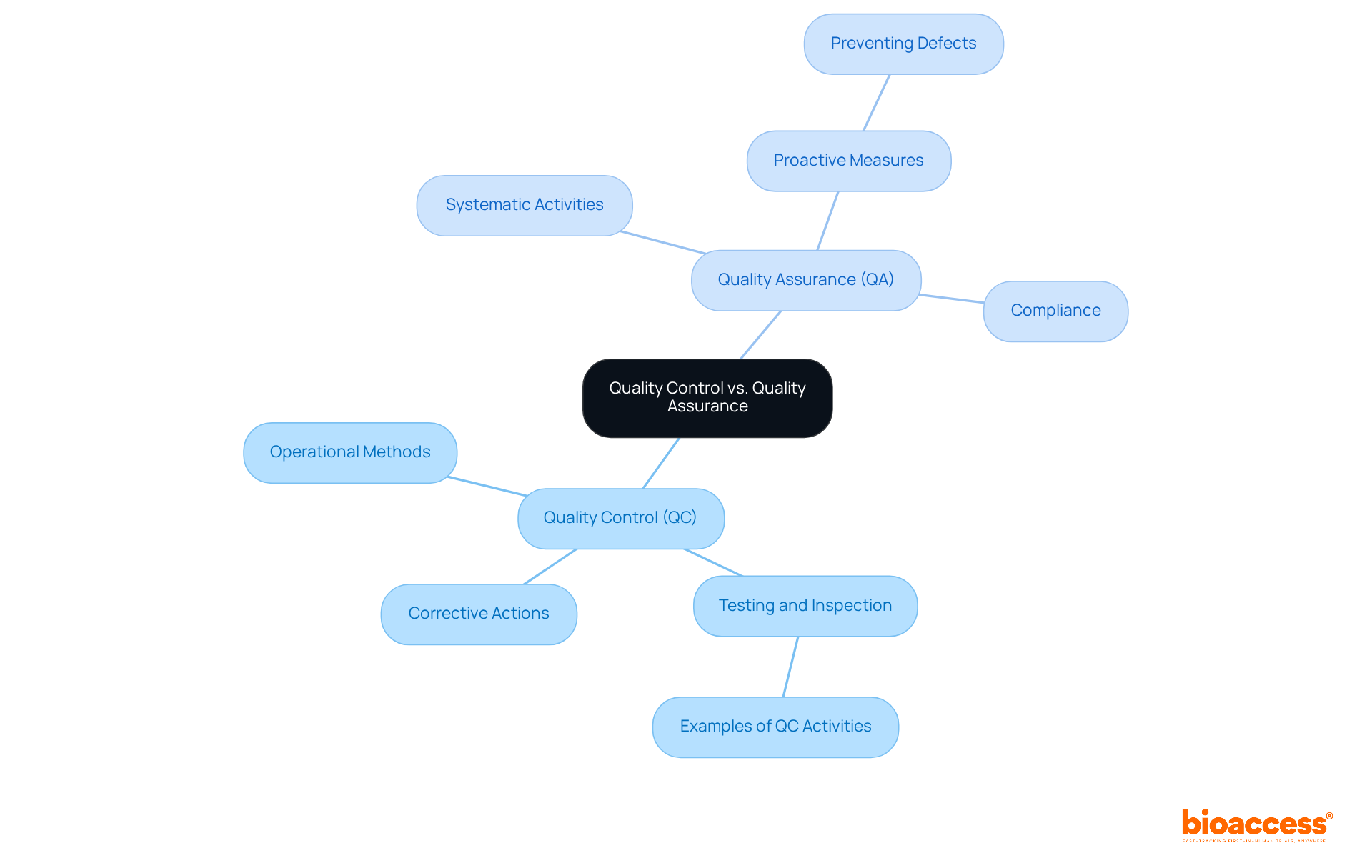
Quality Control (QC) and Quality Assurance (QA) have distinct yet complementary roles within medical device quality control in the industry. Medical device quality control focuses on the operational methods and actions necessary to meet standards for excellence, primarily through rigorous testing and inspection of products. This responsive procedure emphasizes the identification and rectification of flaws to ensure that products adhere to medical device quality control standards. Conversely, medical device quality control encompasses the systematic activities undertaken within the assurance system to ensure compliance with standards. This proactive approach engages the entire organization, ensuring that excellence is woven into every aspect of the product or service. Recognizing this distinction enables organizations to allocate resources effectively for medical device quality control, ensuring that both preventive measures (QA) and corrective actions (QC) are implemented to uphold high standards.
