


This article emphasizes essential strategies for designing effective clinical research case report forms (CRFs), aiming to enhance data collection and compliance. It underscores the significance of incorporating best practices such as clarity, consistency, and usability. Moreover, leveraging technology, particularly electronic CRFs, is shown to significantly improve data accuracy and expedite clinical research outcomes. This addresses the critical need for effective CRF design in the research process, highlighting its relevance in today's Medtech landscape.
Clinical research thrives on precision and efficiency; however, many studies falter due to poorly designed case report forms (CRFs). These forms are not merely bureaucratic necessities; they constitute the backbone of data collection, significantly influencing the integrity and success of clinical trials.
This article presents ten essential tips for crafting effective CRFs, providing insights that could streamline processes, enhance data quality, and ultimately lead to faster, more reliable research outcomes.
What occurs when the very tools intended to facilitate research become obstacles instead? Exploring this question underscores the critical importance of thoughtful design and collaboration in clinical research.
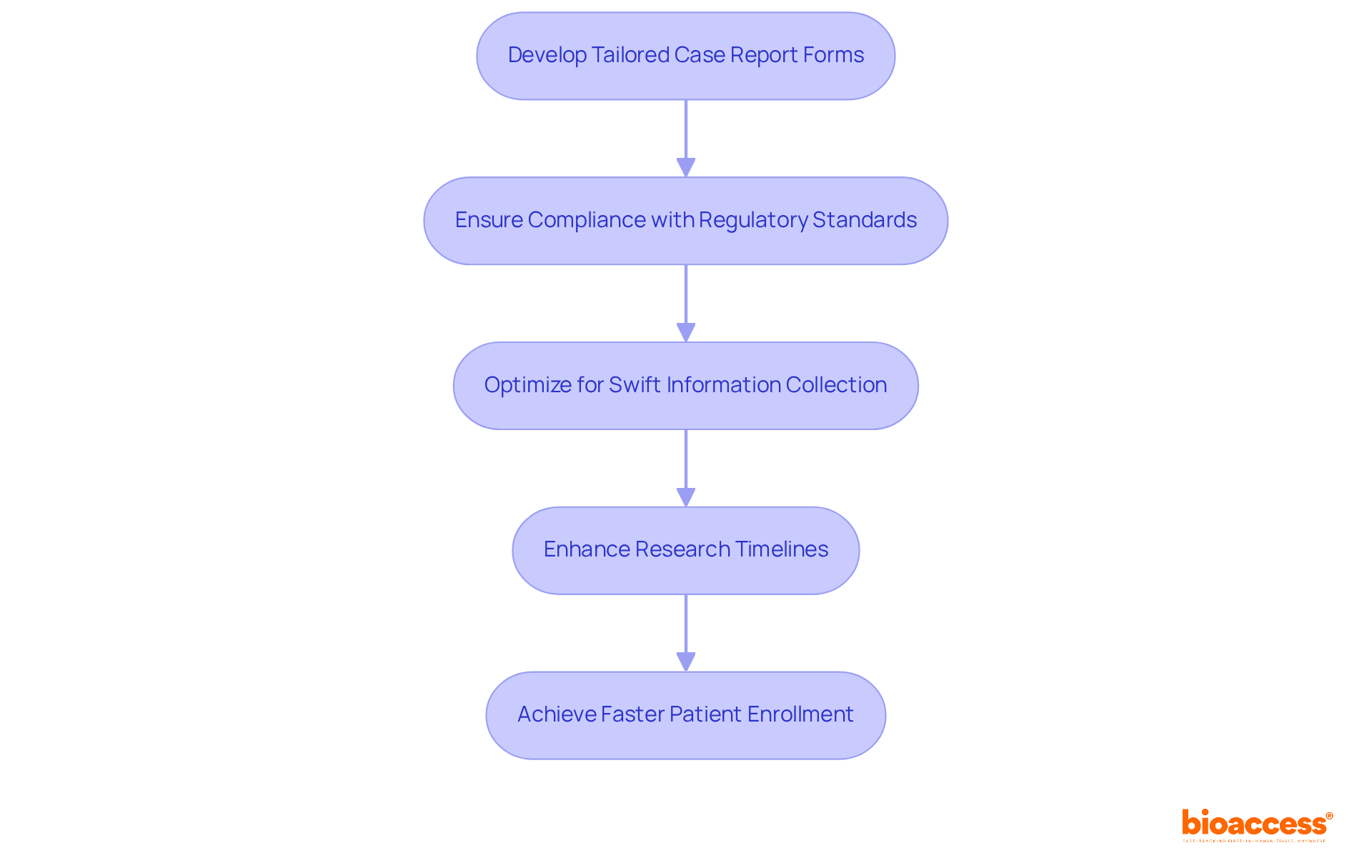
At bioaccess®, we specialize in developing tailored case report templates that address the unique needs of Medtech, Biopharma, and Radiopharma innovators in Latin America. By leveraging regional advantages, we ensure that our case report forms not only comply with regulatory standards but are also optimized for swift information collection and analysis.
With over 20 years of experience in Medtech, our customized approach significantly enhances research timelines, facilitating ethical approvals within 4-6 weeks and achieving patient enrollment rates that are 50% faster than conventional markets.
Established trends show that well-designed electronic case report forms (eCRFs) can reduce costs and improve data quality, making them essential for successful research trials.
Medtech pioneers recognize that tailored case report forms are crucial for addressing specific study requirements, ultimately leading to more efficient and effective research outcomes.
