This article highlights the essential tools necessary for tracking GDPR compliance in clinical research, emphasizing ten specific tools that significantly enhance adherence to data protection regulations. Understanding the importance of these tools is crucial; they include:
Together, these resources facilitate secure data handling, improve team communication, and foster a culture of accountability. Ultimately, they ensure compliance and protect participant information, which is vital in today’s research landscape.
Navigating the complex landscape of clinical research demands unwavering dedication to data protection, particularly in light of the stringent regulations set forth by the General Data Protection Regulation (GDPR). As organizations work diligently to meet these standards, the right tools become essential for ensuring compliance and protecting participant information. This article explores ten crucial tools that not only facilitate GDPR compliance tracking but also bolster the integrity and efficiency of clinical trials. As the regulatory environment evolves, research organizations must consider: how can they stay ahead of potential compliance risks while effectively leveraging these tools?
At bioaccess®, we place a strong emphasis on GDPR adherence, recognizing it as a cornerstone of our services in Latin America. By implementing robust data protection measures, we ensure that all patient information is managed with the utmost care and in strict accordance with GDPR regulations. This commitment not only accelerates the medical study process but also fosters trust among participants and stakeholders.
Our comprehensive services—including site feasibility, investigator selection, trial preparation, project management, and regulatory compliance—enhance the efficiency of research trials. This ultimately leads to more successful outcomes in Medtech, Biopharma, and Radiopharma innovations. In a landscape where data integrity is paramount, bioaccess® stands ready to address the key challenges faced in clinical research, ensuring that our partners can navigate the complexities with confidence.
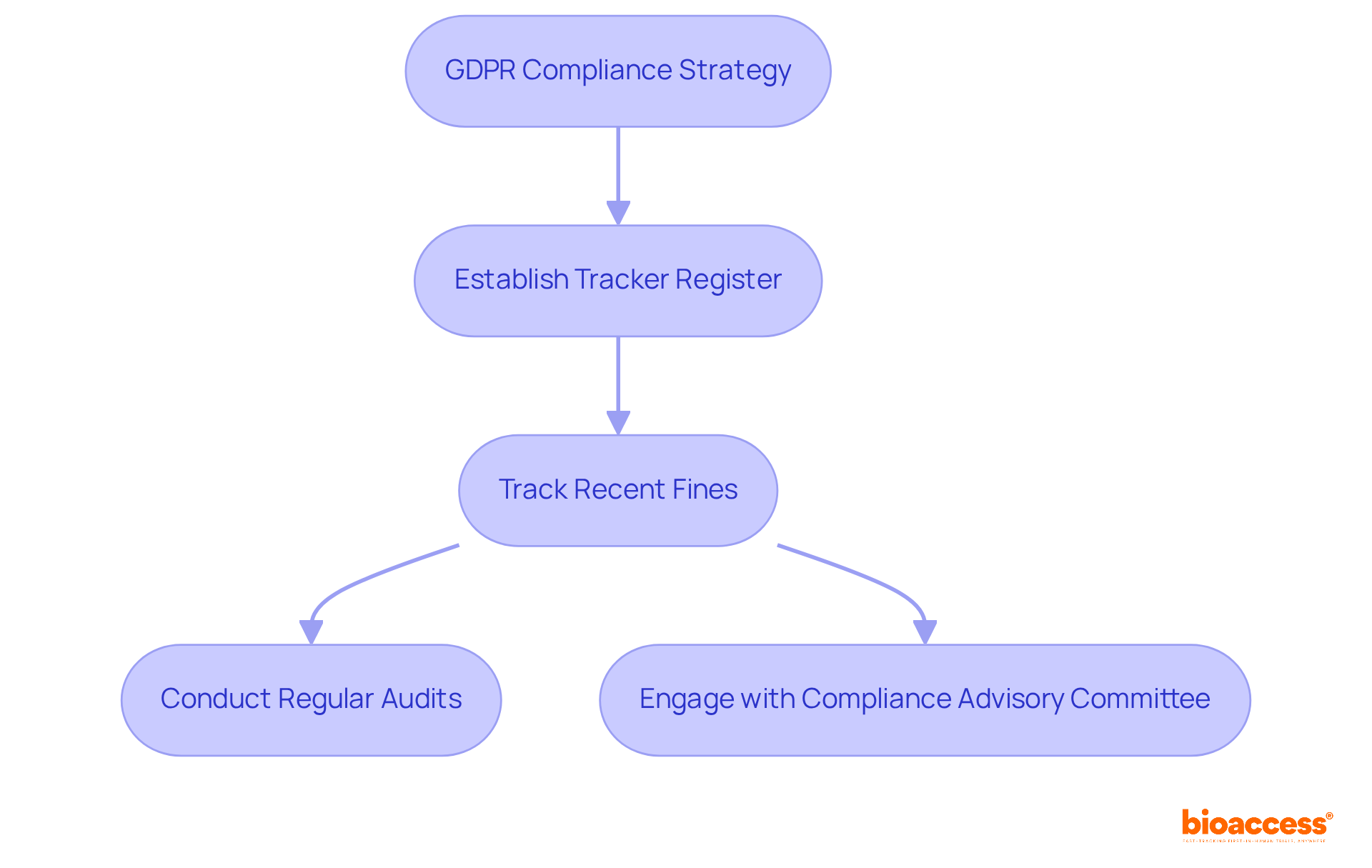
Establishing a tracker register for GDPR enforcement is essential for clinical research organizations navigating the complex landscape of regulations and enforcement across Europe. This tool not only tracks recent fines and regulatory decisions, including the significant penalties imposed in 2025, but also provides vital insights that empower organizations to adapt their compliance strategies proactively.
For example, organizations that have effectively adjusted to GDPR changes have:
By remaining vigilant and informed about enforcement actions, investigation teams can significantly mitigate risks associated with non-compliance, ensuring their practices align with evolving regulatory standards. This proactive approach is crucial for maintaining the integrity of research operations and safeguarding participant information.
Implementing ORCID (Open Researcher and Contributor ID) is crucial for ensuring that all researchers involved in medical trials receive accurate attribution. This unique identifier not only aids in tracking contributions but also ensures compliance with GDPR by linking researchers to their work without compromising personal data. By promoting transparency and accountability, ORCID significantly enhances the integrity of research outputs in healthcare.
Moreover, bioaccess plays a pivotal role by offering extensive trial management services, including:
These services are essential for the effective execution of ORCID, ensuring that all aspects of research trials align with regulatory standards. This alignment underscores the importance of precise researcher attribution and adherence, ultimately fostering a more trustworthy research environment.

The adoption of advanced information management tools is revolutionizing the tracker register for oversight in clinical research. These tools ensure secure storage and organization of sensitive information while enabling rapid retrieval, making compliance with GDPR regulations more manageable. By automating information management processes with a tracker register, organizations can significantly reduce the risk of human errors, thereby enhancing adherence efficiency.
In 2021, a notable 74% of sponsors utilized remote monitoring in research trials, emphasizing a growing trend toward technology integration that incorporates a tracker register for compliance tracking. Furthermore, a 2022 survey indicated that 91% of trial locations planned to implement at least one new technology, reflecting the sector's shift toward more effective information management practices in their tracker register.
Looking ahead to 2025, best practices include leveraging tools that provide real-time data access and automated regulatory workflows, which are increasingly sought after by research sites. By streamlining adherence monitoring through these advanced systems and implementing a tracker register, research organizations can not only meet regulatory requirements but also boost overall operational efficiency.
Additionally, bioaccess enhances this process through its comprehensive clinical trial management services, which encompass:
These services not only facilitate regulation monitoring but also contribute to local economies by fostering job creation, economic growth, and improvements in healthcare.
