


The article titled "10 Examples of Radiopharmaceuticals Transforming Cancer Treatment" emphasizes the revolutionary role of specific radiopharmaceuticals in managing various cancers. It presents key examples, such as Lutetium-177 and Iodine-131, which illustrate significant improvements in treatment efficacy and patient outcomes. These advancements are achieved through targeted therapies that minimize damage to healthy tissues while effectively addressing malignant cells. This focus on precision medicine not only enhances treatment effectiveness but also underscores the critical need for ongoing research and collaboration in the field.
The landscape of cancer treatment is experiencing a significant transformation, propelled by the innovative application of radiopharmaceuticals. These specialized compounds are not only enhancing the precision of therapies but also markedly improving patient outcomes across a variety of cancer types. As healthcare professionals and researchers delve into the potential of these targeted treatments, several pertinent questions emerge:
This article explores ten compelling examples of radiopharmaceuticals that are revolutionizing cancer treatment, emphasizing their unique benefits and the promise they hold for the future of oncology.
bioaccess® plays a crucial role in accelerating clinical research for radiopharmaceuticals, leveraging its strategic presence across Latin America, the Balkans, and Australia. By focusing on early-phase studies, bioaccess® ensures a rapid transition of innovative therapies from concept to clinical application. The organization skillfully navigates complex regulatory environments, utilizing diverse patient populations to secure faster ethical approvals and enhance patient enrollment. This operational flexibility significantly shortens the timeframe for medical products to enter the market, which is vital for the timely advancement of therapies that have the potential to revolutionize the treatment of diseases.
The global market for medical isotopes is projected to reach USD 16.30 billion by 2024, with an anticipated CAGR of 6.50% from 2025 to 2032. This trend underscores the increasing demand for effective tumor treatments. As a leading contract research organization, bioaccess® enhances clinical trial timelines through expedited site activation and patient recruitment, fulfilling the urgent need for innovative cancer solutions. The emphasis on early-phase research is essential for the development of drugs that serve as an example of radiopharmaceuticals, ensuring that promising treatments can be efficiently brought to market.