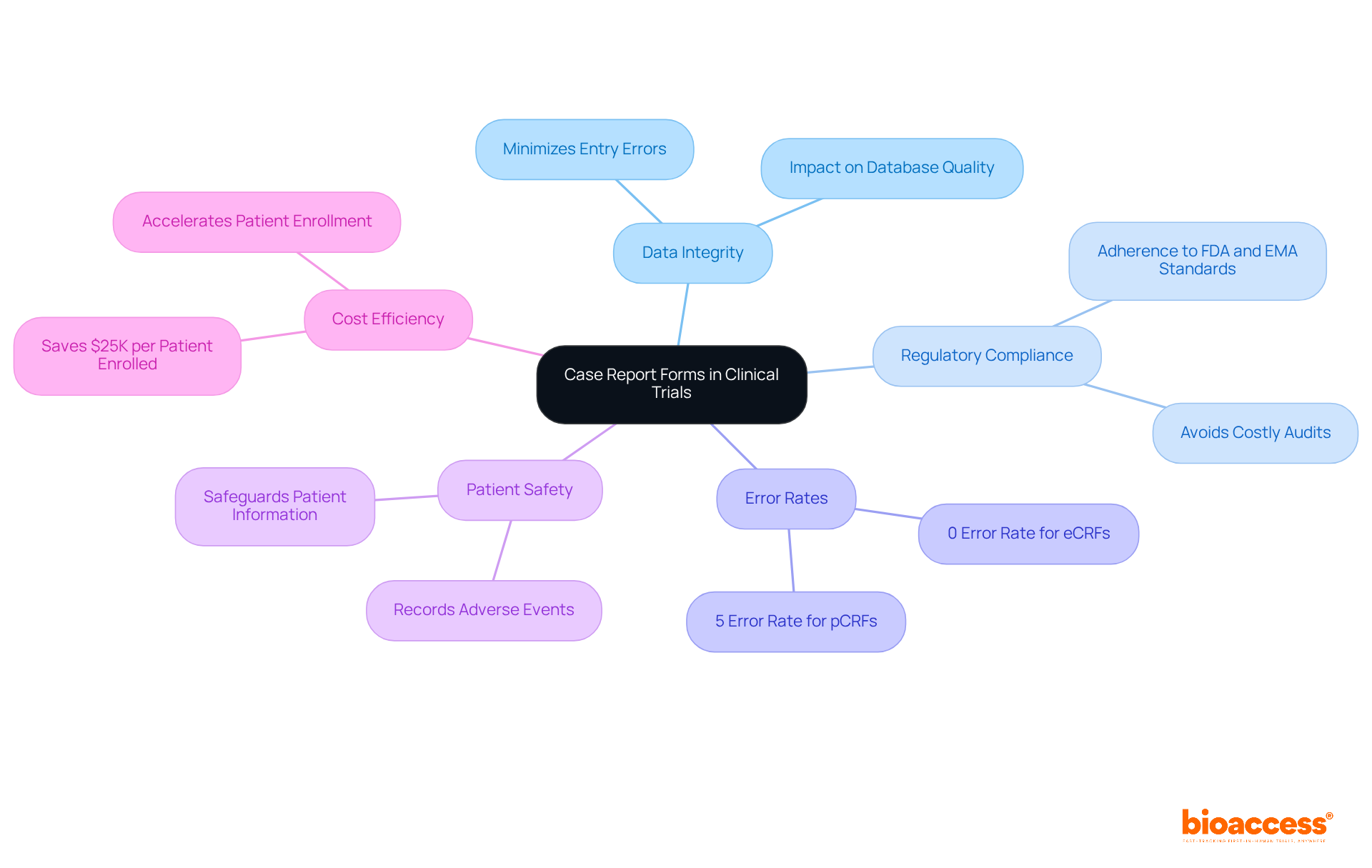
The clinical research case report forms serve as vital instruments in clinical trials, meticulously crafted to collect all protocol-required information from each participant. Their structured format ensures uniform and accurate data collection, which is crucial for maintaining the integrity of research. These forms not only facilitate the tracking of trial progress but also ensure compliance with stringent regulatory standards set by organizations such as the FDA and EMA. Adhering to these standards is imperative, as any lapse can result in delayed approvals or expensive audits.
Indeed, by ensuring high data integrity through well-designed case report forms, the quality of the database can be significantly enhanced, directly influencing the validity of study outcomes. Research suggests that a thoughtfully structured case report form can minimize entry errors by up to 5%, while electronic case report forms (eCRFs) achieve an impressive 0% error rate in contrast to the 5% associated with traditional paper forms.
Furthermore, expert opinions underscore the necessity of case report forms in safeguarding patient safety and optimizing cost efficiency in research studies. By guaranteeing precise information collection, the clinical research case report form is essential to the success of clinical research, ultimately leading to improved patient outcomes and accelerated innovation.
