


The article titled "10 Head to Head Comparisons in Clinical Research Strategies" emphasizes the critical evaluation of various clinical research methodologies and their effectiveness. The title itself captures attention, suggesting a robust comparative analysis of diverse strategies employed in clinical research. This likely includes an exploration of the advantages, limitations, and implications of these methodologies for enhancing research outcomes.
In the evolving Medtech landscape, understanding these strategies is paramount. The article hints at the necessity for clinical researchers to navigate complex challenges effectively. By dissecting different approaches, it aims to provide insights that can drive improvements in research efficacy and patient outcomes.
Ultimately, the significance of collaboration among researchers is underscored, pointing towards the next steps in advancing clinical research practices. This analysis not only serves to inform but also to inspire action among stakeholders committed to elevating the standards of clinical research.
In the rapidly evolving landscape of clinical research, the quest for efficiency and effectiveness has never been more critical. As organizations strive to accelerate their studies and improve patient outcomes, understanding the nuances of various research strategies becomes paramount. This article delves into ten compelling head-to-head comparisons that illuminate the strengths and weaknesses of distinct clinical research methodologies. How can stakeholders harness these insights to optimize their approaches and ultimately enhance the success of medical trials?
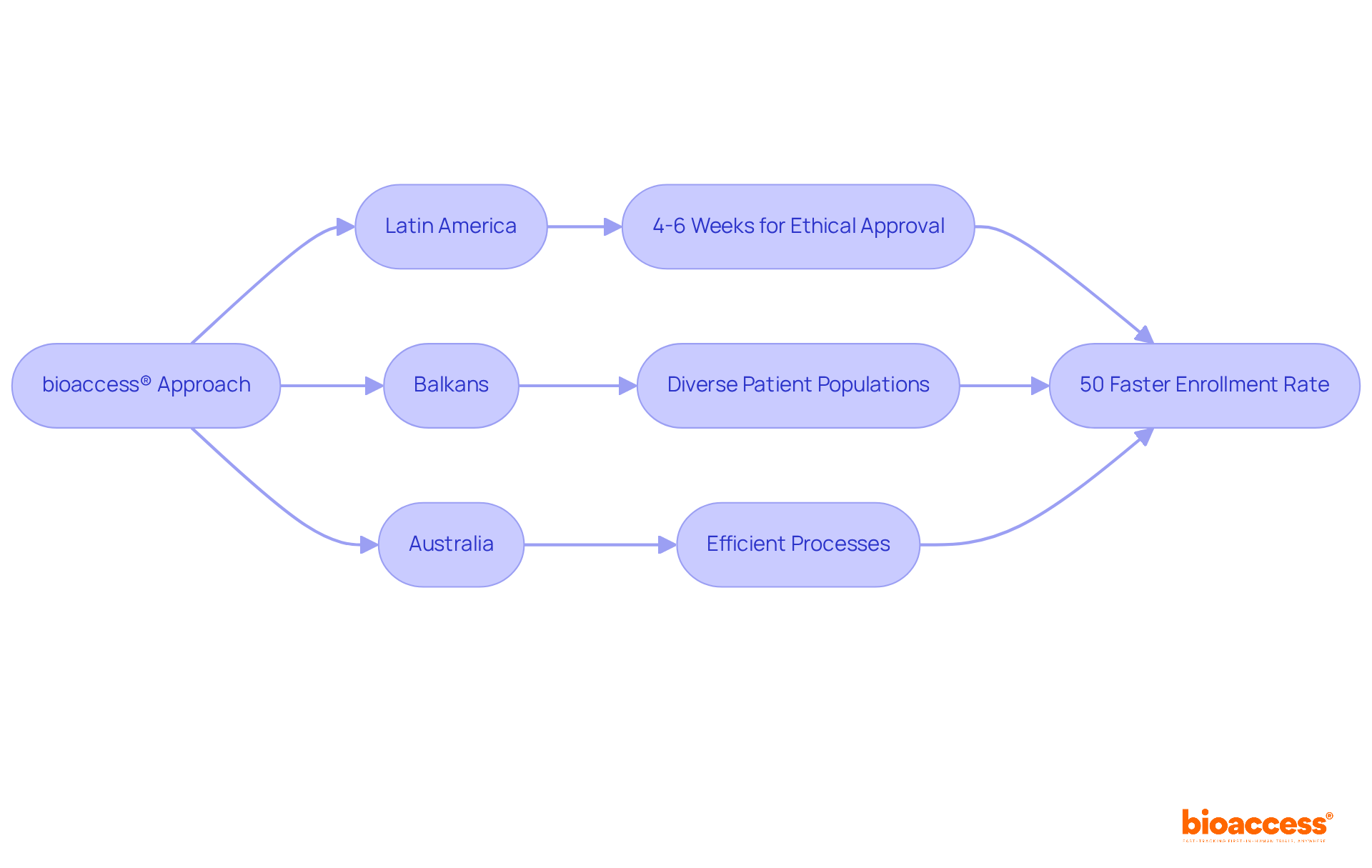
bioaccess® distinguishes itself in the medical investigation landscape by providing unmatched flexibility through its pioneering global-first approach. By harnessing the regulatory speed of Latin America, where ethical approvals can be secured in just 4-6 weeks, and combining this with the diverse patient populations in the Balkans and the efficient processes in Australia, bioaccess® achieves a remarkable 50% faster enrollment rate compared to traditional markets. This exceptional efficiency positions bioaccess® as the ideal partner for Medtech, Biopharma, and Radiopharma innovators who are eager to expedite their research timelines. The region's rich diversity not only enhances the understanding of treatment effects across various ethnic groups but also fosters a robust recruitment environment, establishing it as a vital hub for clinical trials.
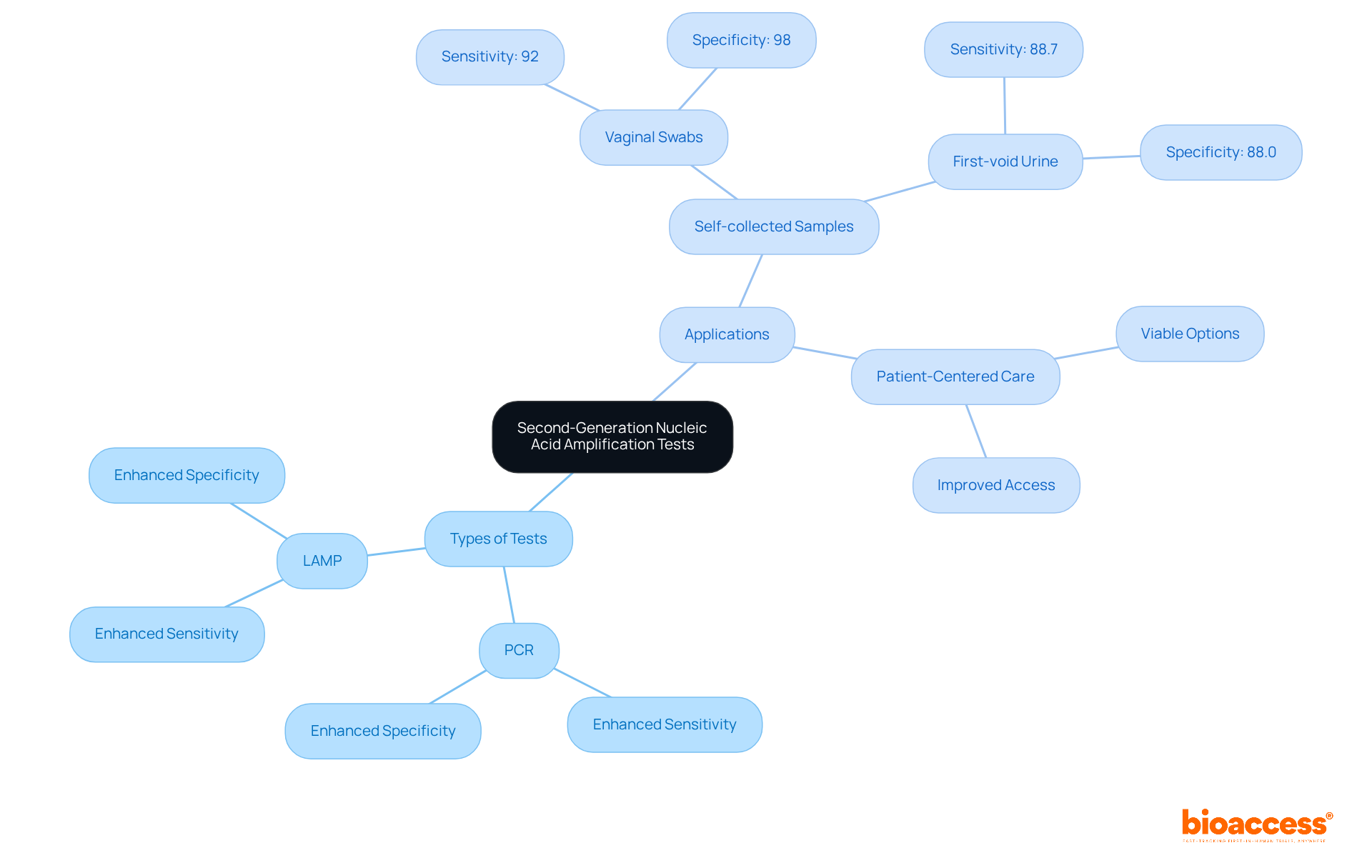