Lutetium-177 is an example of radiopharmaceuticals that represents a significant advancement in the treatment of neuroendocrine tumors. By delivering targeted radiation precisely to malignant cells, Lutetium-177 minimizes damage to surrounding healthy tissue, a critical factor in enhancing patient care.
Clinical studies have consistently demonstrated its effectiveness, leading to improved patient outcomes, including longer survival rates and a better quality of life for those affected by this challenging disease.
This innovative approach not only underscores the importance of targeted therapies in oncology but also highlights the ongoing evolution of treatment options available to patients.

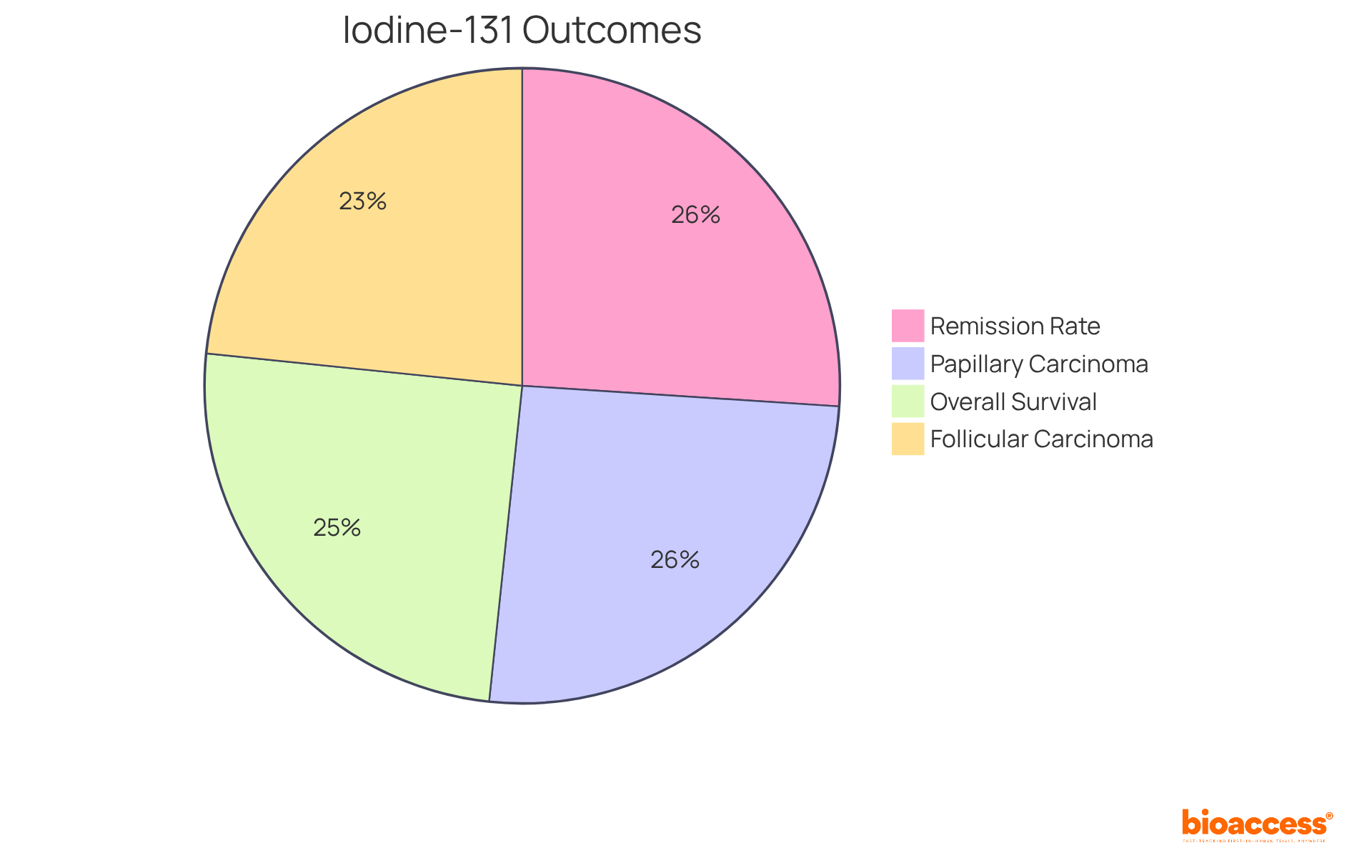
Iodine-131 has significantly transformed the landscape of thyroid cancer treatment, particularly for differentiated thyroid carcinoma (DTC). This is an example of radiopharmaceuticals that selectively target thyroid tissue, facilitating precise ablation of malignant cells. The efficacy of Iodine-131 is underscored by impressive statistics:
Recent studies reveal that high doses of Iodine-131, ranging from 2.75 to 7.4 GBq (74 to 200 mCi), significantly enhance outcomes, boasting an effective rate of 87.9% in patients post-thyroidectomy. Furthermore, the application of Iodine-131 has been associated with a 90% remission rate in well-differentiated thyroid malignancies, achieved following procedures such as thyroidectomies and postoperative iodine therapies. Researchers emphasize the importance of this therapy, noting that it not only eliminates residual thyroid tissue but also targets remaining tumor cells, thereby improving overall survival rates.
Notably, 18.2% of the disease-specific mortality group developed a secondary primary tumor after receiving a diagnosis of thyroid disease, highlighting the inherent risks associated with treatment. Consequently, Iodine-131 is an example of radiopharmaceuticals that remains a cornerstone in the management of thyroid tumors, illustrating its crucial role in enhancing outcomes for patients.
