


The article highlights essential strategies for successful Installation Qualification (IQ), underscoring the critical role of thorough documentation, effective training, and strict adherence to regulatory compliance. These strategies are not merely theoretical; they are bolstered by detailed insights into best practices and quality metrics that are vital for continuous improvement. Collectively, these elements enhance operational efficiency and ensure compliance throughout the installation process. By emphasizing these foundational aspects, the article serves as a guide for organizations looking to optimize their IQ processes, ultimately fostering a culture of excellence and accountability.
In the intricate world of Medtech and Biopharma, the Installation Qualification (IQ) process serves as a critical pillar for ensuring compliance and operational success. Organizations are increasingly recognizing that mastering this process not only streamlines project timelines but also significantly enhances product quality.
However, with the complexities of regulatory frameworks and the potential for costly errors, how can companies effectively navigate the challenges of installation qualification? This article unveils ten essential strategies designed to empower organizations, providing them with the tools necessary to achieve successful installation qualification while fostering a culture of continuous improvement.
At bioaccess®, we excel in expediting Installation Verification (IV) processes by leveraging our in-depth understanding of regulatory frameworks and clinical research protocols. Our expert team provides tailored guidance, ensuring that the installation qualification (IQ) process is executed with precision and efficiency. This not only accelerates project timelines but also enhances outcomes.
By leveraging our extensive experience in the Medtech, Biopharma, and Radiopharma sectors, we empower clients to navigate the complex landscape of installation qualification, ensuring compliance and quality at every stage. This strategic approach aligns with the latest trends in Installation Validation and underscores the critical role of expert insight in achieving successful results.
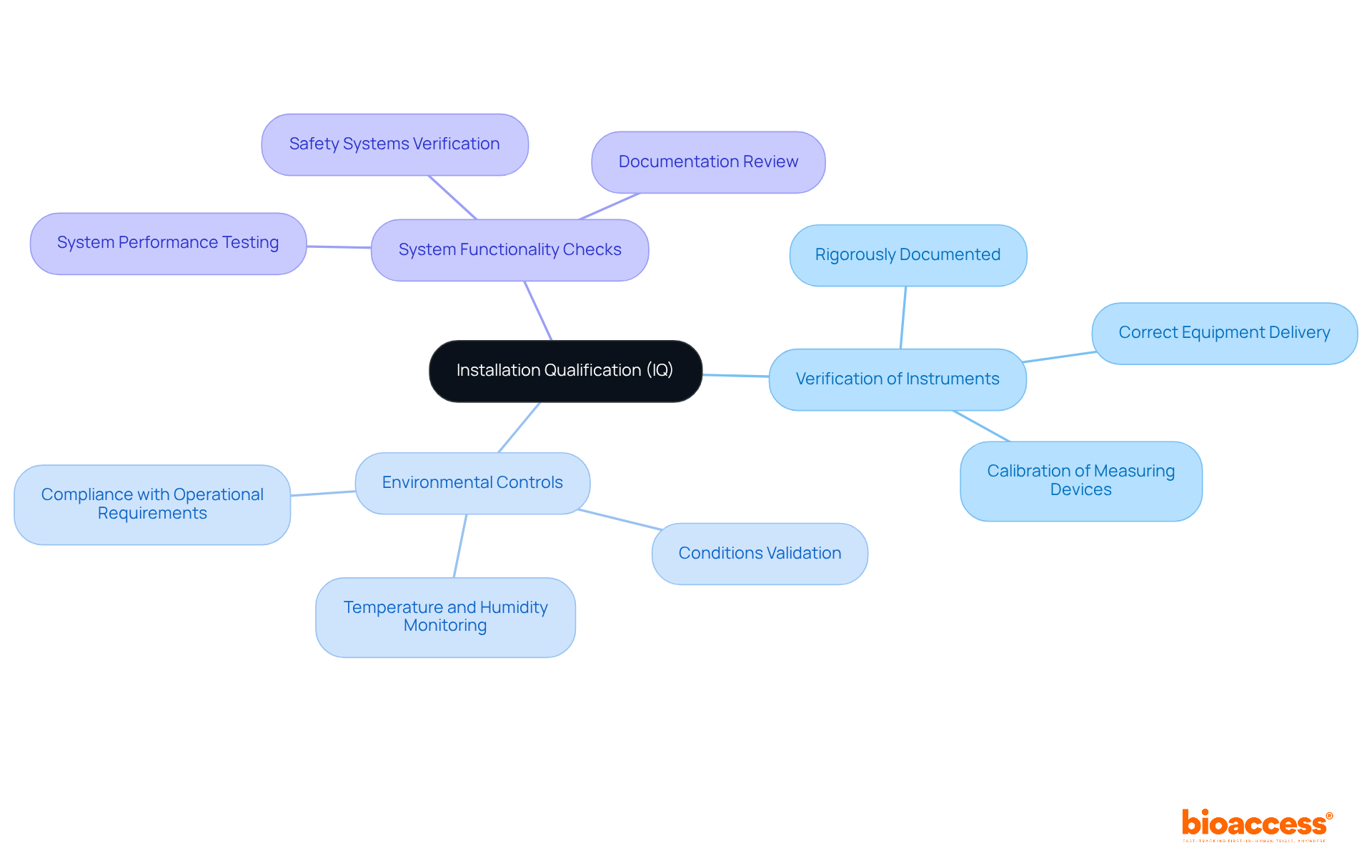
Installation Qualification (IQ) includes several vital elements, such as the verification of instruments, environmental controls, and checks for system functionality. Each of these elements must be rigorously documented to confirm that the installation qualification aligns with predefined specifications.