Recent trends underscore a growing emphasis on the integration of medical device quality control and QA processes to enhance overall efficiency and compliance. Notably, 88% of medtech organizations are prioritizing postmarket improvement of standards, acknowledging the necessity for scalable operations to adapt to market fluctuations. This shift signifies a broader industry movement towards proactive and risk-based quality management, increasingly recognized as a pivotal driver for change.
Moreover, the collaboration between QC and QA is crucial for ensuring that products not only comply with regulatory requirements but also align with medical device quality control to meet customer expectations. Industry experts assert that balancing these two functions is essential for ensuring medical device quality control, as well as maintaining product reliability and consistency. Vishaka Rajaram, Senior Director of Quality, highlights that establishing scalable operations is vital for supporting growth and adapting to market changes. By fostering a culture that values both QC and QA, organizations can navigate the complexities of the healthcare product landscape more effectively, ultimately leading to improved patient outcomes and reduced risk of recalls.
Implementing rigorous testing and validation processes is paramount for medical device quality control in ensuring quality assurance for medical equipment. These processes encompass:
These are essential for maintaining medical device quality control and ensuring that products not only function as intended but also adhere to regulatory standards. Current methodologies, such as Design Verification and Validation (V&V), are crucial for medical device quality control as they systematically evaluate the safety and effectiveness of products.