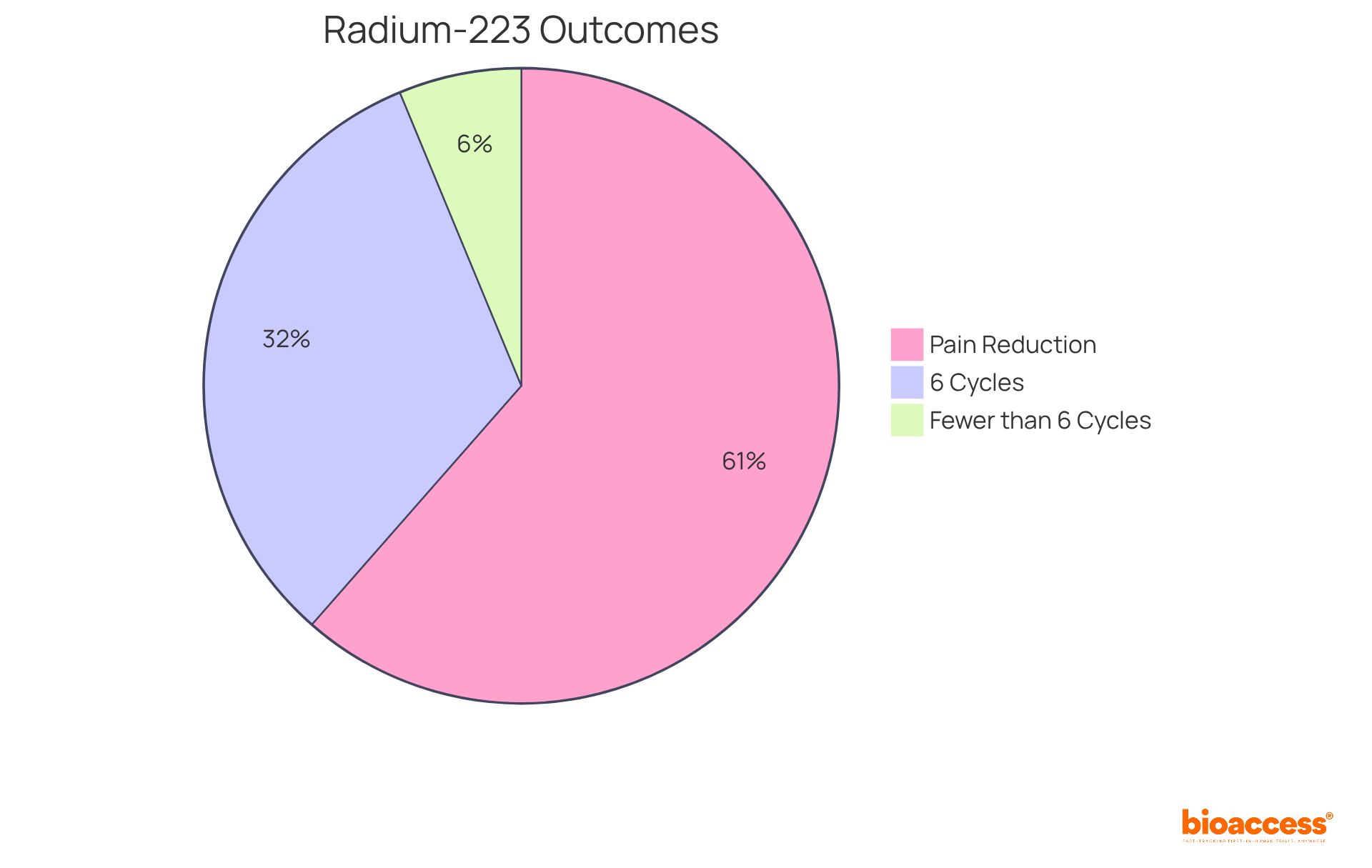
Radium-223 is an example of radiopharmaceuticals designed to combat bone metastases in individuals with prostate tumors. By emitting alpha particles, it delivers localized radiation to bone lesions, significantly alleviating pain and enhancing survival outcomes. Clinical trials have demonstrated that Radium-223 not only mitigates symptoms but also elevates the overall quality of life for patients with advanced prostate cancer.
Notably, research indicates that individuals who complete all six cycles of Radium-223 achieve a median overall survival of 31 months, compared to just 6 months for those who undergo fewer cycles. Furthermore, approximately 59% of patients report a clinically significant reduction in pain, with many noting improvements in their quality of life.
The intervention's effectiveness is underscored by findings that indicate a 55% lower risk of mortality for those completing five or more cycles, underscoring the importance of adhering to the complete treatment regimen. As John Buscombe aptly stated, "Considering the survival benefit of completing the full regimen of all the six cycles, this should be provided if feasible."
Overall, Radium-223 is an example of radiopharmaceuticals that represents a significant advancement in the treatment of metastatic castration-resistant prostate cancer, offering hope for improved patient outcomes.
Phosphorus-32 is an essential compound in the treatment of blood disorders, including polycythemia vera and chronic myeloid leukemia. This isotope selectively targets and eradicates overactive blood cells, providing a precise method for managing these conditions. Recent clinical studies reveal that individuals treated with Phosphorus-32 experience substantial reductions in symptoms, with statistics demonstrating improved hematologic parameters and enhanced overall quality of life.
Hematologists emphasize that Phosphorus-32 is an example of radiopharmaceuticals that not only alleviates the burden of excessive cell proliferation but also enhances outcomes for patients, underscoring the transformative potential of radioactive therapies in hematology. Its targeted mechanism of action positions Phosphorus-32 as a cornerstone in the therapeutic arsenal against these complex blood disorders.