Second-generation nucleic acid amplification tests (NAATs) have revolutionized the detection of infectious diseases. These tests, including PCR and LAMP, demonstrate enhanced sensitivity and specificity compared to traditional methods, establishing their credibility in clinical research. Notably, studies indicate that self-collected samples, such as vaginal swabs and first-void urine, yield comparable results, positioning them as viable options for patient-centered care. This analysis underscores the critical importance of selecting the appropriate diagnostic instrument based on the medical context and individual requirements, prompting healthcare professionals to consider innovative approaches in their practice.
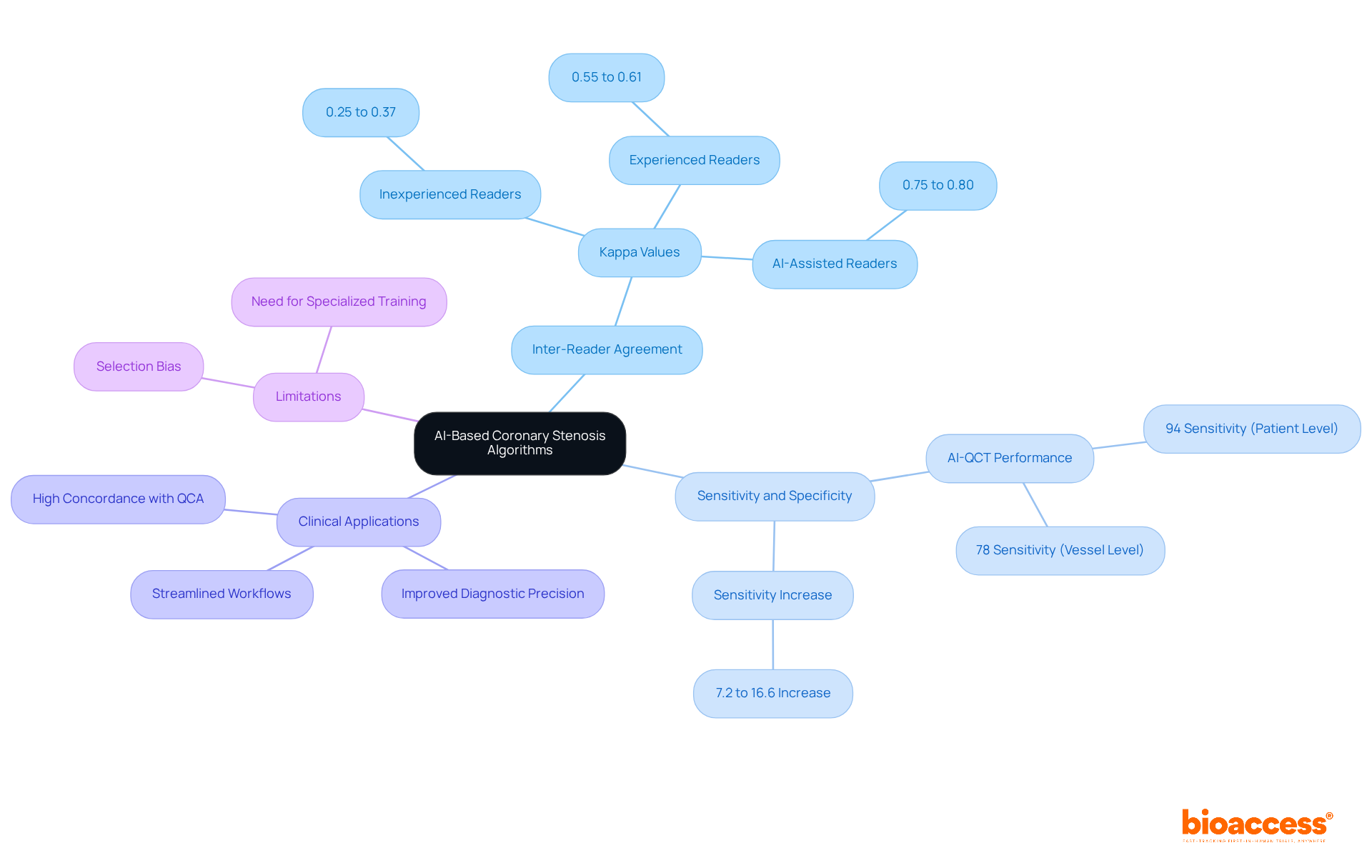
AI-based algorithms for assessing coronary stenosis have significantly improved inter-reader agreement among radiologists. Recent studies highlight that AI-assisted evaluations enhance sensitivity and specificity in detecting obstructive coronary artery disease. Notably, one study reported a sensitivity increase from 7.2% to 16.6% for AI-assisted readers compared to their inexperienced counterparts. This progress not only enhances diagnostic precision but also streamlines workflows in healthcare settings, ultimately leading to improved outcomes for individuals.