Verification confirms that design outputs correspond with design inputs, while validation assesses whether the product meets user requirements in real-world environments. By adopting these methodologies, organizations can significantly reduce the risk of recalls and enhance patient safety, ultimately fostering trust in their medical device quality control practices.
Effective preclinical testing approaches, which include functional and performance evaluations, are essential for detecting potential problems early in the development phase. This proactive strategy facilitates the route to market and ensures adherence to strict standards.

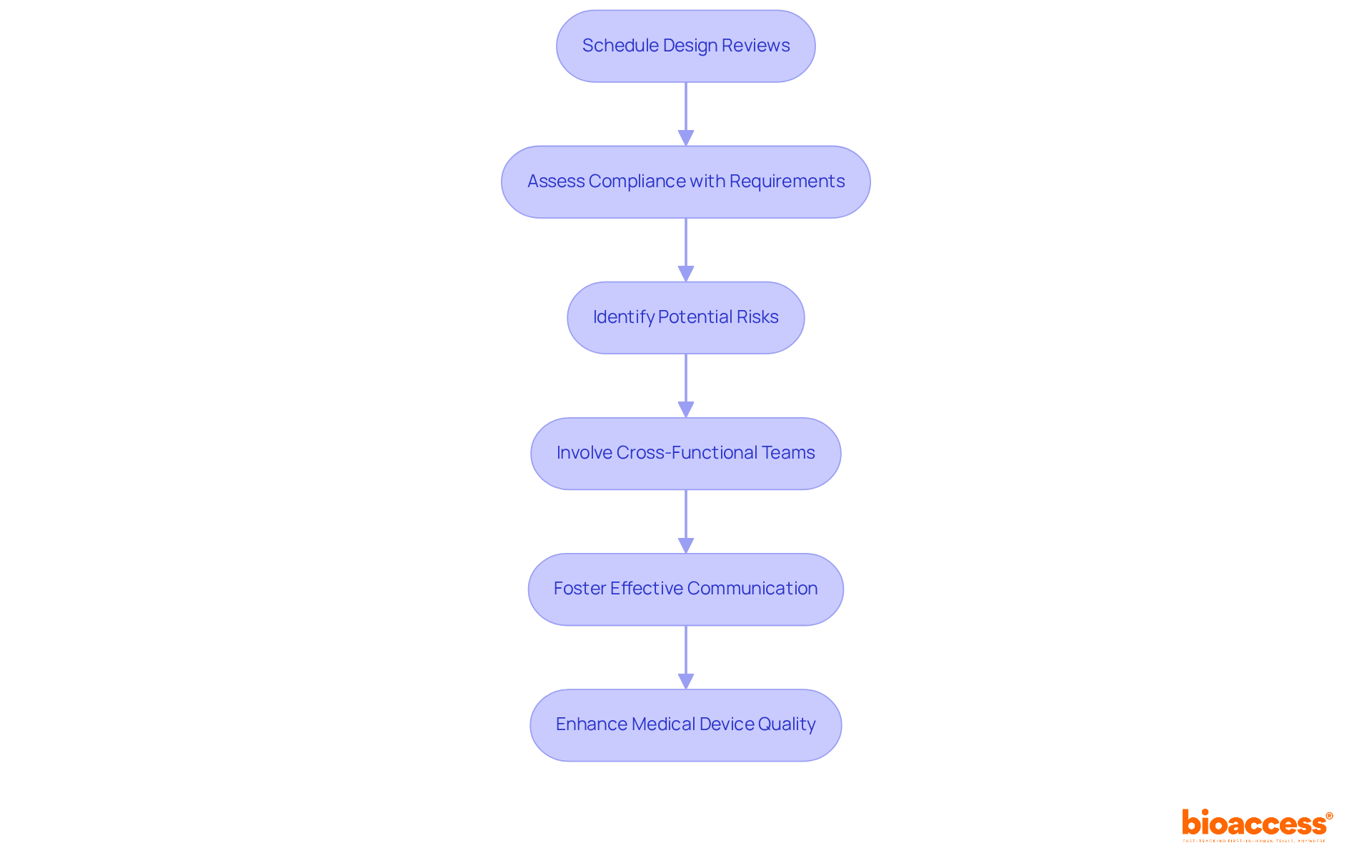
Carrying out design evaluations is crucial for enhancing medical device quality control procedures. These reviews should be strategically scheduled throughout the design process to assess compliance with specified requirements and to pinpoint potential risks.
Involving cross-functional teams in these reviews is essential, as it guarantees a thorough assessment of the product from various viewpoints, including engineering, regulatory, and marketing. This collaborative method not only improves design standards but also accelerates the time to market.
Research indicates that 75% of cross-functional teams face dysfunction due to communication barriers; thus, fostering effective communication within these teams can significantly enhance outcomes. As Mike Drues notes, "Design Reviews are moments in time for you to evaluate the progress of your medical device project as it progresses through development."