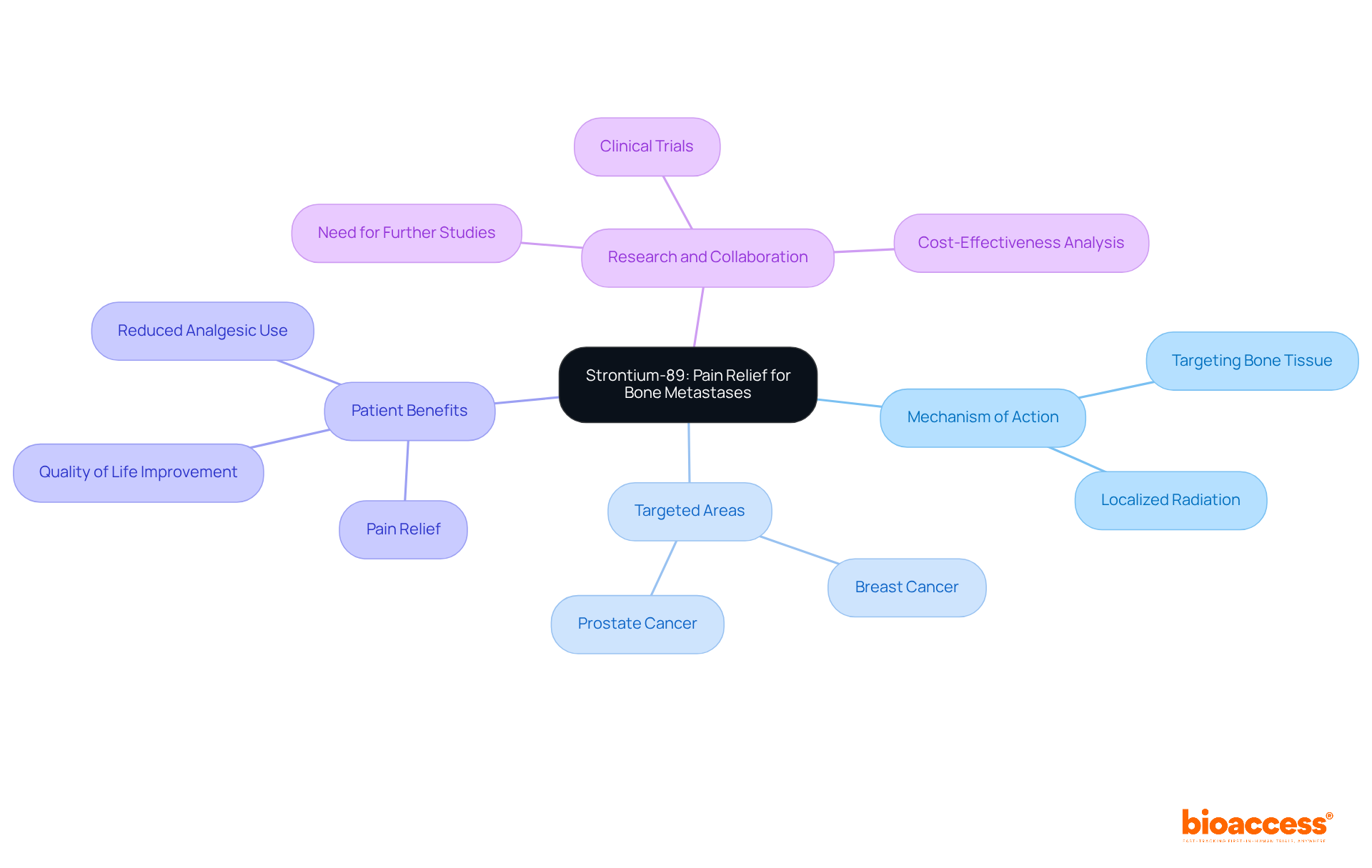
Strontium-89 is a pivotal medical compound that provides effective pain relief for individuals suffering from bone metastases, particularly in cases of prostate and breast tumors. By specifically targeting bone tissue, Strontium-89 delivers localized radiation, which not only alleviates pain but also enhances overall comfort for patients. Its application in palliative care underscores the critical role of such compounds in improving the quality of life for those facing advanced illnesses. This highlights the necessity for continued research and collaboration within the clinical landscape to optimize treatment outcomes.
Samarium-153 is an example of radiopharmaceuticals recognized for its remarkable ability to alleviate bone pain associated with metastatic cancer. By delivering targeted radiation to bone lesions, it significantly reduces pain and enhances mobility for affected individuals. Recent studies reveal that:
In clinical settings, Samarium-153 demonstrates a complete response rate of 80%, with 74% of individuals reporting pain relief within four weeks, effects that can last up to 12 weeks.
Palliative care specialists underscore that Samarium-153 not only effectively controls pain but also diminishes the reliance on opioids, thereby enhancing overall comfort and quality of life. This therapy is particularly beneficial for individuals with prostate and breast tumors; research indicates that:
Such findings highlight the critical role of substances like Samarium-153, which serve as an example of radiopharmaceuticals, in improving the quality of life for individuals undergoing treatment.