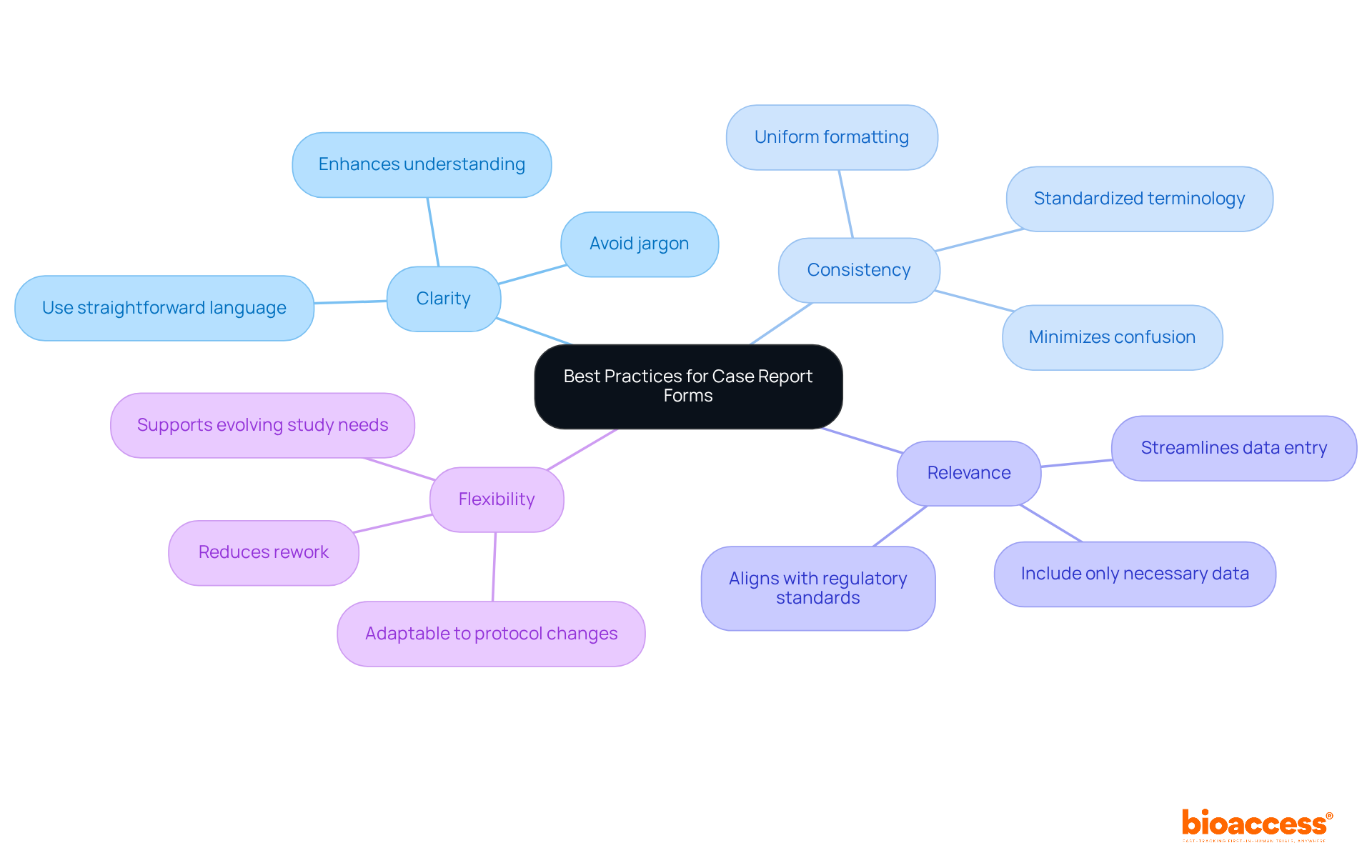
Effective clinical research case report forms must adhere to several best practices to enhance usability and data integrity.
Clarity is paramount; employing straightforward language and avoiding jargon ensures that all users can easily understand the questions. Studies indicate that user-friendly CRFs significantly enhance understanding, resulting in more precise information collection.
Consistency is equally important. Maintaining uniform formatting and terminology throughout the CRF minimizes confusion. Standardization in CRF design is essential for upholding the quality and integrity of information, as it decreases variability in information collection.
Relevance involves including only necessary data points to avoid overwhelming users and streamline data entry. This approach not only enhances the user experience but also aligns with regulatory compliance standards, ensuring that only pertinent information is captured.
Flexibility in design allows CRFs to adapt to changes in study protocols without significant rework. This adaptability is crucial in research trials, where protocols may evolve based on initial findings or regulatory input.
Integrating these principles into the clinical research case report form design results in enhanced information quality and efficiency, ultimately aiding the success of clinical trials. Moreover, the transition from paper-based CRFs to electronic formats has marked improvements in both efficiency and accuracy; for instance, electronic CRFs (eCRFs) reduce entry mistakes to below 1%, compared to a 5% error rate for traditional paper forms. As noted by the Bioaccess Content Team, "eCRFs enable real-time information input and monitoring, significantly improving the accuracy and comprehensiveness of information collected." Furthermore, engaging stakeholders early in the CRF development process can lead to iterative enhancements, resulting in user-friendly forms that facilitate precise data collection. By adopting these best practices, research professionals can ensure their clinical research case report forms are both effective and compliant with evolving regulatory standards.