Effective participation of varied team members in design evaluations has demonstrated to enhance decision-making and creativity, ultimately leading to superior products. By emphasizing design evaluations and embracing a Whole Brain® approach to communication, organizations can establish strong assurance measures that conform to standards and effectively address user needs.
For instance, Johnson Matthey Emission Control Technologies utilized cross-functional collaboration to enhance their catalyst systems development, demonstrating the practical benefits of effective design reviews.
By prioritizing design evaluations, organizations can implement robust medical device quality control measures that align with regulatory standards and effectively meet user needs.
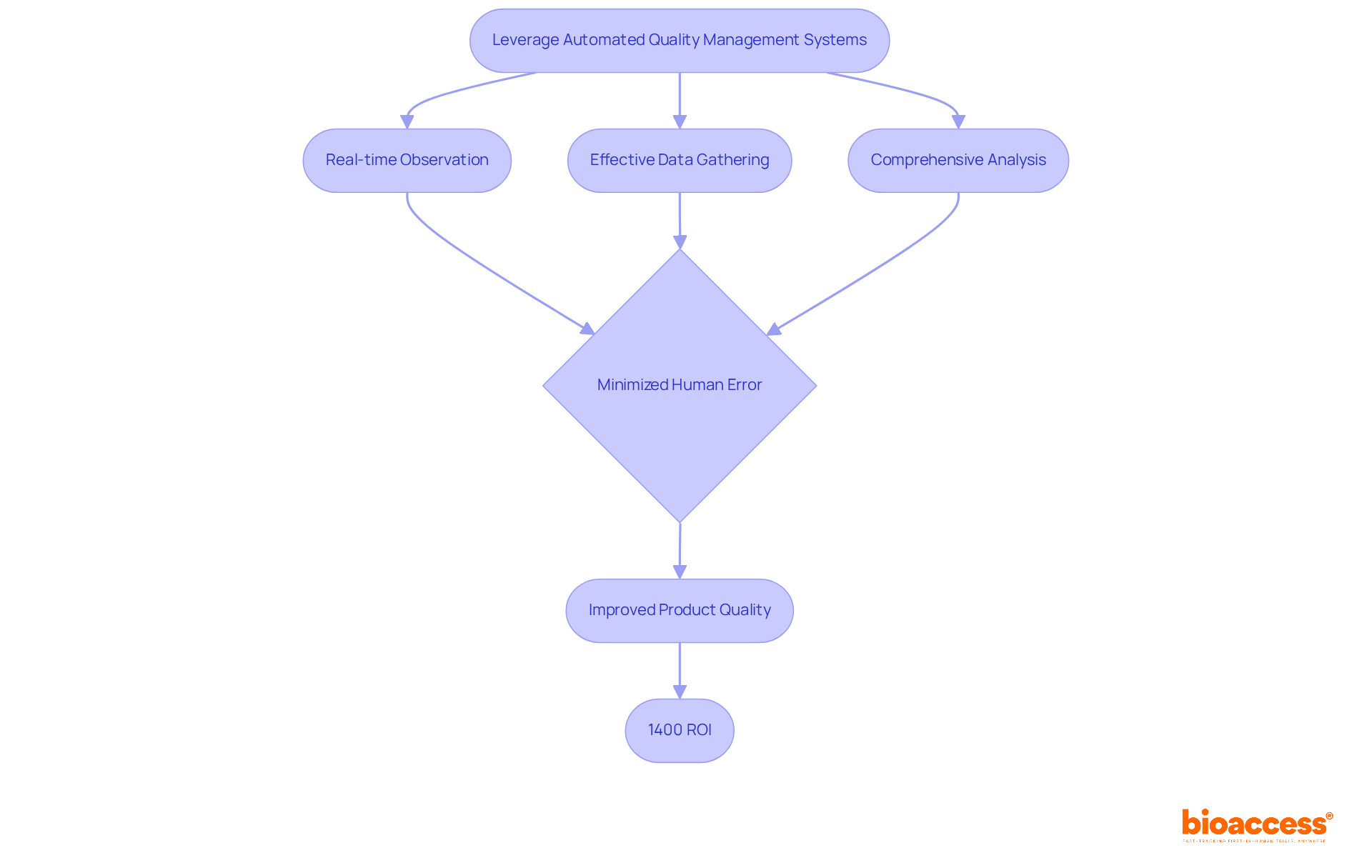
Automated management systems are essential for enhancing control over standards processes in medical device quality control during development. These systems enable real-time observation, effective data gathering, and comprehensive analysis, allowing organizations to swiftly identify and address issues. By minimizing human error, automation significantly improves medical device quality control, resulting in more reliable and consistent product quality.
For example, the Freedom EVO workstation has fully automated numerous sample preparation workflows, showcasing increased efficiency and accuracy in laboratory environments. Additionally, integrating software solutions with existing workflows not only streamlines operations but also boosts overall efficiency, ultimately leading to superior outcomes in control processes.
The implementation of such systems has demonstrated substantial returns on investment, with some organizations reporting a remarkable 1400% ROI from enhanced performance measurement initiatives. As the Medtech sector evolves, leveraging automation in medical device quality control will be vital for maintaining high benchmarks.