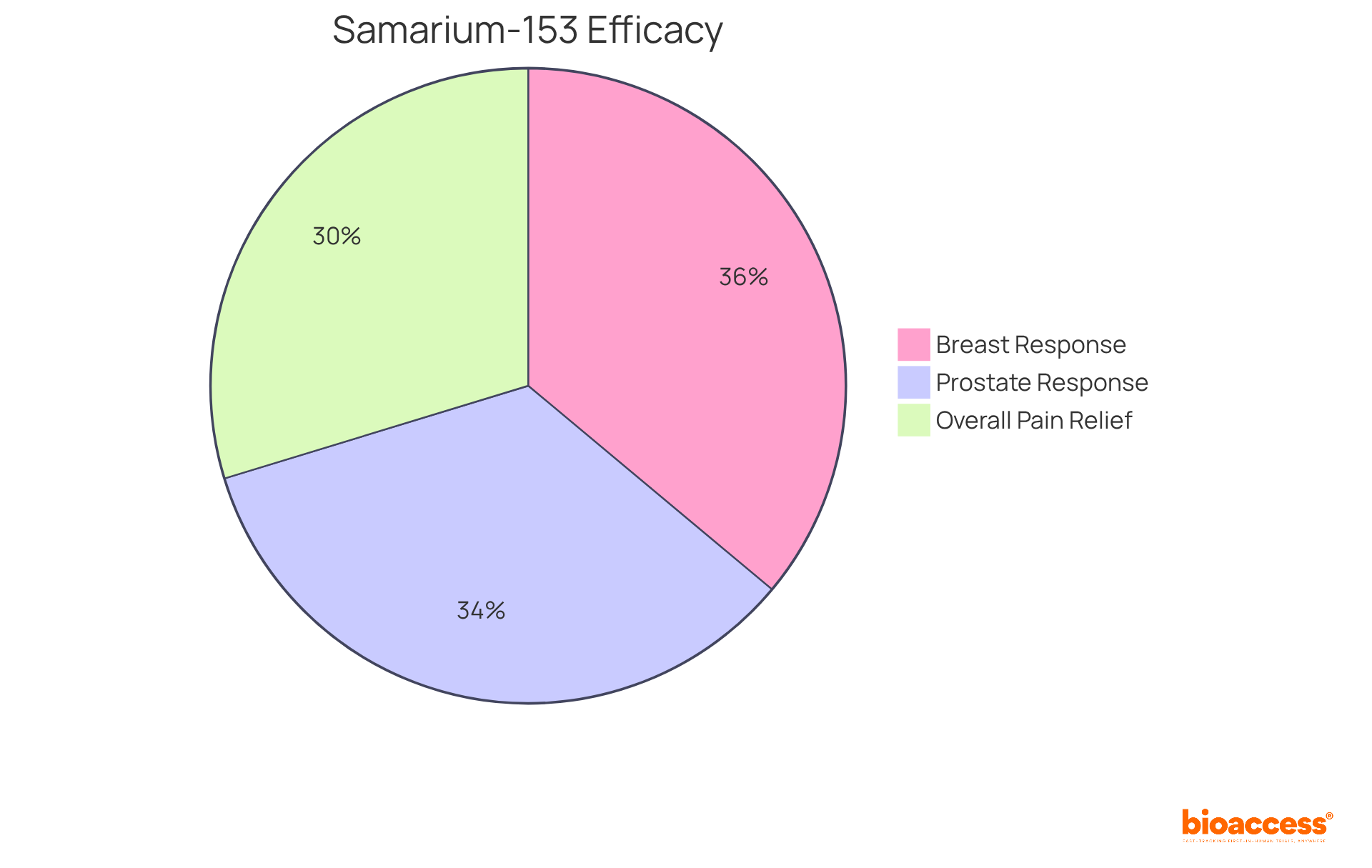
Yttrium-90 serves as an example of radiopharmaceuticals that has significantly advanced the field of radioimmunotherapy by merging the precise targeting capabilities of antibodies with the therapeutic effects of radiation. This innovative strategy allows for targeted delivery to malignant cells while minimizing harm to surrounding healthy tissue, ultimately resulting in improved treatment outcomes.
Recent studies indicate that Yttrium-90 has shown promising efficacy across various cancers, including non-Hodgkin's lymphoma and hepatocellular carcinoma. For instance, clinical trials have demonstrated that individuals treated with Yttrium-90 following two lines of systemic therapy achieve a median overall survival of roughly one year. Moreover, the pooled hazard ratio for disease advancement in individuals receiving Yttrium-90 is approximately 0.48, signifying a 52% decrease in the risk of progression. Additionally, 55% of studies indicated progression-free survival (PFS), further endorsing the method's effectiveness.