Effective equipment verification is crucial; it ensures that the correct equipment is delivered and installed according to the manufacturer's guidelines, thus preventing discrepancies that could affect the installation qualification phases. As noted by regulatory experts, proper installation not only safeguards product quality but also reinforces compliance with industry standards.
Furthermore, environmental controls must be validated to ensure that conditions such as temperature and humidity meet operational requirements. This meticulous approach establishes a solid foundation for the Operational Assessment (OQ) and Performance Assessment (PQ) phases, ensuring that all systems function as intended and are fully prepared for operational use.
By adhering to these best practices, organizations can significantly reduce the risk of non-compliance and enhance overall product integrity.
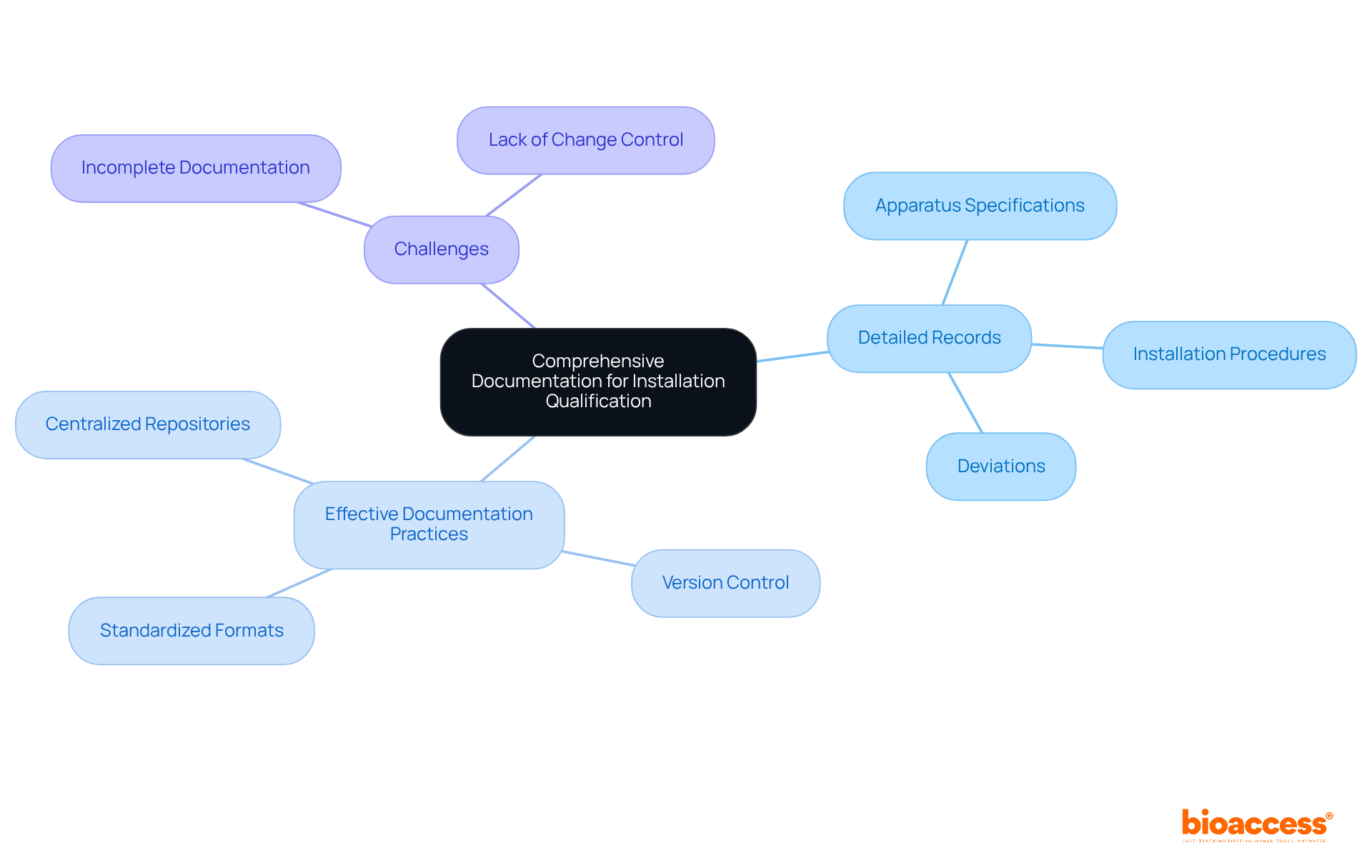
To guarantee a successful installation qualification (IQ), it is imperative to maintain thorough documentation throughout the procedure. This documentation must include:
Proper documentation not only aids adherence to regulatory standards but also provides a clear audit trail, which is vital for maintaining the credibility of the installation qualification procedure. As compliance specialist Theophilus Onyejiaku asserts, "IQ ensures that equipment is installed properly, all the necessary parts are present, and each meets specifications."