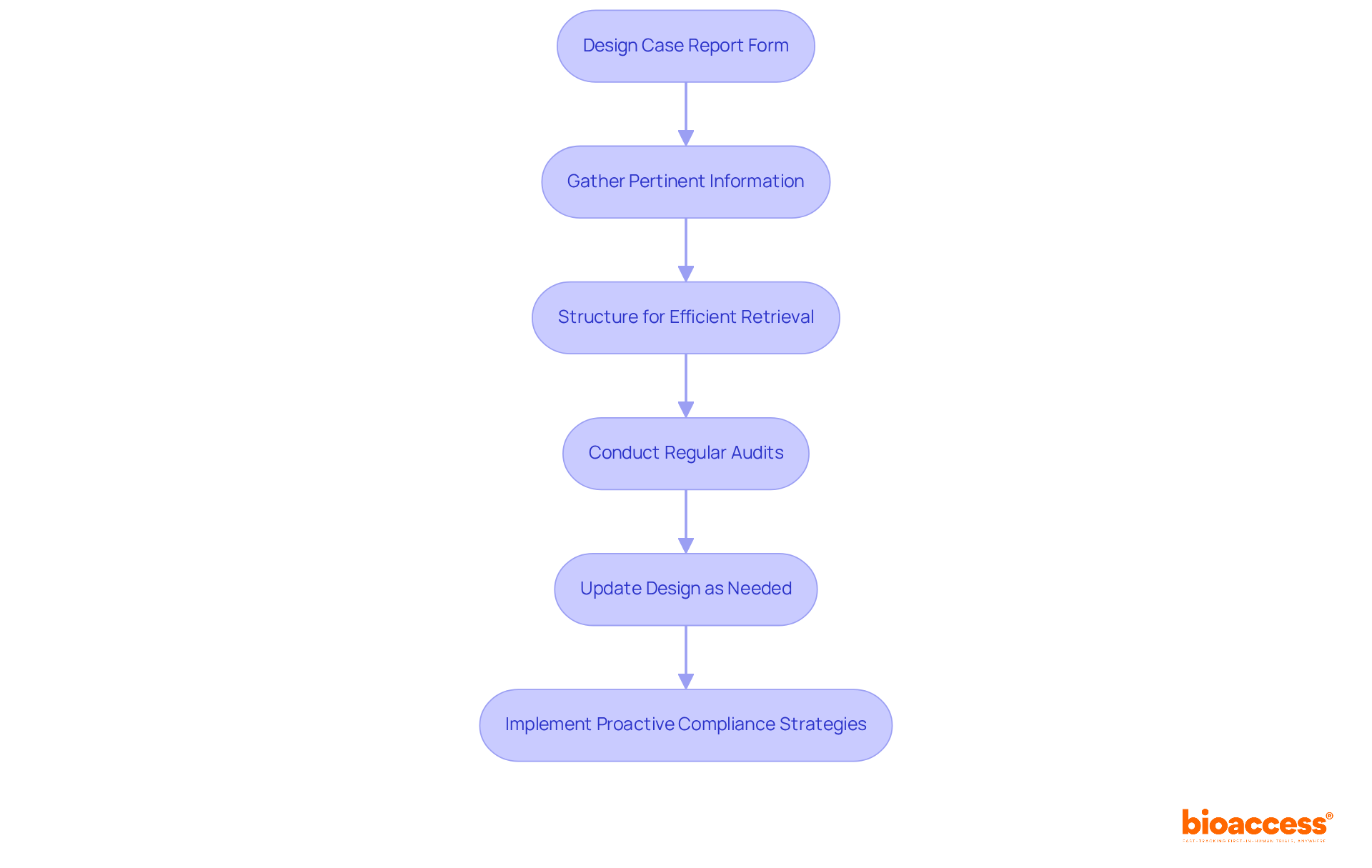
Ensuring adherence in the design of the clinical research case report form is essential for successful medical research. Adhering to regulatory standards such as ICH-GCP and FDA guidelines is paramount. This involves gathering information that is directly pertinent to the study objectives and structuring the clinical research case report form to facilitate efficient retrieval and analysis.
Regular audits and updates to the clinical research case report form design are crucial for maintaining compliance and adapting to evolving regulatory requirements. Organizations that implement a proactive compliance strategy can significantly reduce the risk of penalties while enhancing the quality of their reporting.
Furthermore, information from recent studies suggests that following ICH-GCP and FDA guidelines not only enhances compliance rates but also accelerates the overall trial process, resulting in quicker enrollment and more dependable outcomes. By prioritizing compliance in the clinical research case report form design, researchers can ensure that their studies adhere to the highest standards of regulatory scrutiny.
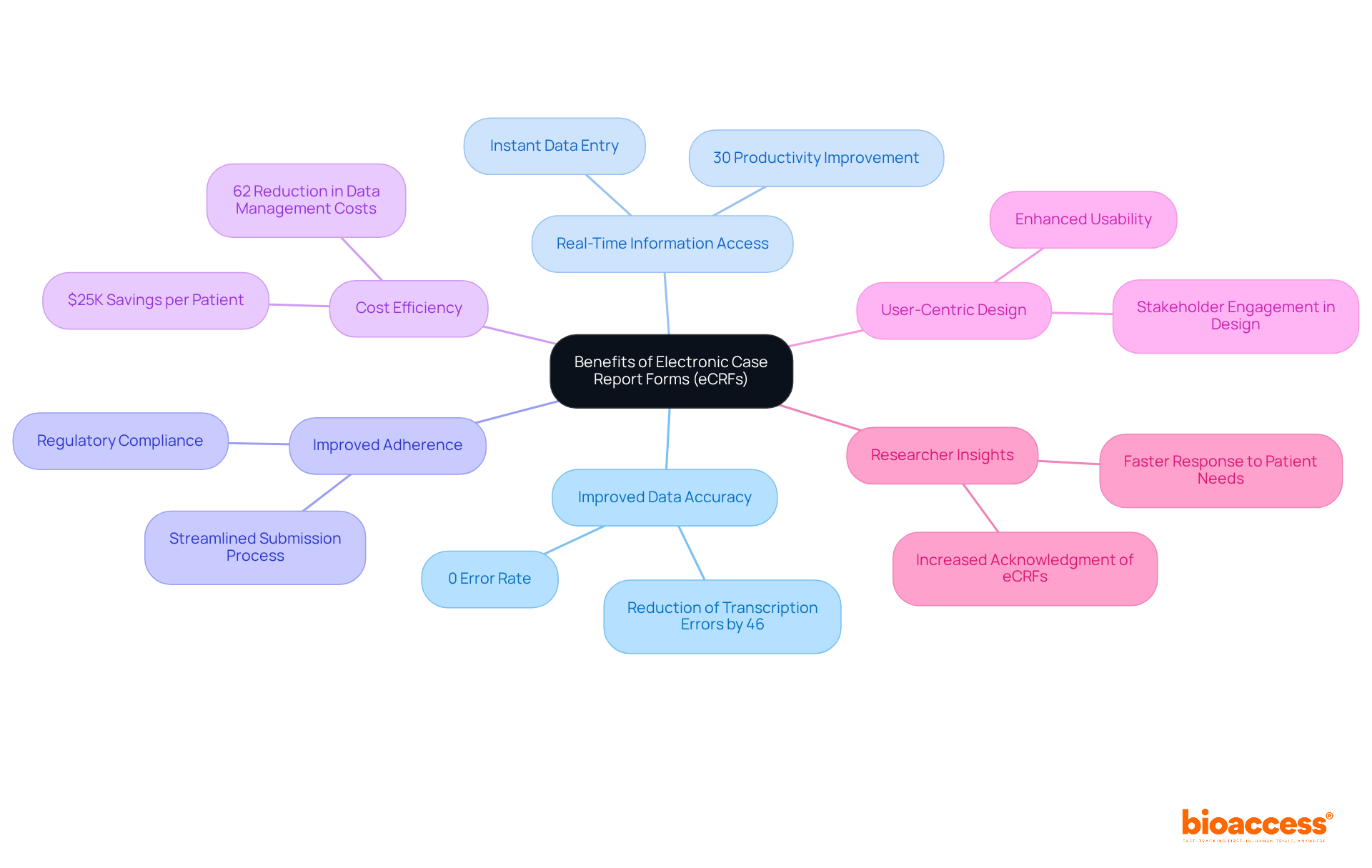
Electronic case report forms (eCRFs) represent a transformative shift in clinical research, offering significant advantages over traditional paper forms:
Improved Data Accuracy: eCRFs minimize transcription errors through automated data entry and integrated validation checks, achieving a remarkable 0% error rate compared to the 5% error rate associated with paper forms. This improvement in accuracy is essential for preserving the integrity of clinical information.