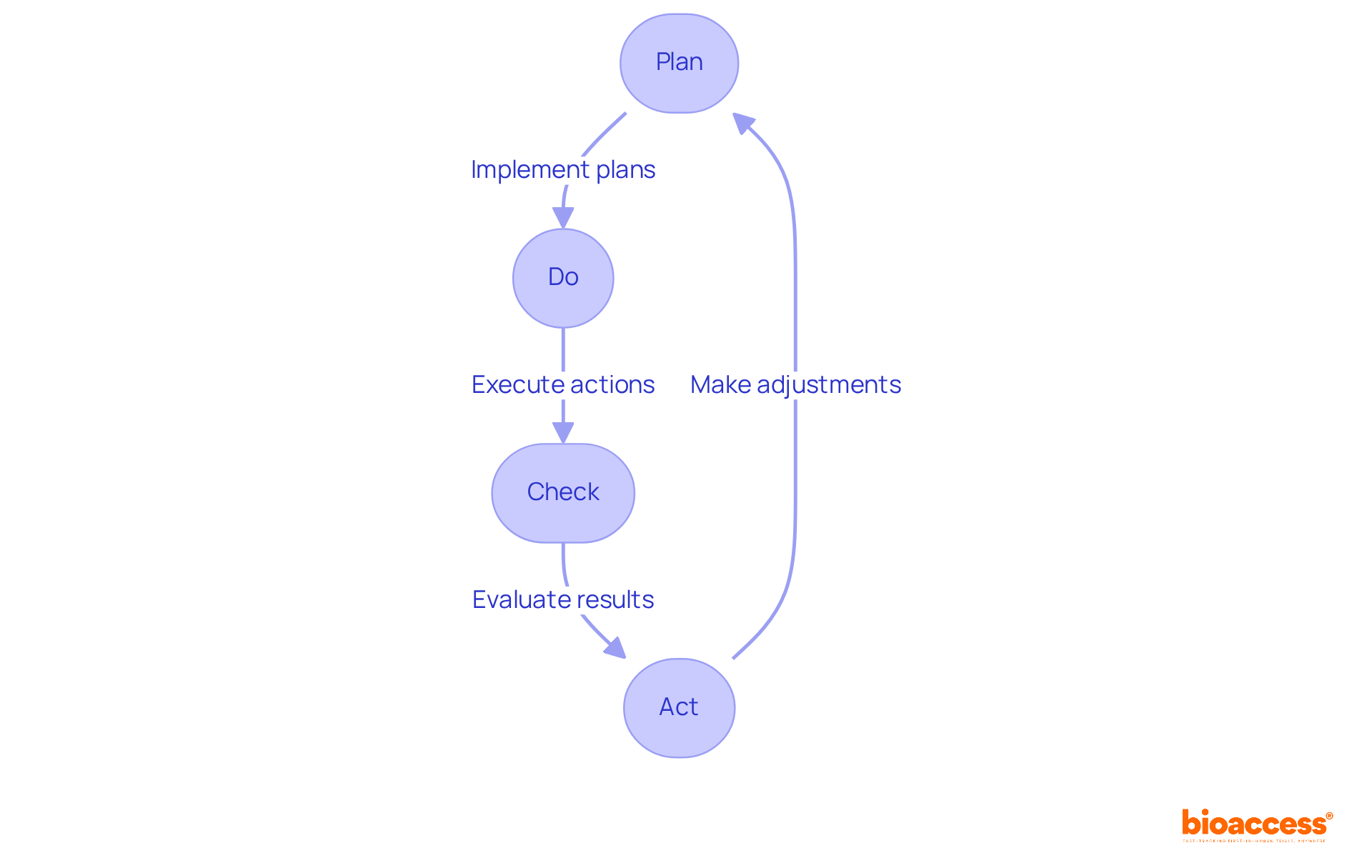
Highlighting the ongoing enhancement of medical device quality control procedures is essential for companies in the medical device sector, particularly in light of the frameworks established by agencies such as INVIMA. By adopting methodologies like Plan-Do-Check-Act (PDCA), companies can systematically assess their practices and implement necessary changes to comply with INVIMA's stringent standards.
Frequent evaluations, feedback mechanisms, and staff development foster a culture of excellence that encourages innovation and adaptability to compliance changes, ensuring that products consistently meet high standards.
As a Level 4 health authority acknowledged by PAHO/WHO, INVIMA's supervision underscores the significance of maintaining strict medical device quality control systems to achieve compliance excellence in the healthcare equipment sector.
Establishing thorough training programs for control staff is essential for ensuring effective medical device quality control and maintaining high standards in medical device development. These programs must encompass:
Investing in employee development not only equips teams with the necessary knowledge but also enhances their capability to implement effective medical device quality control processes. Regular refresher courses and certifications are crucial for sustaining competency among skilled personnel in medical device quality control, fostering a culture of continuous improvement. This approach leads to elevated product standards and ensures compliance with evolving regulations, such as the forthcoming Quality Management System Regulation (QMSR) by implementing medical device quality control, which is set for full implementation by February 2026.
Organizations that prioritize regulatory training for their control teams focused on medical device quality control are better positioned to navigate the complexities of FDA 21 CFR Part 820, ultimately safeguarding patient safety and enhancing product efficacy.