For instance, AI-QCT has demonstrated a remarkable 94% sensitivity at the individual level and 78% at the vessel level, surpassing conventional methods and showcasing AI's potential to minimize variability in evaluations. Furthermore, AI-QCT achieved the highest concordance with invasive quantitative coronary angiography (QCA), underscoring its effectiveness in clinical applications.
However, it is essential to acknowledge potential limitations, such as selection bias stemming from the use of invasive coronary angiography (ICA) as the reference standard. Additionally, specialized training is vital for enhancing diagnostic performance, particularly for inexperienced readers. The study involved 196 individuals who underwent both coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA) within a six-month period, providing critical context for these findings. Enhancements in inter-reader agreement are particularly crucial in environments where experienced readers may be scarce, thereby facilitating more reliable diagnoses and timely interventions.
A head to head comparisons of coronary CT angiography techniques, including dual-source and single-source CT, reveals significant differences in diagnostic performance. Notably, dual-source CT provides enhanced image quality and quicker acquisition times, making it more suitable for individuals with elevated heart rates, high calcium scores, or obesity. Recent advancements in dual-source technology, such as iterative reconstruction algorithms and improved temporal resolution, have significantly reduced motion artifacts. Studies indicate a decrease from 15.9% in single-source CT to just 4.8% in dual-source CT. Moreover, the effective radiation dose for dual-source CT is about 36% less than that of single-source CT, reinforcing its role in optimizing safety while maintaining high diagnostic accuracy. As highlighted in the literature, "the image quality achieved with dual source was considerably superior to that obtained with single source," emphasizing the importance of selecting the appropriate technology based on medical requirements. Clinicians are encouraged to incorporate the CAD-RADS scoring system into their evaluations to enhance diagnostic strategies effectively.