Real-Time Information Access: Researchers benefit from instant access to information, enabling immediate analysis and facilitating quicker decision-making. This capability can lead to productivity improvements of up to 30%, significantly accelerating the research process.
Improved Adherence: eCRFs can be meticulously crafted to automatically conform to regulatory requirements, ensuring that all necessary information is captured accurately. This adherence to standards not only streamlines the regulatory submission process but also bolsters the credibility of the outcomes documented in the clinical research case report form.
Cost Efficiency: Utilizing eCRFs can result in substantial savings, with studies indicating that data management costs can be reduced by up to 62% compared to traditional methods. This financial advantage is particularly beneficial for Medtech companies aiming to optimize their research budgets.
User-Centric Design: The design of eCRFs prioritizes clarity and simplicity, enhancing usability and encouraging adherence among researchers. Engaging stakeholders early in the design phase can identify potential usability issues, ensuring that the forms meet the needs of all users.
Quotes from Researchers: Numerous researchers have highlighted the benefits of eCRFs, with one asserting, "The speed of eCRF trials enables us to address patient needs more quickly, ultimately benefiting all parties involved in the eCRF trials." Such insights emphasize the increasing acknowledgment of eCRFs as an essential instrument in contemporary research.
In summary, the adoption of clinical research case report forms not only enhances information quality and compliance but also boosts the overall efficiency of trials, making them a vital element for successful Medtech studies.
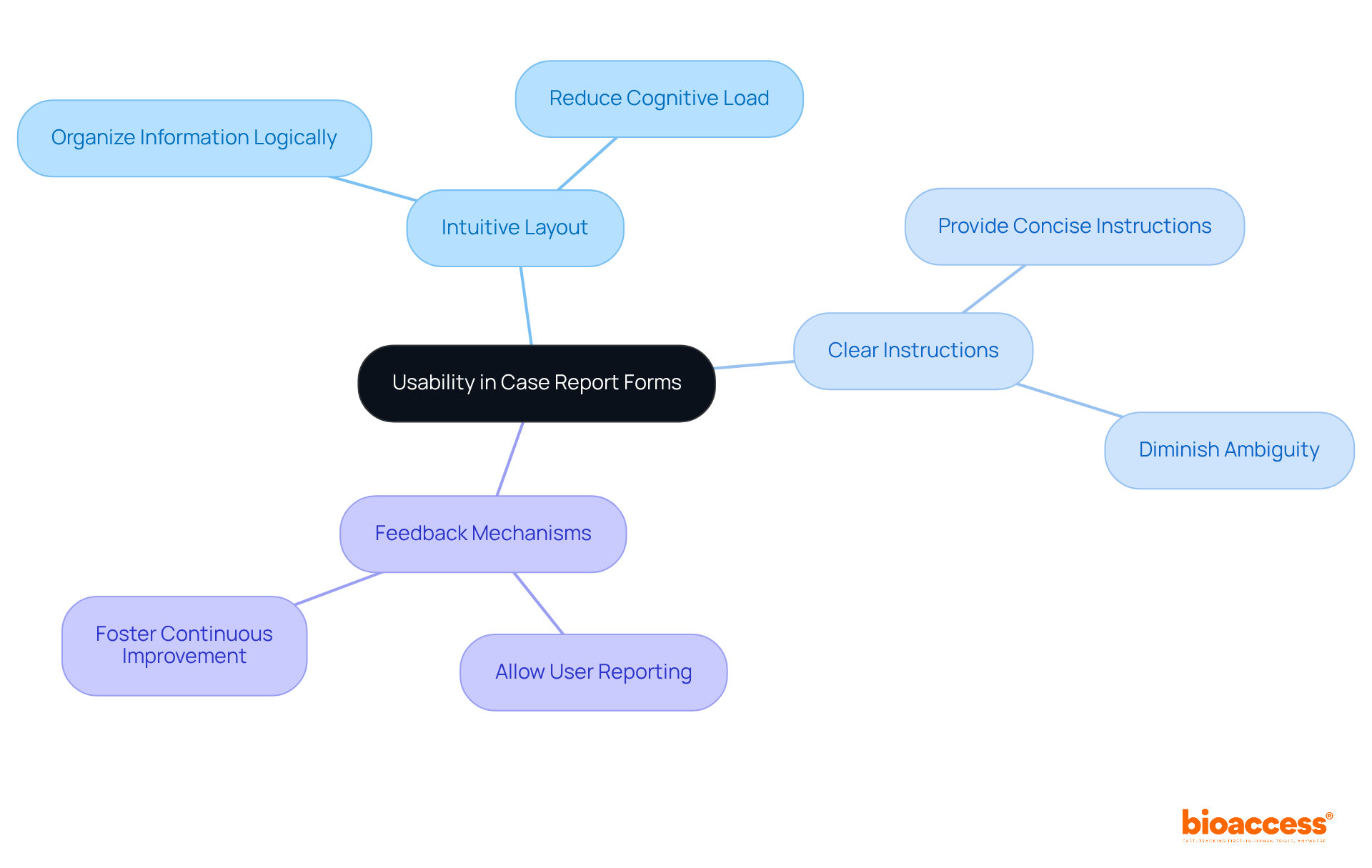