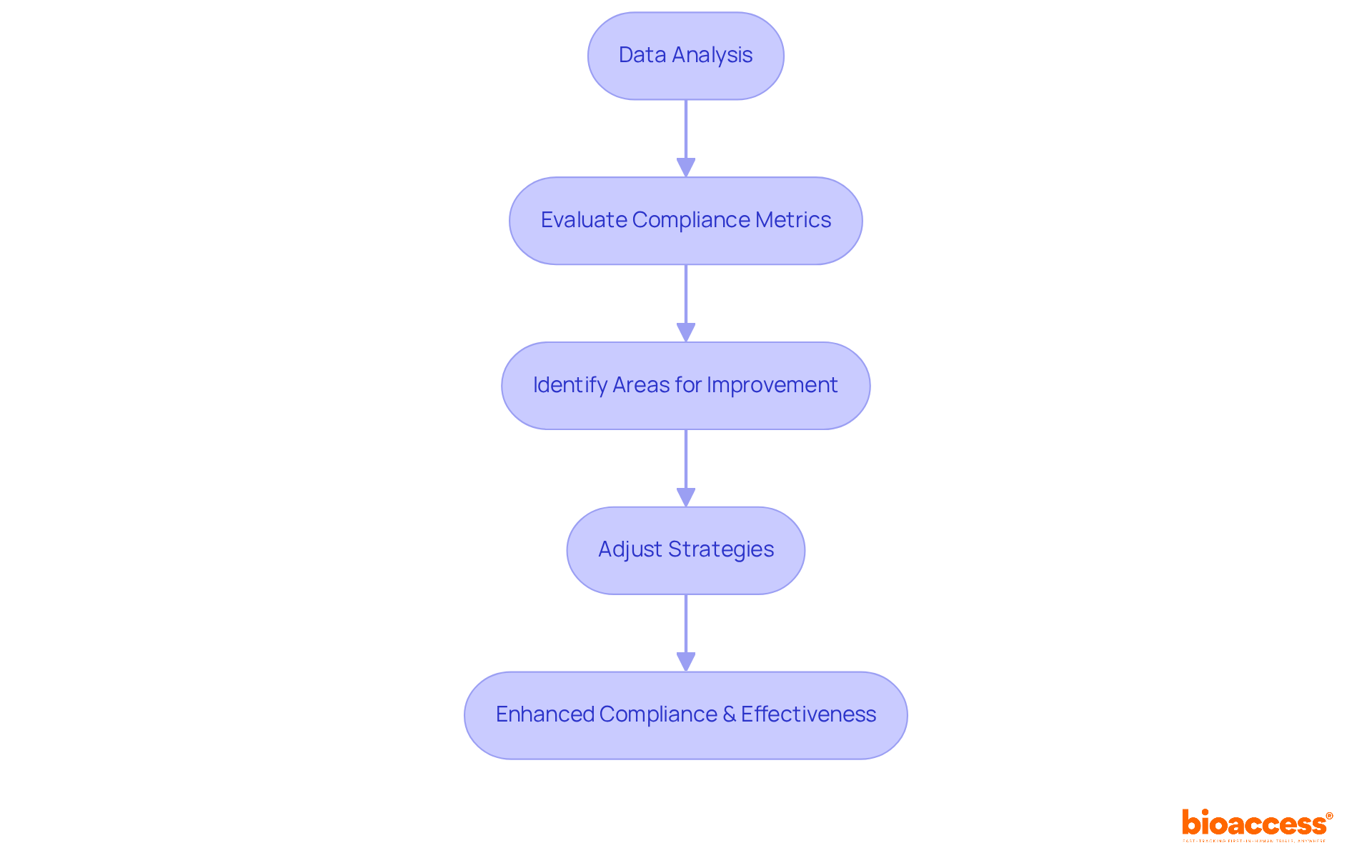
Analytics platforms empower organizations to evaluate the impact of GDPR on their investigative efforts. By analyzing data patterns and compliance metrics, study teams can identify areas for improvement and adjust their strategies accordingly. This proactive approach not only enhances compliance but also boosts the overall effectiveness of clinical operations. In a landscape where regulatory adherence is paramount, leveraging such tools is essential for maintaining operational excellence.