Effective documentation practices—including:
further enhance the reliability of the installation qualification procedure. It is crucial to recognize that incomplete documentation is one of the most frequent challenges faced during installation qualification, highlighting the need for meticulous record-keeping. Ultimately, these practices bolster the overall quality assurance framework in clinical research.
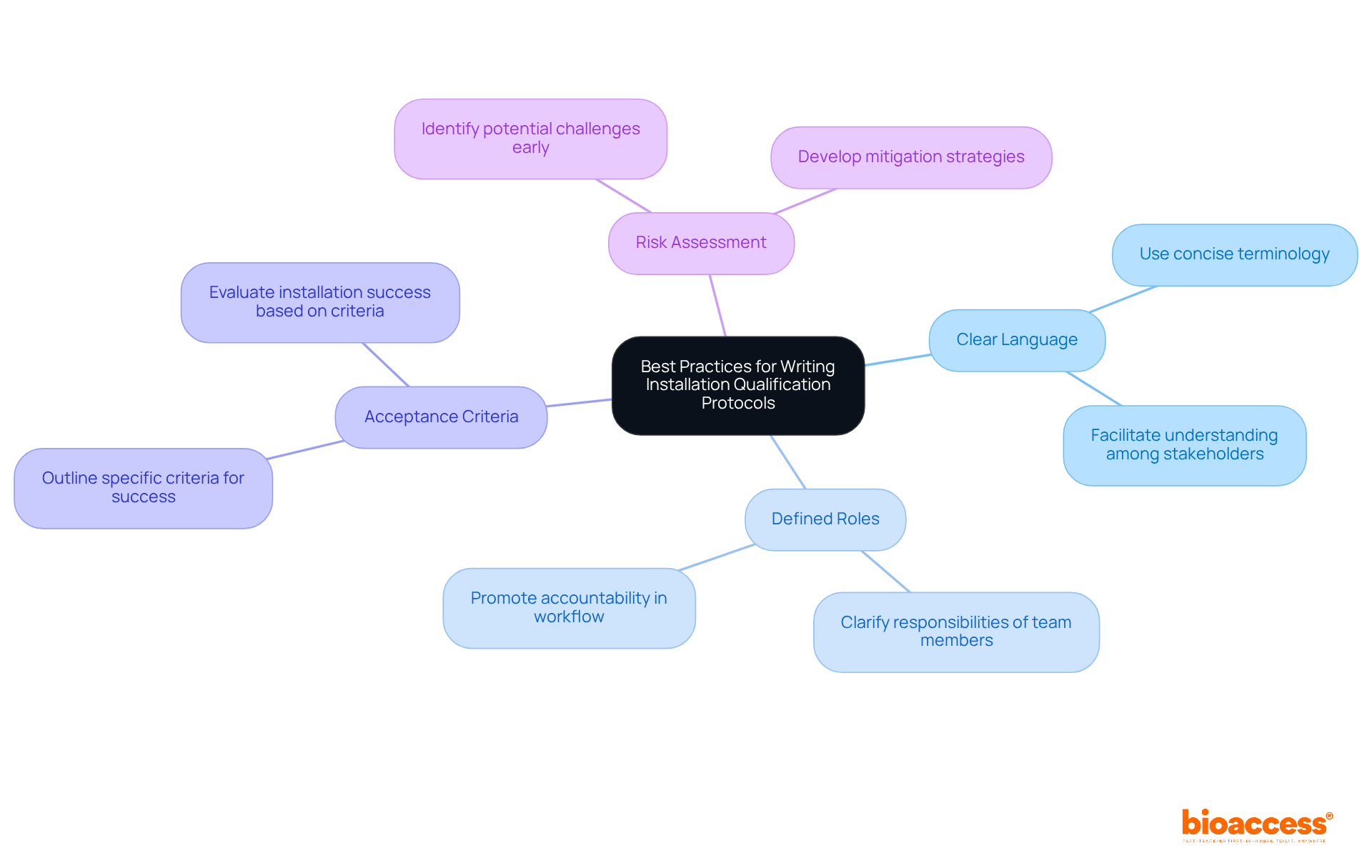
To develop effective installation qualification (IQ) protocols, it is essential to adhere to best practices that enhance clarity and effectiveness. Begin by employing clear and concise language, facilitating understanding among all stakeholders. Clearly defining roles and responsibilities is crucial; this ensures that each team member comprehends their specific tasks, thereby promoting accountability throughout the workflow. Furthermore, outlining specific acceptance criteria is vital for evaluating the success of the installation.