User-friendly clinical research case report form templates are crucial for minimizing errors and facilitating effective information gathering in clinical research. To enhance usability, consider the following strategies:
Research indicates that common errors in CRFs frequently arise from usability issues, such as unclear instructions or poorly organized layouts. For instance, a study revealed that inconsistencies in information entry could reach as high as 27%, underscoring the necessity for effective design strategies. According to Saveli I Goldberg, "An obvious approach for decreasing entry mistakes would be the minimization of manual input by utilizing direct transfers from electronic medical records into research databases." By prioritizing usability, clinical researchers can create clinical research case report forms that not only promote precise information gathering but also enhance overall study integrity. Furthermore, statistics show that error rates can vary significantly, with some methods recording up to 2,784 mistakes per 10,000 fields, emphasizing the essential role of usability in ensuring accuracy.
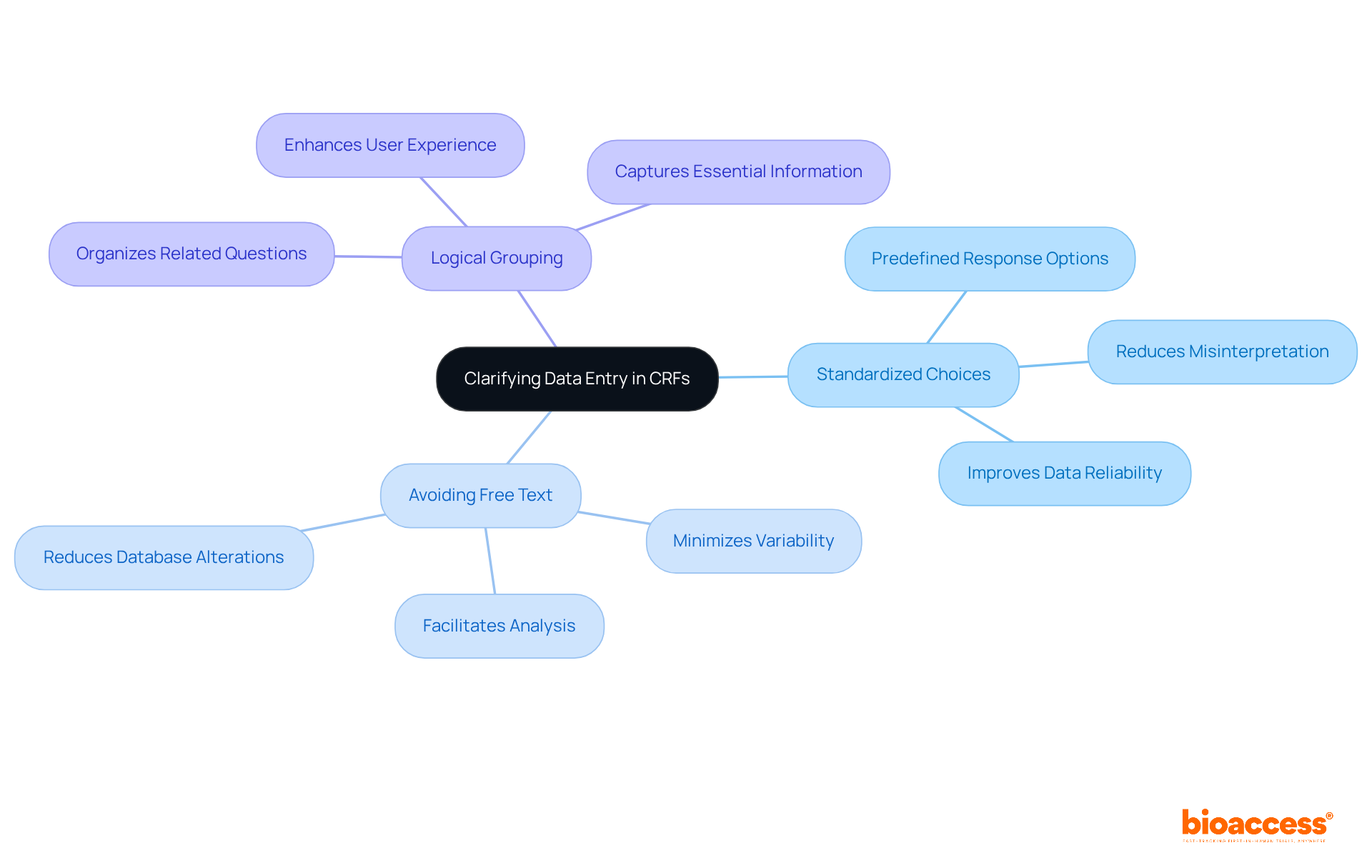
To ensure precise information entry, case report forms (CRFs) must include clear and coherent response options. Implementing standardized options is essential for reducing confusion and improving information quality. Here are key strategies:
Integrating these practices into CRF design can result in enhanced information precision and effectiveness, ultimately aiding the successful implementation of trials. For example, a well-organized CRF can decrease the time invested in cleaning and improve the efficiency of regulatory submissions, as demonstrated by the use of standardized templates in multiple studies. Moreover, the incorporation of visual signals and clear guidelines within case report forms has been demonstrated to reduce the occurrence of entry mistakes, emphasizing the significance of careful design in medical research.