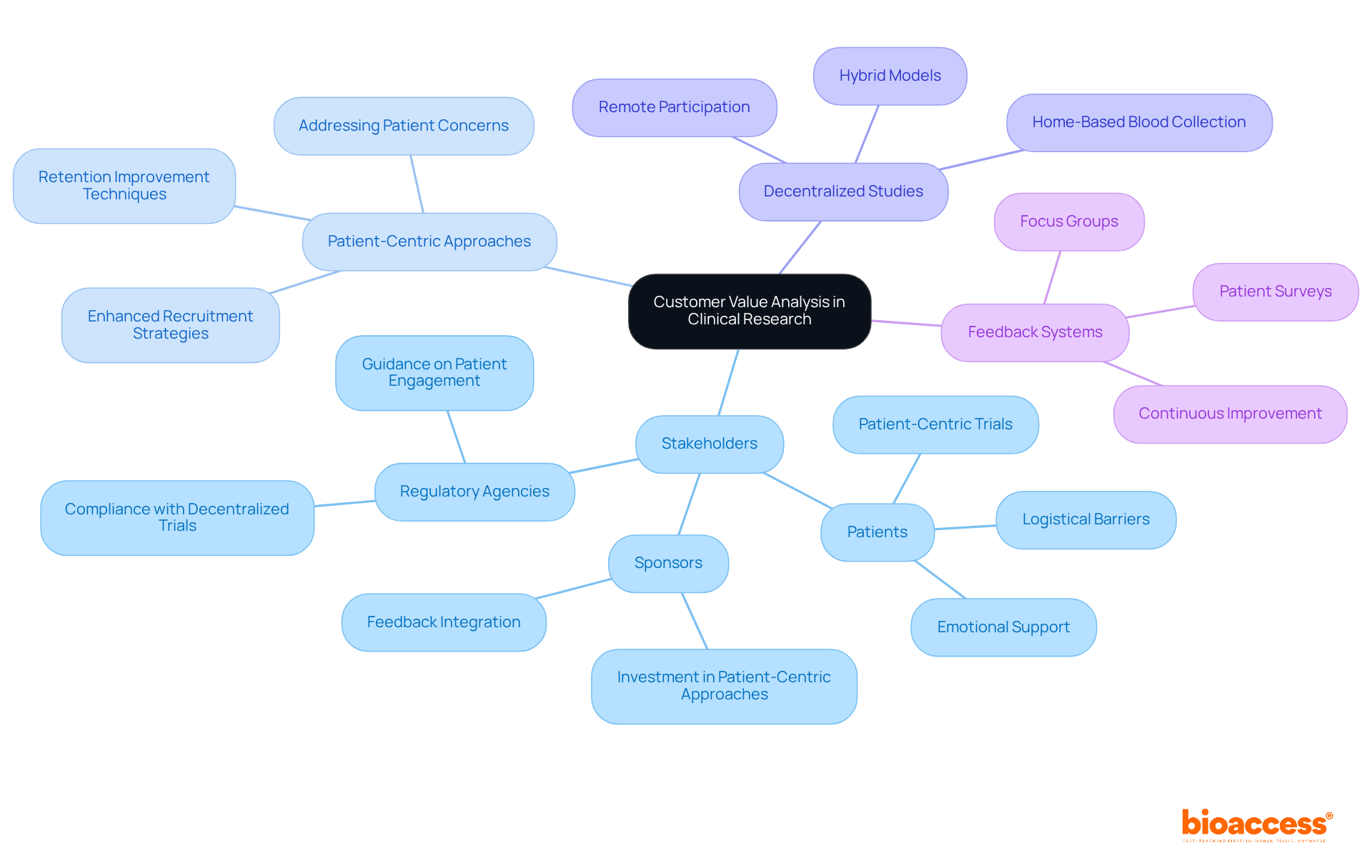
Customer value assessment in medical studies underscores the critical importance of understanding the expectations and requirements of all stakeholders, particularly patients, sponsors, and regulatory agencies. Organizations that prioritize patient-centric approaches consistently report higher satisfaction rates and improved recruitment outcomes. Research indicates that patient-focused studies can attract larger groups of participants by addressing their concerns and minimizing barriers to involvement. By integrating feedback systems and value-oriented measures, healthcare organizations can enhance their service offerings, ultimately fostering stronger connections with clients and elevating overall study success.
Moreover, the shift towards decentralized studies, which facilitate remote involvement, illustrates how adapting to individual needs can yield more effective recruitment strategies and enhanced retention rates. This comprehensive approach not only boosts participant engagement but also aligns medical studies with the evolving landscape of patient expectations. As the clinical research environment continues to change, it is imperative for organizations to embrace these strategies to ensure successful outcomes.
Ethical approvals in research studies exhibit substantial variability across regions, significantly impacting study schedules and overall viability. Notably, Latin America distinguishes itself with expedited ethical review processes, typically achieving approvals within 4 to 6 weeks. This efficiency offers a marked advantage compared to North America and Europe, where stringent regulatory frameworks often result in extended timelines, occasionally exceeding six months.
For example, Colombia's regulatory environment is recognized for its swift assessments, with the IRB/EC and INVIMA review processes completed in just 90 to 120 days. Understanding these regional variations is crucial for sponsors aiming to enhance study designs and schedules, as head to head comparisons of the pace of ethical approvals can directly influence the success of medical initiatives.