Incorporating a risk assessment section is another essential element. This proactive approach enables teams to identify potential challenges early, allowing for the development of mitigation strategies that can address issues before they escalate. As emphasized by industry professionals, a well-structured protocol not only enhances compliance but also increases the likelihood of successful outcomes. By adhering to these optimal methods, organizations can significantly improve the efficiency of their installation qualification procedures, ultimately resulting in enhanced operational effectiveness and regulatory adherence.
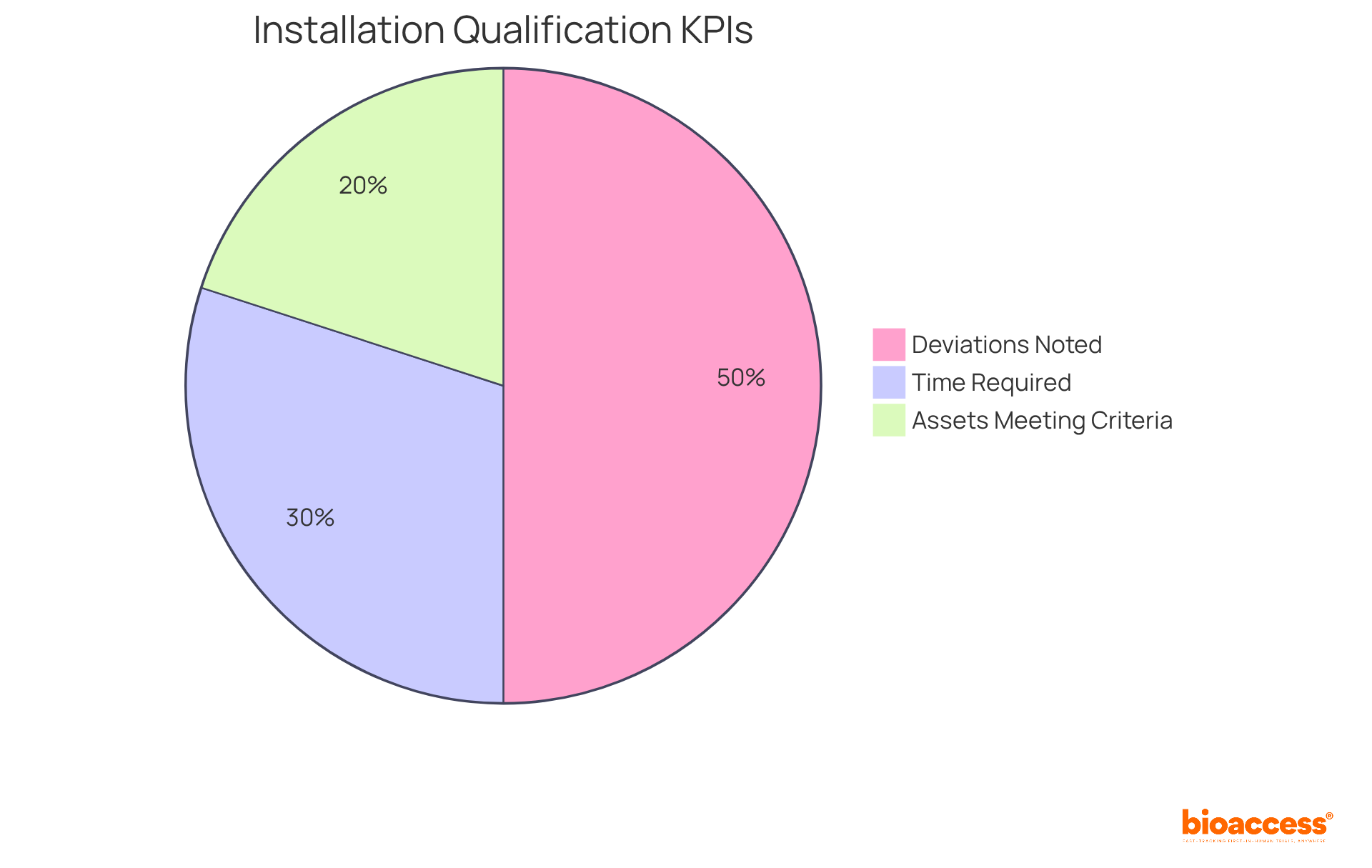
To effectively gauge the success of installation qualification, organizations must implement quality metrics that act as key performance indicators (KPIs). These KPIs include:
A recent analysis revealed that organizations often report a significant number of deviations, with 167 forms documented, underscoring the necessity for meticulous documentation and adherence to protocols. Additionally, environmental condition tests assess the reliability of devices under varying conditions, further emphasizing the importance of thorough evaluations.