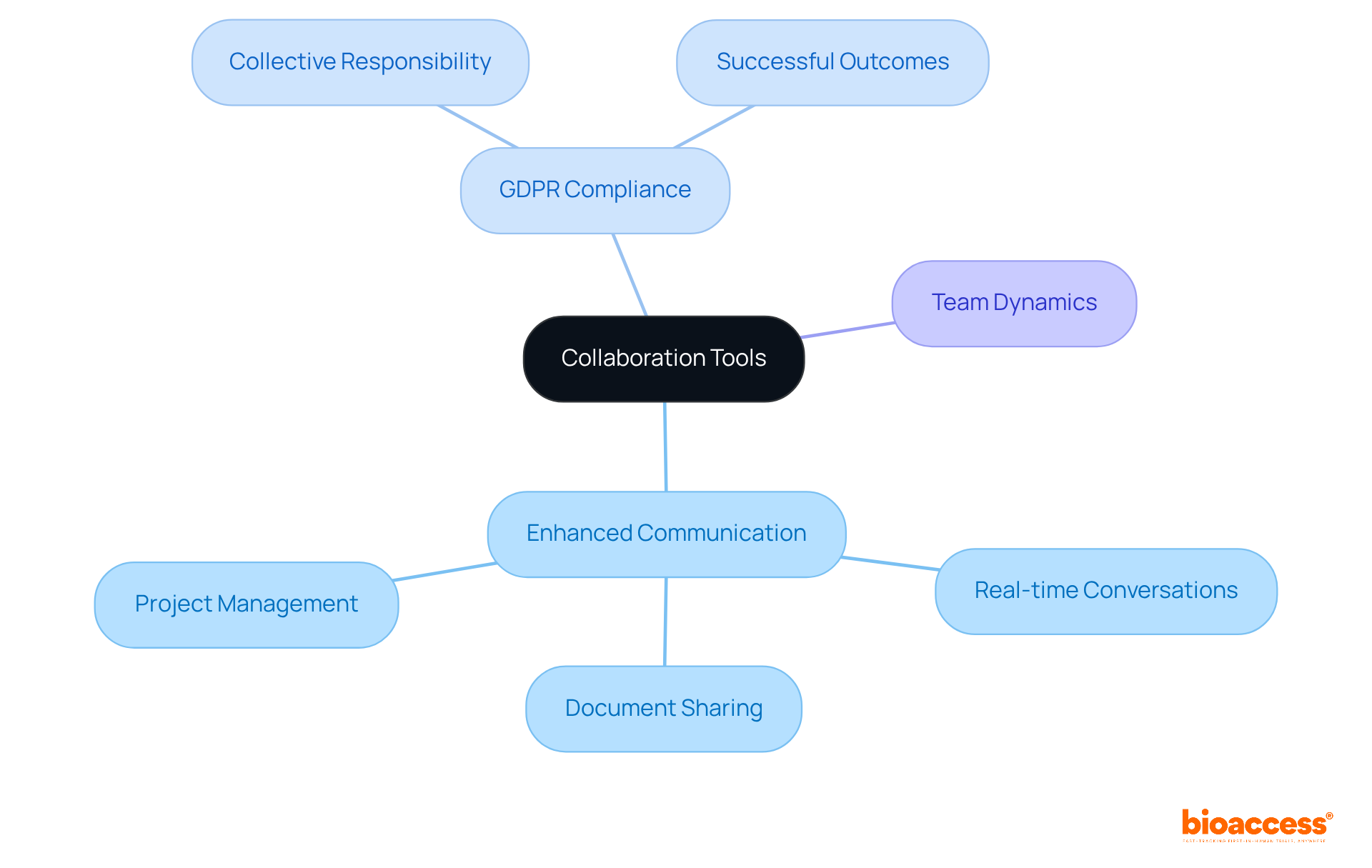
Collaboration tools play a pivotal role in enhancing communication among study teams, ensuring that every member understands the requirements for GDPR adherence. These tools facilitate real-time conversations, document sharing, and project management—elements that are essential for maintaining compliance across various study activities. By cultivating a collaborative environment, organizations can make compliance a collective responsibility, reinforcing the notion that every team member plays a crucial part in upholding regulations.
In the ever-evolving Medtech landscape, the integration of such tools is not just beneficial; it is necessary. As clinical research becomes increasingly complex, the ability to communicate effectively and share information seamlessly is paramount. Organizations that prioritize collaboration are better equipped to navigate the challenges of compliance, ultimately leading to more successful study outcomes.
In conclusion, fostering collaboration through effective tools is vital for ensuring that all team members are aligned with compliance requirements. As you consider your own challenges in clinical research, reflect on how enhancing communication can lead to improved adherence to regulations and better overall results.