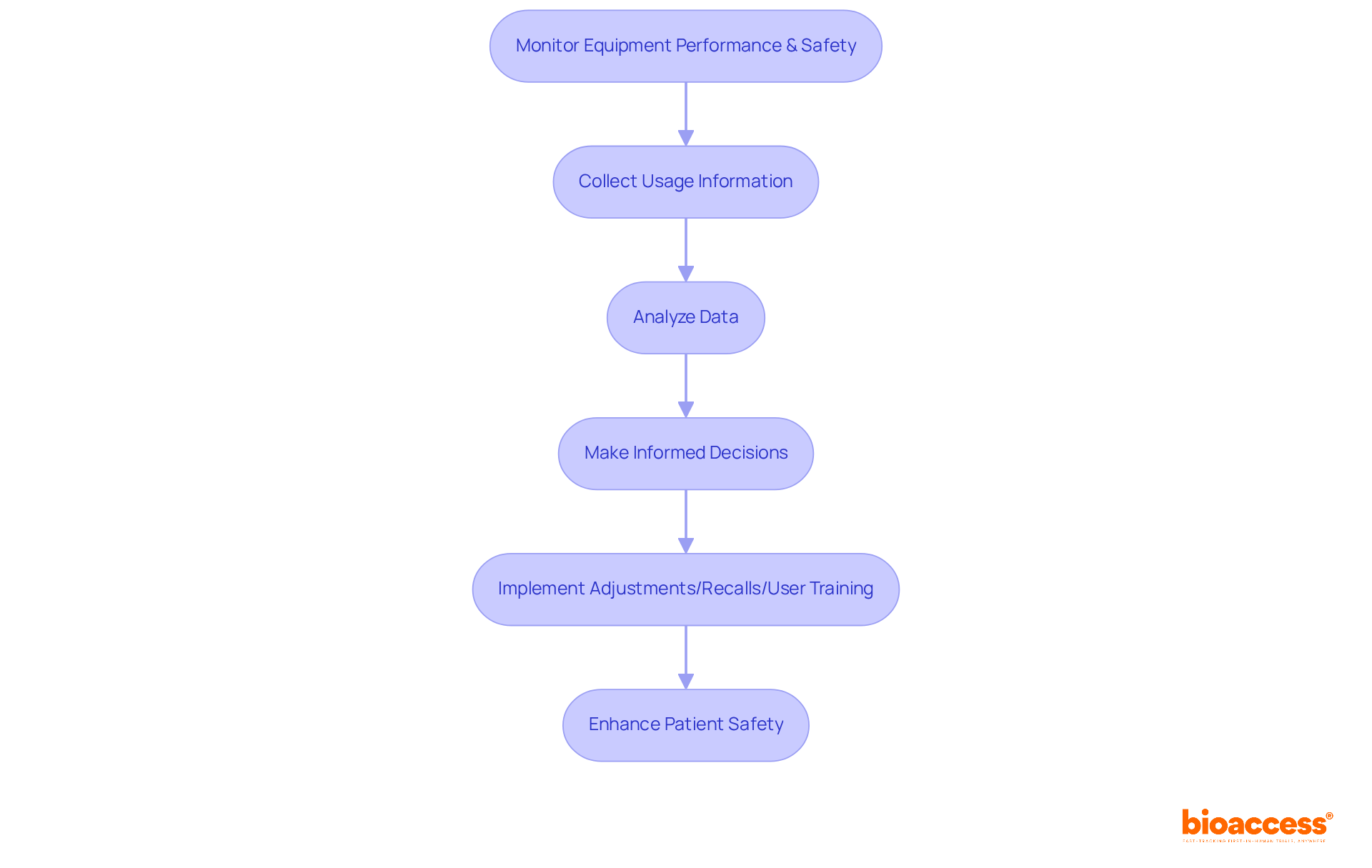
Post-market surveillance is essential for ensuring continuous medical device quality control in the management of medical equipment. This systematic process of medical device quality control involves monitoring equipment performance and safety after market release, which allows organizations to swiftly identify potential issues.
By collecting and analyzing information on equipment usage, companies can make informed decisions regarding necessary adjustments, recalls, or further user training. This proactive approach not only enhances patient safety but also ensures compliance with regulatory standards, particularly through effective medical device quality control, especially since approximately 70% of healthcare instruments in Brazil are subject to continuous oversight.