By consistently reviewing these metrics, teams can identify areas needing enhancement, ensuring that their IQ processes not only remain efficient but also comply with standards. This proactive approach fosters a culture of continuous improvement, aligning with industry best practices and enhancing overall operational effectiveness. As Kazi, a professional in the pharmaceutical sector, states, 'The process of installation qualification is crucial for ensuring the safety, performance, and reliability of machinery and systems in regulated fields such as biotechnology, pharmaceuticals, and medical devices.' This highlights the critical nature of IQ in maintaining product quality and compliance.
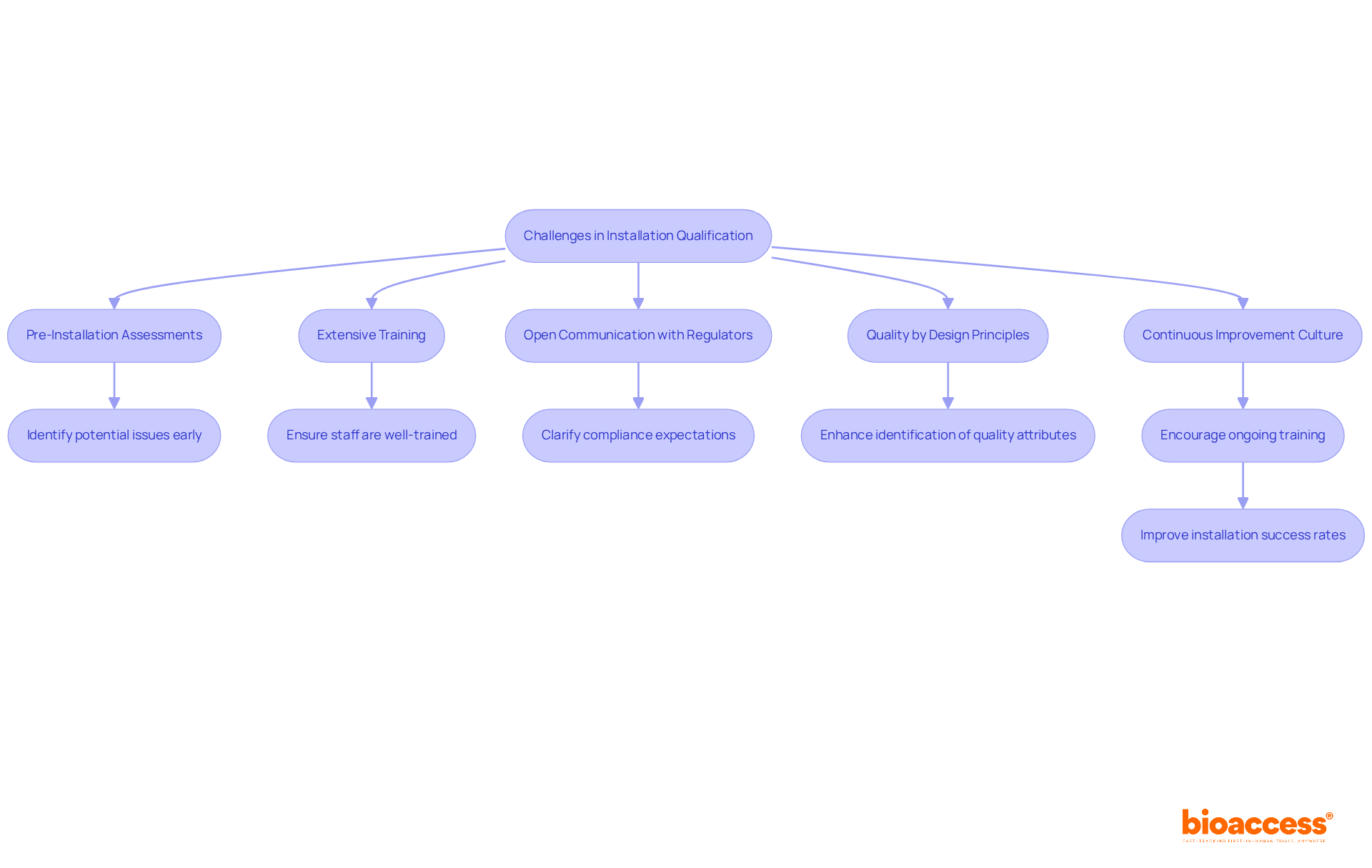
Installation Qualification (IQ) often faces challenges including device malfunctions, inadequate training, and hurdles related to regulatory compliance. To effectively navigate these issues, organizations should implement several key strategies:
By proactively tackling these challenges, organizations can simplify the installation qualification process, which ensures compliance and operational efficiency.