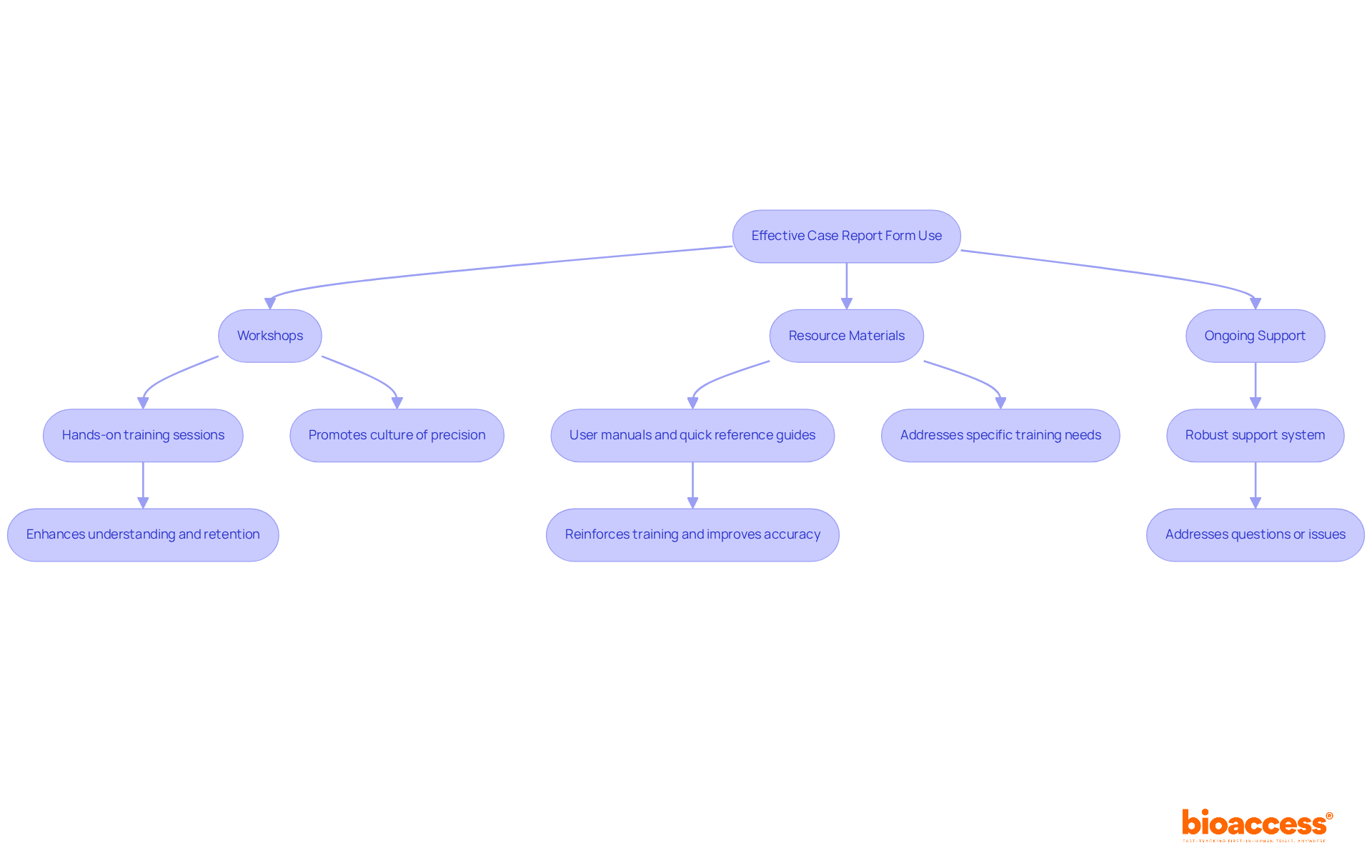
Offering comprehensive training for researchers on the effective use of case report forms (CRFs) is crucial for ensuring information accuracy and integrity in clinical trials. Key strategies include:
Workshops: Conduct hands-on training sessions that familiarize researchers with the CRF layout and data entry processes. These interactive sessions can enhance understanding and retention of critical information. A study found that visual checking resulted in 2958% more errors than double entry, underscoring the need for effective training methods.
Resource Materials: Create thorough user manuals and quick reference guides that researchers can refer to during information entry. Such materials serve as valuable tools to reinforce training and improve accuracy. According to Judy L. Jarrell, the immediate use of information taught in training maximizes retention, highlighting the importance of accessible resources.
Ongoing Support: Establish a robust support system for researchers to address questions or issues that arise during the trial. This ongoing support can significantly decrease mistakes and improve overall information quality. Notably, 72% of respondents reported positive behavior changes due to training, emphasizing the long-term benefits of ongoing support.
Applying these strategies not only provides researchers with the essential skills but also promotes a culture of precision and responsibility in clinical research information entry. By addressing specific training needs, such as data statistics and cohort construction, organizations can further enhance the effectiveness of their training programs.
Regular assessment and improvement of the clinical research case report form based on user feedback is crucial for enhancing its effectiveness in clinical research. Key strategies for achieving this include:
Feedback Surveys: Distributing surveys to researchers post-trial is essential for gathering insights on CRF usability and identifying areas for improvement. Studies indicate that 83% of trials with post-feedback consultations reported enhancements, underscoring the value of structured feedback mechanisms.