Ongoing research continues to explore the full potential of Yttrium-90, focusing on optimizing its targeting mechanisms and improving patient outcomes. Significantly, the TRACE trial has demonstrated enhanced overall survival with Yttrium-90 compared to other therapies. As the landscape of oncology treatment evolves, Yttrium-90 is an example of radiopharmaceuticals that stands out as a promising option, supported by a growing body of evidence from clinical trials and expert insights.
However, it is crucial to note that adverse events linked to Yttrium-90 therapy are predominantly hematological and generally reversible, underscoring the need for careful management. The significance of a multidisciplinary approach in managing hepatocellular carcinoma (HCC) is also vital, emphasizing the cooperative aspect of successful strategies for addressing the disease.
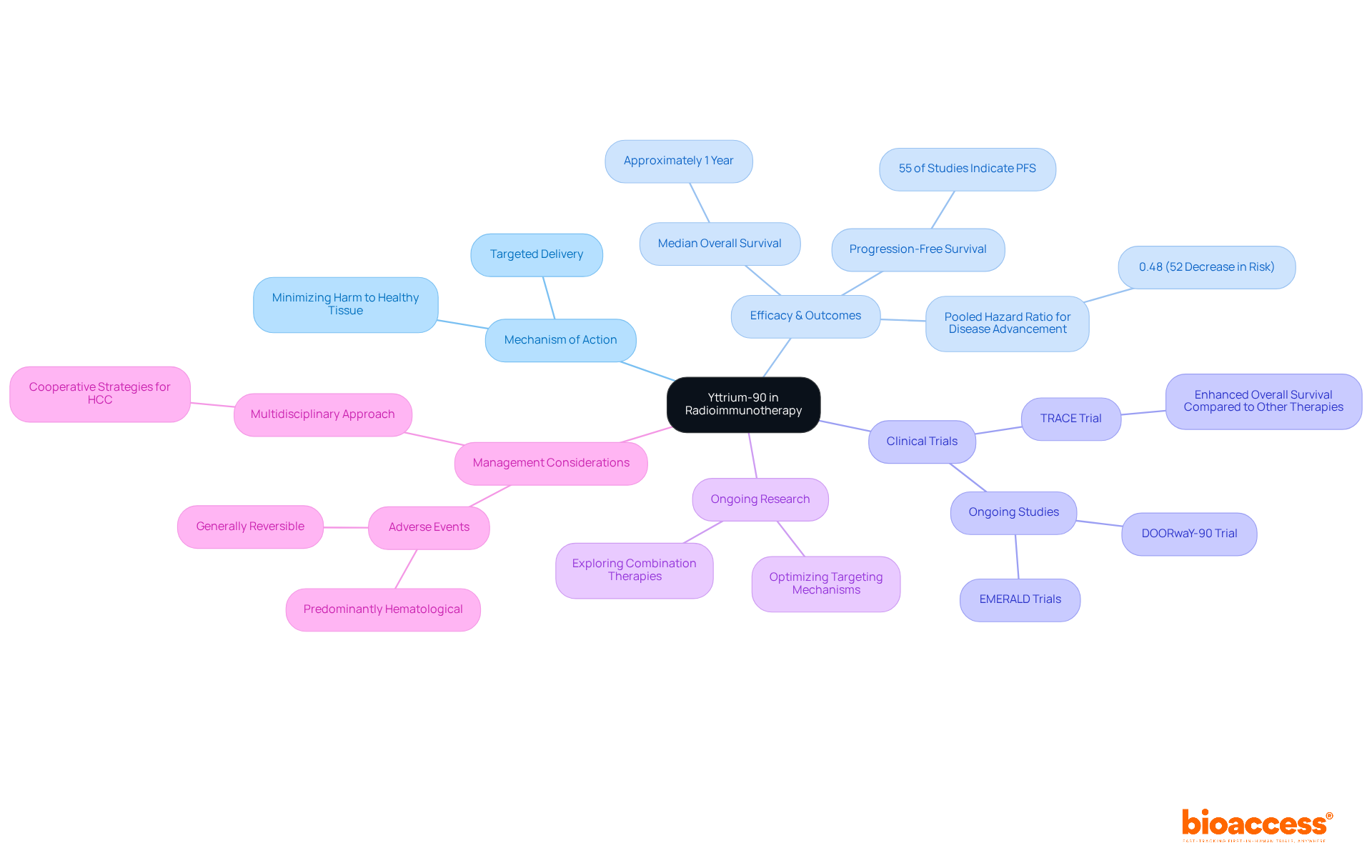
Theranostics represents a groundbreaking approach that integrates diagnostic imaging with targeted treatment through radioactive compounds. This innovative method in personalized medicine enables the identification of unique tumor characteristics, which facilitates the development of tailored treatment plans. Such strategies not only enhance therapeutic efficacy but also minimize adverse effects. The fusion of diagnostics and therapeutics within theranostics signifies a crucial advancement in cancer management, showcasing an important example of radiopharmaceuticals.