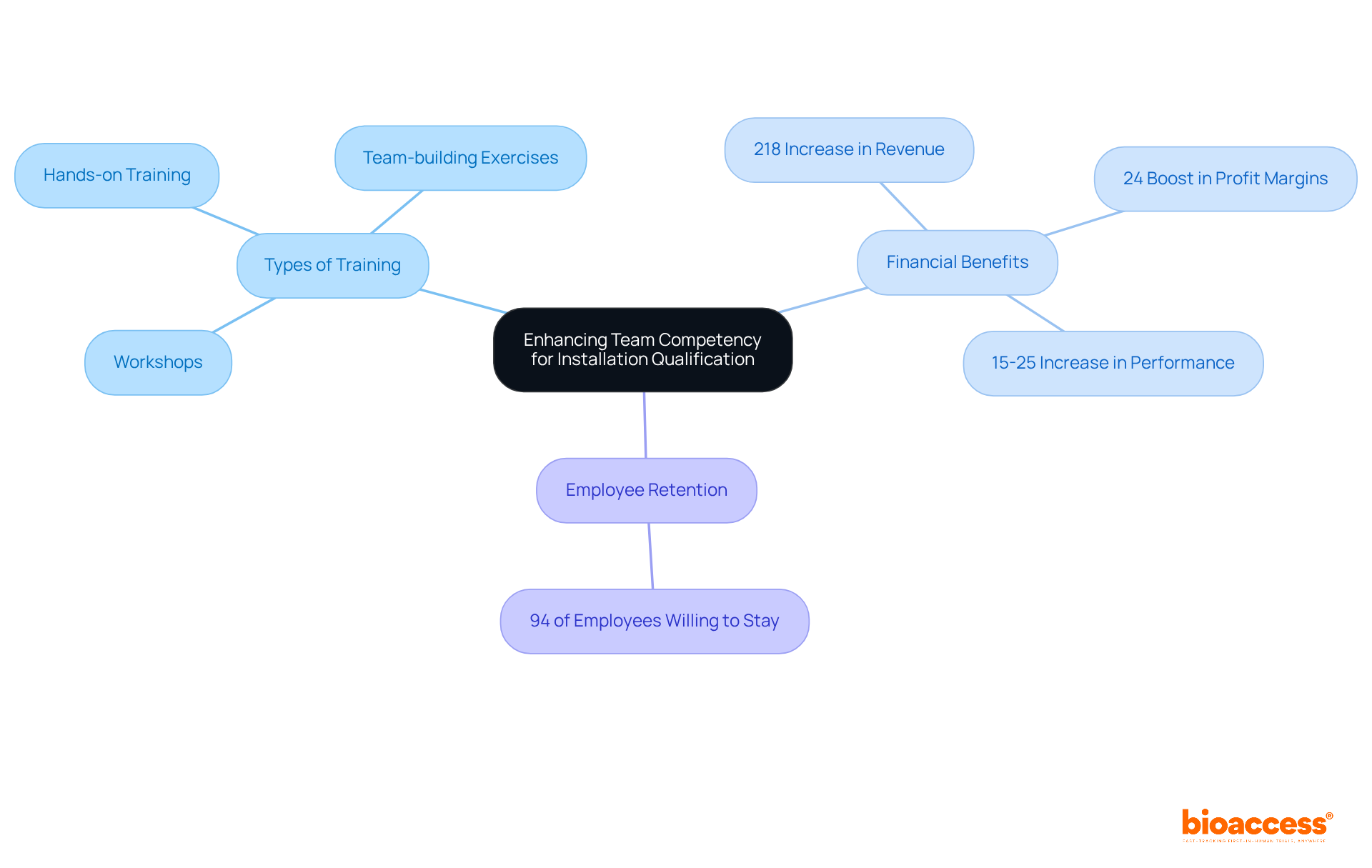
Enhancing team competency for installation qualification requires a steadfast commitment to continuous training and development. This commitment encompasses a variety of opportunities, including:
Investing in these development initiatives not only equips staff with the essential skills needed to navigate the complexities of the qualification journey but also significantly enhances overall performance.