Furthermore, effective post-market monitoring practices contribute significantly to medical device quality control, enhancing the overall reliability of healthcare instruments and facilitating timely actions and improvements based on real-world performance data.
As the healthcare equipment market continues to grow, projected to reach $955.49 billion by 2030, the importance of robust monitoring systems cannot be overstated.
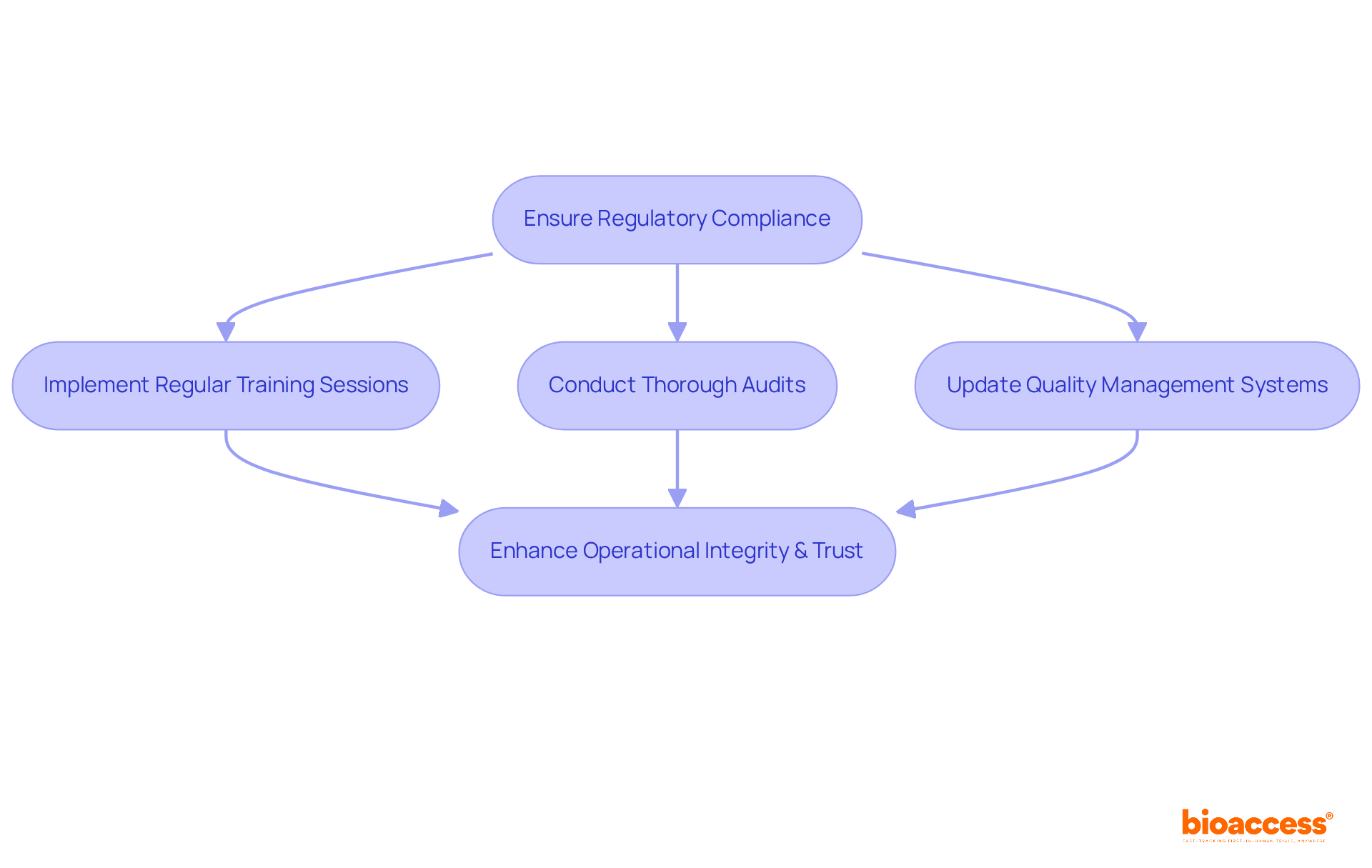
Regulatory compliance is a cornerstone for effective medical device quality control within the medical device sector. Organizations must remain vigilant regarding evolving regulations from the FDA and ISO to ensure their products adhere to stringent safety and efficacy standards. This commitment involves:
By prioritizing adherence to these regulations, organizations not only enhance their operational integrity but also foster trust among stakeholders, ensuring that their medical device quality control measures make products safe and effective for patient use.