Recent studies reveal that therapies like 225Ac-PSMA-617 have produced exceptional clinical outcomes, with over 80% of individuals who have not undergone chemotherapy experiencing a PSA decline of 90% or more. Oncologists emphasize that this dual strategy not only improves patient outcomes but also fosters a more precise therapeutic model, aligning with the growing trend towards personalized medicine.
As the field continues to evolve, the ongoing development of novel therapeutic agents is expected to further enhance the efficacy of theranostic applications. This progression paves the way for more customized and effective treatments for tumors, reinforcing the critical nature of collaboration and innovation in clinical research.
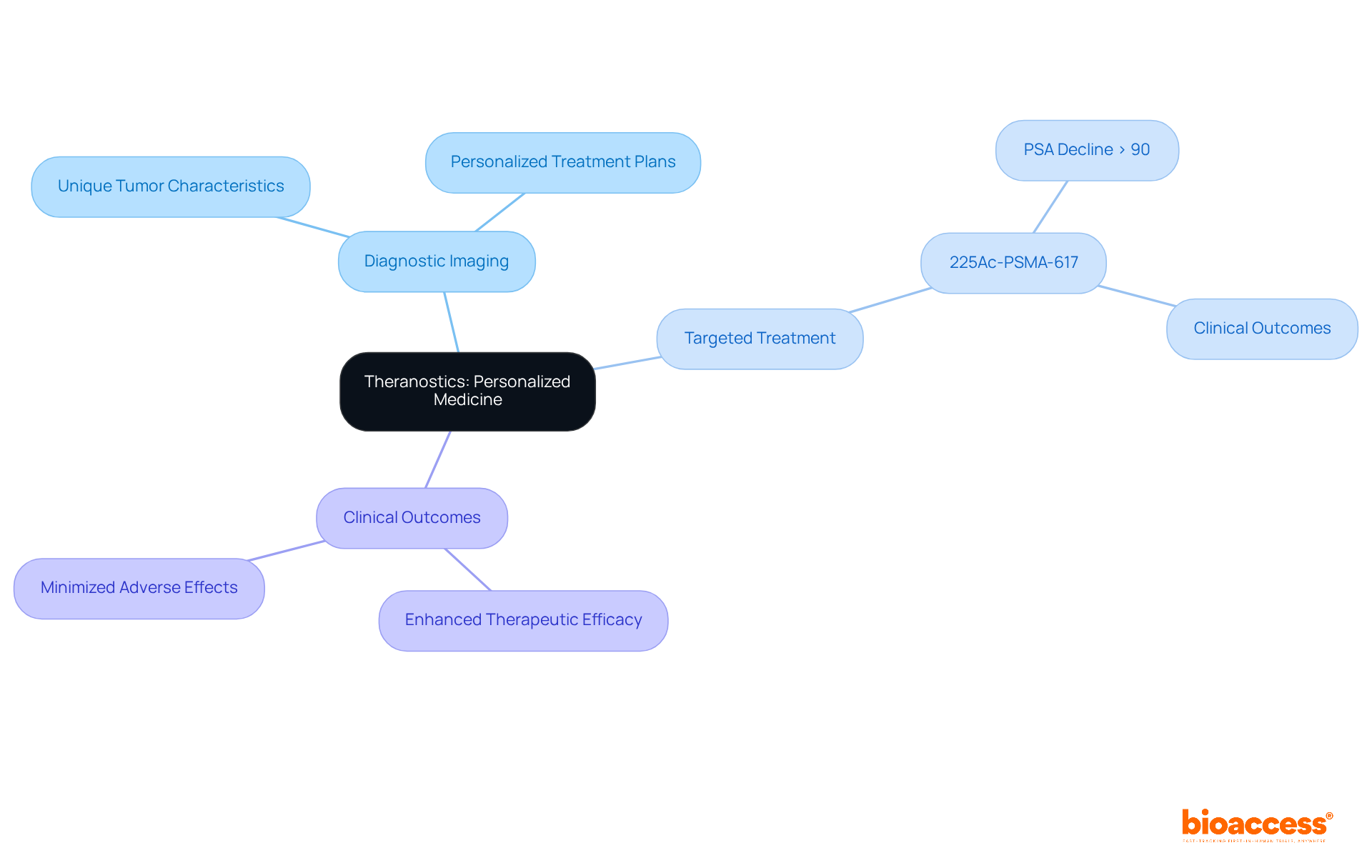
The future of radiopharmaceuticals is exceptionally promising, with ongoing research and technological advancements serving as a prime example of radiopharmaceuticals transforming oncology treatment. Targeted alpha therapy stands out as a groundbreaking approach, utilizing alpha particles to deliver potent radiation directly to malignant cells, thereby minimizing damage to surrounding healthy tissue. This innovative method has demonstrated considerable promise in clinical trials, achieving a disease control rate of 94.3% for RLT-naïve patients, emphasizing its efficacy in addressing difficult tumors.