Research indicates that organizations prioritizing employee training can experience a remarkable 218% increase in revenue and a 24% boost in profit margins, underscoring the financial advantages of a well-trained team. Furthermore, 94% of employees express a willingness to remain with a company that invests in their learning and development, highlighting the critical importance of fostering a supportive learning environment. Additionally, companies with robust training systems enhance employee performance by 15 to 25%, demonstrating the direct impact of training on operational efficiency.
By implementing effective training programs tailored to the unique needs of teams involved in installation qualification, organizations can achieve more favorable outcomes and enhance their operational efficiency.
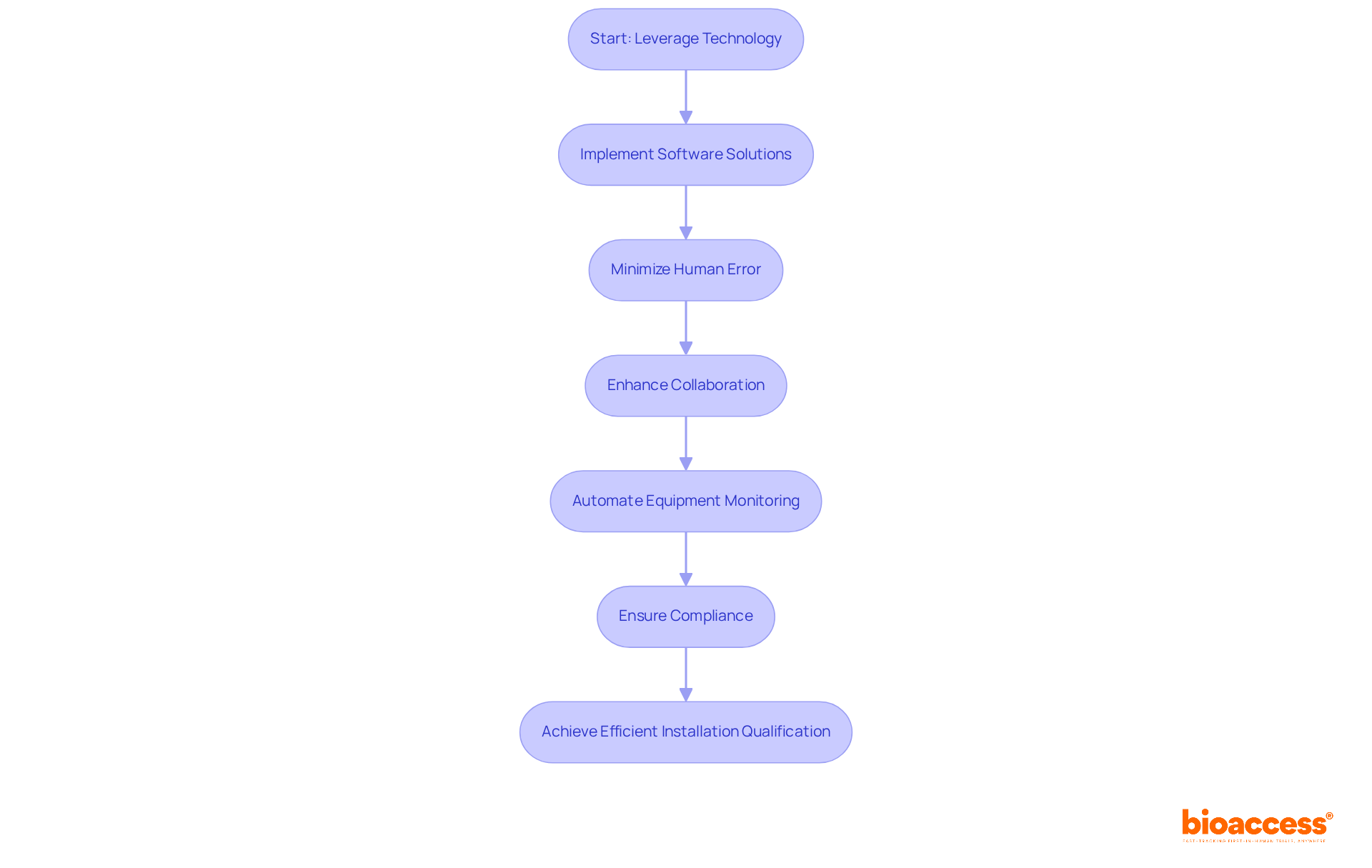
Utilizing technology in installation qualification significantly enhances operational efficiency and accuracy. By implementing software solutions for documentation, data collection, and project management, organizations can minimize human error and foster improved collaboration among team members. As Bill Gates aptly noted, "It’s fine to celebrate success but it is more important to heed the lessons of failure," underscoring the necessity for continuous improvement through technology.