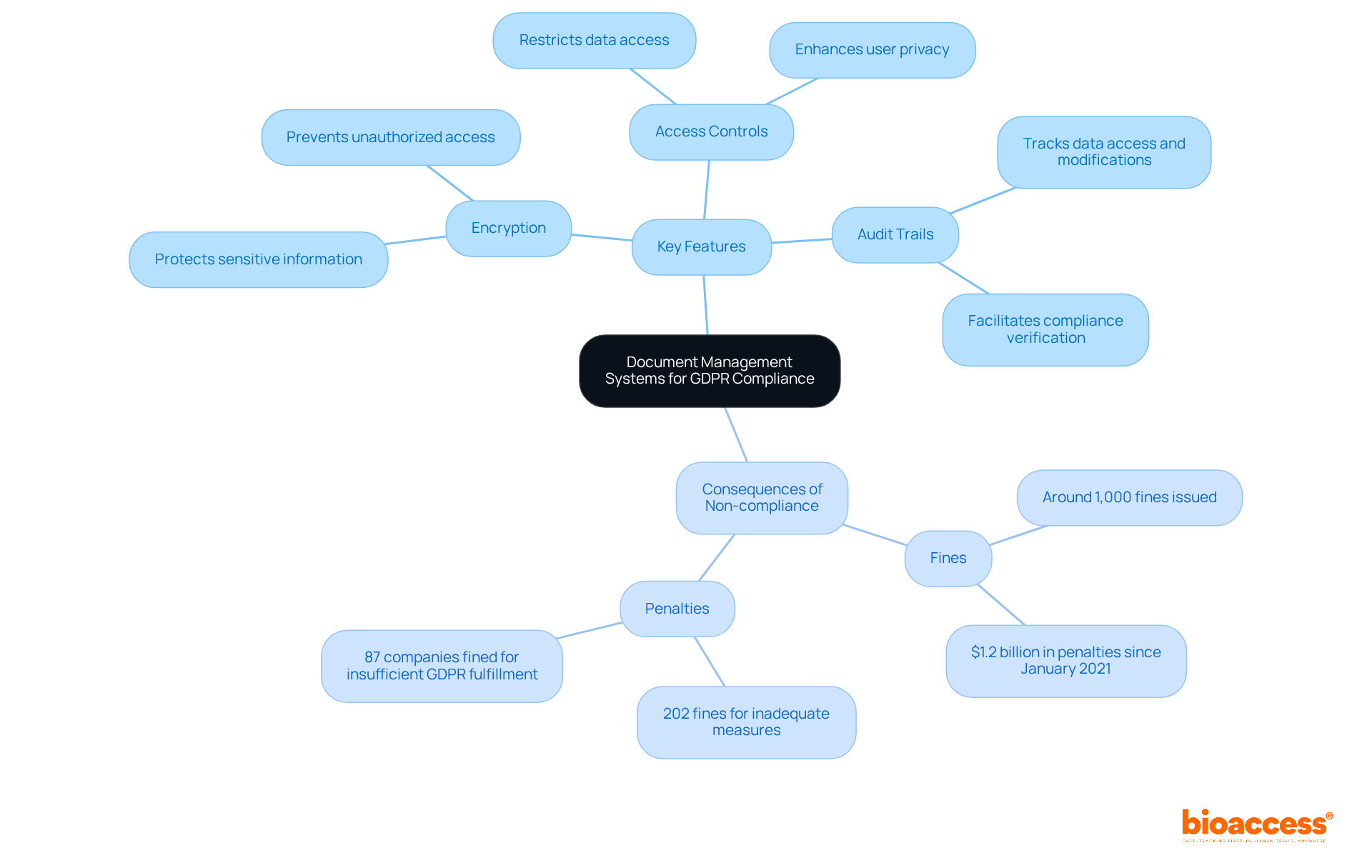
Establishing a robust document management system is crucial for securely storing information in line with GDPR requirements. These systems provide vital features such as encryption, access controls, and comprehensive audit trails, all of which work together to protect sensitive information from unauthorized access. With GDPR authorities imposing nearly $1.2 billion in penalties since January 2021 and around 1,000 fines for infractions as of February 2022, organizations must prioritize secure storage solutions to effectively safeguard participant information.