Iterative Testing: Implementing a cycle of testing and revising CRFs allows for the prompt addressing of identified issues. This approach not only improves usability but also ensures that the forms remain aligned with evolving study protocols. For instance, 84% of studies that incorporated education as a co-intervention noted improvements, highlighting the effectiveness of iterative adjustments.
Information Analysis: Examining entry mistakes is essential for recognizing prevalent issues within CRFs. By understanding these issues, researchers can make informed adjustments to the design, thereby enhancing data accuracy and reliability. A well-structured CRF can lead to a significant reduction in errors, as user-friendly designs increase compliance and encourage accurate completion.
Incorporating user feedback into the clinical research case report form design process fosters a culture of continuous improvement, ultimately contributing to the success and validity of clinical trials.
Engaging stakeholders in the design of the clinical research case report form is essential for capturing diverse perspectives and ensuring that the form meets the needs of all parties involved. Effective strategies include:
Collaborative Workshops: Organizing workshops that unite investigators, data managers, and regulatory experts significantly enhances CRF design. These sessions facilitate open dialogue, allowing stakeholders to share insights and address potential challenges early in the process. Research has demonstrated that engaging stakeholders in the initial phases of the clinical research case report form design can decrease mistakes and enhance information accuracy.
Stakeholder Feedback: Continuously soliciting feedback from all stakeholders throughout the trial ensures that the CRF remains relevant and effective. This iterative method enables modifications based on real-time feedback, improving the overall quality of information gathering. Research indicates that 24 out of 29 investigators would accept an electronic CRF in the future, highlighting the importance of adapting to stakeholder preferences.
Cross-Disciplinary Teams: Forming teams that encompass various disciplines fosters a rich exchange of ideas and perspectives. This variety is crucial for creating forms that are not only thorough but also easy to use, ultimately enhancing information quality and trial results. For instance, the average expense per patient with electronic case report forms was 374€ in contrast to 1,135€ for paper forms, illustrating the financial advantages of efficient design.
Current Practices: Many organizations are adopting collaborative approaches to CRF development, recognizing that stakeholder engagement leads to better-designed forms. The overall average expense of a trial utilizing a clinical research case report form was noted at 88,222€, suggesting a trend towards more effective collection methods.
Examples of Collaborative Workshops: Successful case studies illustrate the effectiveness of collaborative workshops in refining CRF elements. For example, the research "Cost Analysis of eCRFs vs. pCRFs" demonstrated that eCRFs not only lowered expenses but also enhanced stakeholder satisfaction with information collection techniques.
By prioritizing collaboration in the clinical research case report form design, clinical research teams can ensure that their data collection tools are robust, relevant, and capable of supporting high-quality research outcomes. Incorporating stakeholder perspectives is essential for achieving trial validity and participant safety.
Effective clinical research case report forms (CRFs) are integral to the success of clinical trials, serving as the backbone for data collection and compliance with regulatory standards. By prioritizing tailored designs that cater to specific study requirements, researchers can significantly enhance both the efficiency and accuracy of their trials. The insights shared throughout this article underscore the importance of understanding the role of CRFs in clinical research, highlighting how well-structured forms can lead to improved patient outcomes and accelerated innovations.
Key strategies discussed include implementing best practices in CRF design, such as clarity, consistency, and usability, which are essential for minimizing errors and ensuring high-quality data collection. The transition to electronic case report forms (eCRFs) exemplifies the benefits of leveraging technology in research, offering advantages like real-time information access and improved data accuracy. Additionally, fostering collaboration among stakeholders during the design process ensures that CRFs meet the diverse needs of all parties involved, ultimately enhancing the quality of information gathered.
As the landscape of clinical research continues to evolve, the significance of well-designed case report forms cannot be overstated. Researchers are encouraged to adopt these essential tips and best practices to refine their CRF designs continually. By doing so, they not only improve the integrity of their studies but also contribute to the advancement of medical knowledge and patient care. Embracing these principles will pave the way for more effective clinical trials and better health outcomes for patients worldwide.
What is bioaccess® and what do they specialize in?
bioaccess® specializes in developing tailored case report forms for Medtech, Biopharma, and Radiopharma innovators in Latin America, ensuring compliance with regulatory standards while optimizing information collection and analysis.
How does bioaccess® improve clinical research timelines?
With over 20 years of experience in Medtech, bioaccess® enhances research timelines by facilitating ethical approvals within 4-6 weeks and achieving patient enrollment rates that are 50% faster than conventional markets.
What are the benefits of well-designed electronic case report forms (eCRFs)?
Well-designed eCRFs can reduce costs, improve data quality, minimize entry errors to nearly 0%, and are essential for successful research trials.
Why are case report forms important in clinical trials?
Case report forms are crucial for collecting protocol-required information from participants, ensuring uniform and accurate data collection, tracking trial progress, and maintaining compliance with regulatory standards.
What are the consequences of not adhering to regulatory standards in clinical research?
Failing to adhere to regulatory standards can lead to delayed approvals and expensive audits, impacting the overall success of clinical research.
What best practices should be followed when designing case report forms?
Best practices include ensuring clarity, consistency, relevance, and flexibility in design, which enhances usability and data integrity.
How does the transition from paper-based CRFs to electronic formats impact clinical trials?
The transition to electronic CRFs improves efficiency and accuracy, reducing entry mistakes to below 1% compared to a 5% error rate for traditional paper forms.
Why is stakeholder engagement important in the development of case report forms?
Engaging stakeholders early in the CRF development process can lead to iterative enhancements, resulting in user-friendly forms that facilitate precise data collection.