Automated systems for equipment monitoring provide real-time data, ensuring that all systems operate optimally during the qualification phase. This strategy not only streamlines workflows but also aids in compliance with regulatory standards, ultimately resulting in faster project completion and better outcomes. Insights from the industry reveal that flexibility with new technologies is crucial for sustained success, and simplifying procedures can enhance user experience. By leveraging these technological advancements, organizations can achieve a more efficient installation qualification process.
To attain compliance with regulations during installation qualification, organizations must remain vigilant regarding relevant guidelines and standards, particularly Good Manufacturing Practices (GMP). In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in overseeing the framework for health products, including medical devices. Established under the Ministry of Health and Social Protection, INVIMA ensures that equipment and systems are installed correctly and function as intended, aligning with international standards set by the Pan American Health Organization/World Health Organization, which classifies INVIMA as a Level 4 health authority.
Adhering to GMP is essential, as it lays the groundwork for ensuring conformity with legal requirements for installation qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), as detailed in 21 CFR Part 211 and 21 CFR Part 820. This underscores the necessity for documented evidence and strict adherence to established protocols. Regular audits and comprehensive reviews of compliance documentation are vital for identifying potential issues early, thus preventing escalation and ensuring alignment with regulatory requirements. This proactive approach not only safeguards the integrity of the qualification process but also enhances product quality and patient safety.
Moreover, organizations that implement a performance metric of 95% system uptime significantly improve project outcomes, illustrating the tangible benefits of adhering to GMP standards. Training personnel involved in the installation process is critical to ensuring that all team members comprehend their roles and responsibilities, which is vital for effective and compliant installation processes. Frequent errors in installation qualification, such as inadequate documentation and failure to meet acceptance criteria, can lead to scrutiny from authorities, highlighting the importance of thorough preparation. As noted by industry experts like Ana Criado, who possesses extensive experience in oversight matters and compliance, effective adherence to GMP standards is crucial for building trust among healthcare providers and regulators, ultimately contributing to the success of pharmaceutical manufacturing.
Promoting ongoing enhancement in installation qualification practices requires a structured method for evaluating procedures and results. Organizations should actively solicit feedback from team members and stakeholders, leveraging this input to refine protocols and procedures. Studies show that organizations with well-trained staff face fewer compliance challenges, underscoring the importance of effective feedback systems.
By fostering a culture of ongoing enhancement, teams can adjust quickly to shifts in the compliance environment, thereby improving the effectiveness and efficiency of installation qualification processes. Industry leaders emphasize that quality control systems are essential for early detection of variations, preventing defects from reaching customers. This proactive stance not only mitigates risks but also aligns with regulatory expectations, ultimately leading to superior outcomes in clinical research.
In conclusion, the significance of installation qualification cannot be overstated. By embracing expert guidance, maintaining rigorous documentation, and fostering a culture of continuous improvement, organizations can not only enhance their operational efficiency but also ensure the safety and reliability of their products. The commitment to these strategies will pave the way for successful installation qualification, ultimately benefiting both the organization and the end-users.
What is bioaccess® and how does it assist with Installation Qualification (IQ)?
bioaccess® specializes in expediting Installation Verification (IV) processes by providing expert guidance that aligns with regulatory frameworks and clinical research protocols. Their team ensures that the IQ process is executed efficiently, accelerating project timelines and enhancing outcomes.
What are the key components of Installation Qualification (IQ)?
The key components of Installation Qualification (IQ) include the verification of instruments, environmental controls, and checks for system functionality. Each element must be documented to confirm alignment with predefined specifications.
Why is equipment verification important in the IQ process?
Equipment verification is crucial because it ensures that the correct equipment is delivered and installed according to the manufacturer's guidelines, preventing discrepancies that could affect the installation qualification phases and ultimately safeguarding product quality.
What role do environmental controls play in Installation Qualification?
Environmental controls must be validated to ensure that conditions such as temperature and humidity meet operational requirements. This validation establishes a solid foundation for the subsequent Operational Assessment (OQ) and Performance Assessment (PQ) phases.
What documentation is necessary for a successful Installation Qualification?
Comprehensive documentation for a successful Installation Qualification should include detailed records of apparatus specifications, installation procedures, and any deviations encountered during the process.
How can organizations ensure effective documentation practices during Installation Qualification?
Organizations can ensure effective documentation by using standardized formats, implementing version control, and utilizing centralized document repositories for secure storage and easy access.
What are the common challenges faced during Installation Qualification?
One of the most frequent challenges is incomplete documentation, which can hinder compliance and affect the credibility of the installation qualification procedure.
How does proper documentation contribute to Installation Qualification?
Proper documentation aids adherence to regulatory standards and provides a clear audit trail, which is vital for maintaining the credibility of the installation qualification procedure and bolstering the overall quality assurance framework in clinical research.