By implementing these secure systems, medical investigation organizations not only protect personal information—including names, addresses, contact numbers, health data, biometric data, and other personal identification details—but also enhance their compliance with regulatory standards. This proactive approach can significantly improve adherence rates, as evidenced by the 202 fines related to inadequate technical and organizational measures for user privacy. Ultimately, a well-executed document management system serves as a fundamental component in achieving GDPR compliance and fostering trust in clinical practices.
As Doug Bonderud states, 'We meet the highest security standards, so you can be assured that your information is safe with us and that you’re complying with GDPR and other privacy regulations.
Training platforms play a vital role in educating research teams about their responsibilities under GDPR, fostering a culture of accountability. These platforms provide essential resources and training modules that cover critical regulatory topics, ensuring that every team member is well-informed about their role in protecting participant information. By prioritizing education, organizations can significantly mitigate the risk of non-compliance, ultimately safeguarding both their participants and their reputation.

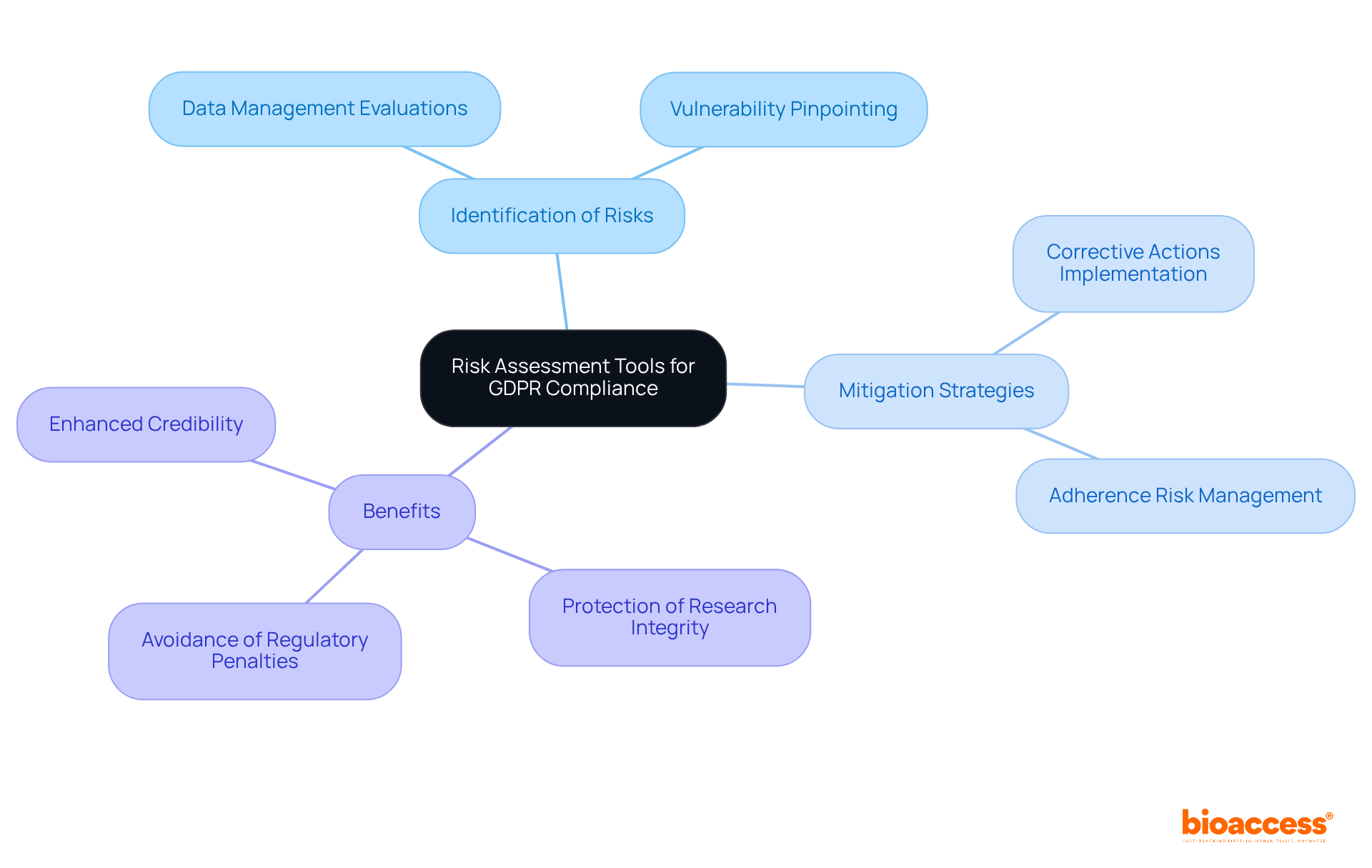
Risk assessment tools play a crucial role in helping organizations identify and mitigate potential GDPR compliance risks. These tools facilitate comprehensive evaluations of data management practices, enabling project teams to pinpoint vulnerabilities and implement corrective actions. By addressing adherence risks head-on, organizations not only protect their research integrity but also steer clear of costly regulatory penalties.