Recent advancements also include enhanced imaging methods that improve the accuracy of medical applications, enabling better tumor localization and planning. The worldwide market for radiopharmaceuticals, as an example of radiopharmaceuticals, is expected to attain $12.18 billion by 2030, indicating the rising demand for advanced treatments for tumors. Major pharmaceutical companies are heavily investing in this sector, with approximately $10 billion spent on recent deals, underscoring the industry's confidence in the potential of targeted therapies, which serve as an example of radiopharmaceuticals.
Researchers are optimistic about the future of targeted alpha therapy, with Dr. François Bénard highlighting its capability to deliver therapeutic responses after only a few injections, contrasting sharply with conventional methods that often necessitate extended administration. As the landscape of oncology evolves, these innovations promise to deliver more personalized and effective treatment options, ultimately improving patient outcomes and revolutionizing cancer care.
The exploration of radiopharmaceuticals has illuminated their transformative potential in cancer treatment, showcasing innovative solutions that significantly enhance patient outcomes. By leveraging precise targeting mechanisms, these compounds not only improve the efficacy of therapies but also minimize adverse effects, marking a significant advancement in oncology.
Throughout the article, various examples of radiopharmaceuticals such as Lutetium-177, Iodine-131, Radium-223, and others have been highlighted for their specific applications and impressive clinical results. Each compound demonstrates a unique ability to address different cancer types, including:
This reinforces the critical role of targeted therapies in modern medicine. The ongoing advancements in this field, including the integration of theranostics and the promising future of targeted alpha therapy, underscore the importance of continued research and innovation.
As the landscape of cancer treatment evolves, the commitment to developing and optimizing radiopharmaceuticals remains paramount. The insights gained from recent studies and clinical trials not only pave the way for more personalized and effective treatment options but also emphasize the need for collaboration within the medical community. Embracing these advancements will be crucial in improving patient care and outcomes, ultimately revolutionizing how cancer is treated.
What is bioaccess® and its role in clinical research for radiopharmaceuticals?
bioaccess® is a contract research organization that accelerates clinical research for radiopharmaceuticals by focusing on early-phase studies and navigating complex regulatory environments. It operates strategically across Latin America, the Balkans, and Australia to ensure a rapid transition of innovative therapies from concept to clinical application.
How does bioaccess® enhance clinical trial timelines?
bioaccess® enhances clinical trial timelines through expedited site activation and patient recruitment, which helps fulfill the urgent need for innovative cancer solutions and shortens the timeframe for medical products to enter the market.
What is the projected market size for medical isotopes by 2024?
The global market for medical isotopes is projected to reach USD 16.30 billion by 2024, with an anticipated compound annual growth rate (CAGR) of 6.50% from 2025 to 2032.
What is Lutetium-177, and how does it benefit patients with neuroendocrine tumors?
Lutetium-177 is a radiopharmaceutical that delivers targeted radiation directly to malignant cells in neuroendocrine tumors, minimizing damage to surrounding healthy tissue. Clinical studies have shown its effectiveness in improving patient outcomes, including longer survival rates and better quality of life.
How has Iodine-131 transformed thyroid cancer treatment?
Iodine-131 has revolutionized thyroid cancer treatment, particularly for differentiated thyroid carcinoma (DTC), by selectively targeting thyroid tissue for precise ablation of malignant cells. Its effectiveness is highlighted by high disease-specific survival rates and significant remission rates in well-differentiated thyroid malignancies.
What are the survival rates associated with Iodine-131 treatment?
The disease-specific survival rates at five years are 86.3% for all individuals, 88.6% for those diagnosed with papillary thyroid carcinoma, and 80.8% for follicular thyroid carcinoma. High doses of Iodine-131 have shown an effective rate of 87.9% in patients post-thyroidectomy.
What risks are associated with Iodine-131 treatment?
There is a noted risk of developing a secondary primary tumor, as 18.2% of the disease-specific mortality group developed one after being diagnosed with thyroid disease. Despite this, Iodine-131 remains a cornerstone in managing thyroid tumors.