Looking ahead to 2025, challenges such as cybersecurity, data privacy, and logistical hurdles will demand a proactive approach to compliance. It is imperative for companies to stay informed about regulatory changes and leverage technology to streamline their processes.
The landscape of medical device quality control is increasingly shaped by strategic methodologies and practices that ensure safety, efficacy, and compliance. Implementing essential strategies such as:
allows organizations to significantly enhance their product development processes. These approaches not only streamline operations but also foster a culture of continuous improvement, ultimately leading to better patient outcomes and increased trust in medical technologies.
Key insights from the article highlight the importance of:
Each of these elements plays a vital part in maintaining high standards of quality control, ensuring that medical devices not only meet regulatory requirements but also align with user needs and expectations. Furthermore, the emphasis on ongoing training for quality control personnel underscores the industry's commitment to excellence and compliance.
As the medical device sector continues to evolve, the significance of adhering to regulatory compliance cannot be overstated. Staying informed about changing regulations and leveraging technology will be crucial for organizations aiming to navigate the complexities of the healthcare landscape. By prioritizing these essential strategies, stakeholders can contribute to a future where medical devices are not only innovative but also safe and reliable, ultimately enhancing the overall quality of patient care.
What is bioaccess® and what role does it play in medical device clinical research?
bioaccess® is an organization that pioneers medical device quality control standards for clinical research, leveraging geographical advantages in Latin America, the Balkans, and Australia to secure ethical approvals quickly, typically within 4-6 weeks.
How does bioaccess® enhance the clinical research process?
bioaccess® enhances the clinical research process by utilizing pre-qualified networks of over 50 activated sites, which improves the standard of clinical data and facilitates faster patient enrollment, crucial for maintaining research integrity.
What initiative is bioaccess® collaborating on to improve clinical research in Latin America?
bioaccess® is collaborating with Caribbean Health Group to position Barranquilla as a premier destination for clinical research in Latin America, significantly enhancing recruitment potential and supported by the Colombian Minister of Health.
What is the projected growth of the clinical trials sector in Latin America by 2030?
The clinical trials sector in Latin America is projected to reach USD 2,654.0 million by 2030, with a compound annual growth rate (CAGR) of 6.7% from 2024 to 2030.
Why is implementing a Quality Management System (QMS) important in medical device quality control?
Implementing a QMS is essential for efficient medical device quality control as it specifies necessary processes, responsibilities, and procedures, ensuring meticulous recording and oversight of production and research elements, thereby enhancing operational efficiency and product quality.
How can organizations benefit from adopting ISO 13485 standards?
By adopting ISO 13485 standards, organizations can elevate their management practices, improve patient outcomes, and streamline quality processes, which aligns with the FDA's QMSR final rule effective February 2, 2026.
What distinguishes Quality Control (QC) from Quality Assurance (QA) in medical devices?
Quality Control (QC) focuses on the operational methods and actions to meet standards through testing and inspection, while Quality Assurance (QA) involves systematic activities to ensure compliance with standards, engaging the entire organization in maintaining quality.
Why is the integration of QC and QA processes emphasized in the medtech industry?
The integration of QC and QA processes is emphasized to enhance overall efficiency and compliance, as 88% of medtech organizations are prioritizing postmarket improvements to adapt to market fluctuations and ensure product reliability.
What are the benefits of balancing QC and QA functions in organizations?
Balancing QC and QA functions is essential for ensuring compliance with regulatory requirements, meeting customer expectations, and fostering a culture that supports growth and adapts to market changes, ultimately leading to improved patient outcomes and reduced risk of recalls.
Case Study: Munich Leukemia Lab - MLL - Streamlining medical genetics with the aid of automation (https://diagnostics.tecan.com/case_studies/mll_munich_leukemia_laboratory)
Case Study: Reinier de Graaf Hospital - A helping hand for vitamin testing (https://diagnostics.tecan.com/case_studies/reinier_de_graaf_hospital_vitamin_testing)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
How AI is reshaping pharma quality management system (https://pwc.be/en/news-publications/2025/how-ai-is-reshaping-pharma-qms.html)