In the ever-evolving Medtech landscape, the importance of such tools cannot be overstated. They empower organizations to navigate the complexities of data protection, ensuring that compliance is not just a checkbox but a fundamental aspect of their operations. As you consider your own challenges in clinical research, ask yourself: Are you doing enough to safeguard your data management practices?
Ultimately, the proactive use of risk assessment tools is essential for maintaining compliance and fostering trust in your research endeavors. By prioritizing these evaluations, organizations can enhance their credibility and ensure a robust framework for data protection.
Utilizing feedback and survey tools is essential for organizations aiming to gather valuable insights from participants while ensuring compliance with GDPR. These tools facilitate data collection in a way that respects participant privacy and adheres to regulatory standards.
In addition to participant feedback, comprehensive trial management services—including:
are crucial for enhancing the quality of research studies. By integrating participant feedback into these processes, organizations can significantly improve study outcomes and ensure adherence to regulatory requirements, ultimately leading to more successful clinical trials.
The pursuit of GDPR compliance in clinical research transcends mere regulatory obligation; it embodies a commitment to safeguarding participant data and enhancing research integrity. By leveraging essential tools and strategies, organizations can adeptly navigate the complexities of GDPR, ensuring their practices align with evolving standards while fostering trust among stakeholders.
Key tools such as:
are pivotal in streamlining compliance tracking, protecting sensitive information, and educating teams on their responsibilities. Moreover, integrating collaboration and feedback tools significantly enhances communication and data collection, all while adhering to regulatory requirements.
In a landscape where compliance is paramount, organizations are urged to proactively adopt these tools and strategies. By prioritizing GDPR adherence, clinical research teams not only mitigate risks but also bolster their credibility and operational efficiency. Embracing these practices will ultimately lead to more successful outcomes in clinical trials, fostering a culture of accountability and trust within the research community.
What is bioaccess®'s approach to GDPR compliance in clinical research?
bioaccess® emphasizes GDPR adherence as a cornerstone of its services in Latin America, implementing robust data protection measures to manage patient information in strict accordance with GDPR regulations.
How does bioaccess® enhance the efficiency of clinical research trials?
bioaccess® offers comprehensive services such as site feasibility, investigator selection, trial preparation, project management, and regulatory compliance, which enhance the efficiency of research trials and lead to more successful outcomes in Medtech, Biopharma, and Radiopharma innovations.
What is the purpose of the GDPR Enforcement Tracker?
The GDPR Enforcement Tracker is a tool for clinical research organizations to monitor compliance and enforcement actions across Europe, tracking recent fines and regulatory decisions to help organizations adapt their compliance strategies proactively.
How can organizations mitigate risks associated with GDPR non-compliance?
Organizations can mitigate risks by instituting regular compliance audits and engaging with the proposed Compliance Advisory Committee to enhance their understanding of regulatory expectations.
What role does ORCID play in clinical research?
ORCID (Open Researcher and Contributor ID) ensures that all researchers receive accurate attribution for their contributions, linking them to their work while maintaining compliance with GDPR by protecting personal data.
What trial management services does bioaccess® provide in relation to ORCID?
bioaccess® offers extensive trial management services, including feasibility studies, site selection, regulatory reviews, and project management, which are essential for effective ORCID implementation and adherence to regulatory standards.