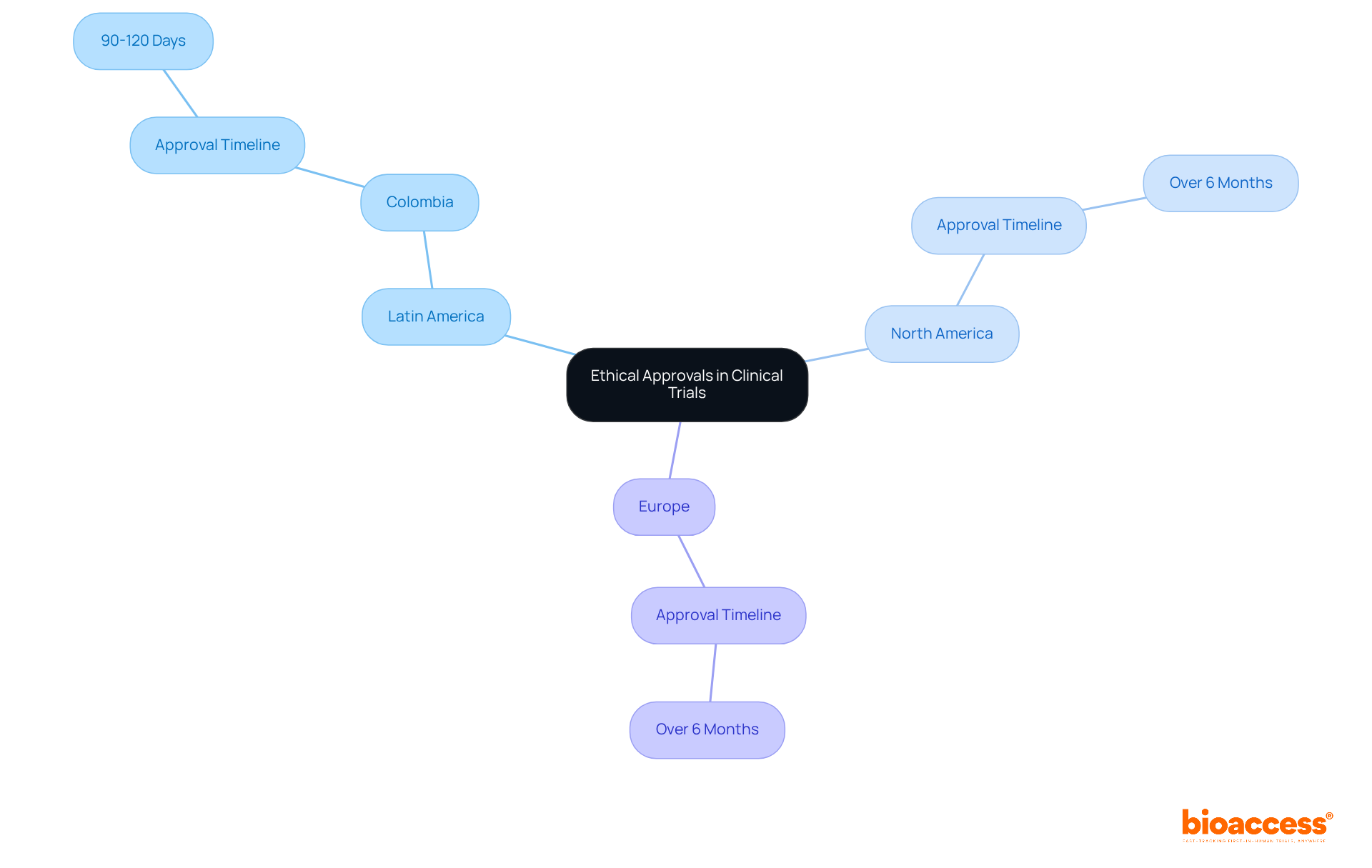
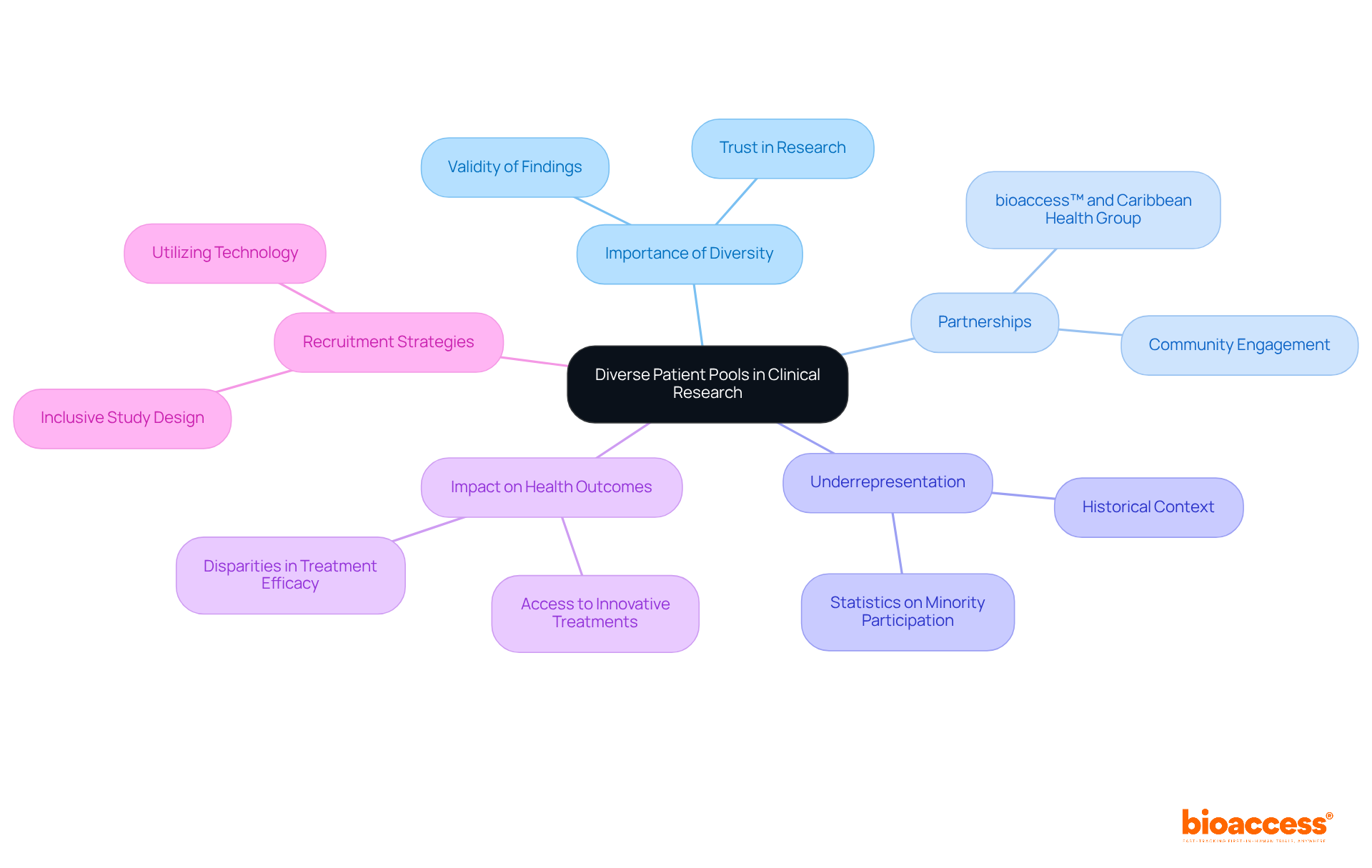
Enrollment approaches in research studies vary significantly across regions, shaped by cultural, economic, and regulatory factors. In Latin America, community engagement is crucial for recruitment success. Local partnerships, exemplified by the collaboration between bioaccess™ and Caribbean Health Group, play a vital role in fostering trust and awareness, which in turn enhances enrollment rates. This collaboration aims to position Barranquilla as a premier destination for medical studies in Latin America, supported by Colombia's Minister of Health, who advocates for the expansion of research initiatives in the region. Remarkably, this partnership has achieved over a 50% reduction in recruitment time and boasts a 95% retention rate, underscoring its effectiveness.
Research indicates that recruitment rates in Latin America can rival those in North America, where digital marketing and patient registries are more commonly utilized. However, nearly 80% of medical studies globally fail to meet their initial enrollment targets, with delays costing sponsors between $600,000 and $8 million each day. Cultural factors also play a significant role in recruitment; for instance, while 16% of the U.S. population is Hispanic, they constitute only 1% of research study participants. Similarly, although African-Americans make up 12% of the U.S. population, they represent only 5% of research participants. This disparity highlights the urgent need for tailored recruitment strategies that resonate with specific communities.
Engaging local healthcare providers and employing culturally relevant messaging can enhance participation rates and ensure a more representative sample in medical studies. The experience of leaders like Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during bioaccess®'s inaugural human trial in Colombia further illustrates the potential for successful outcomes through strategic collaborations.
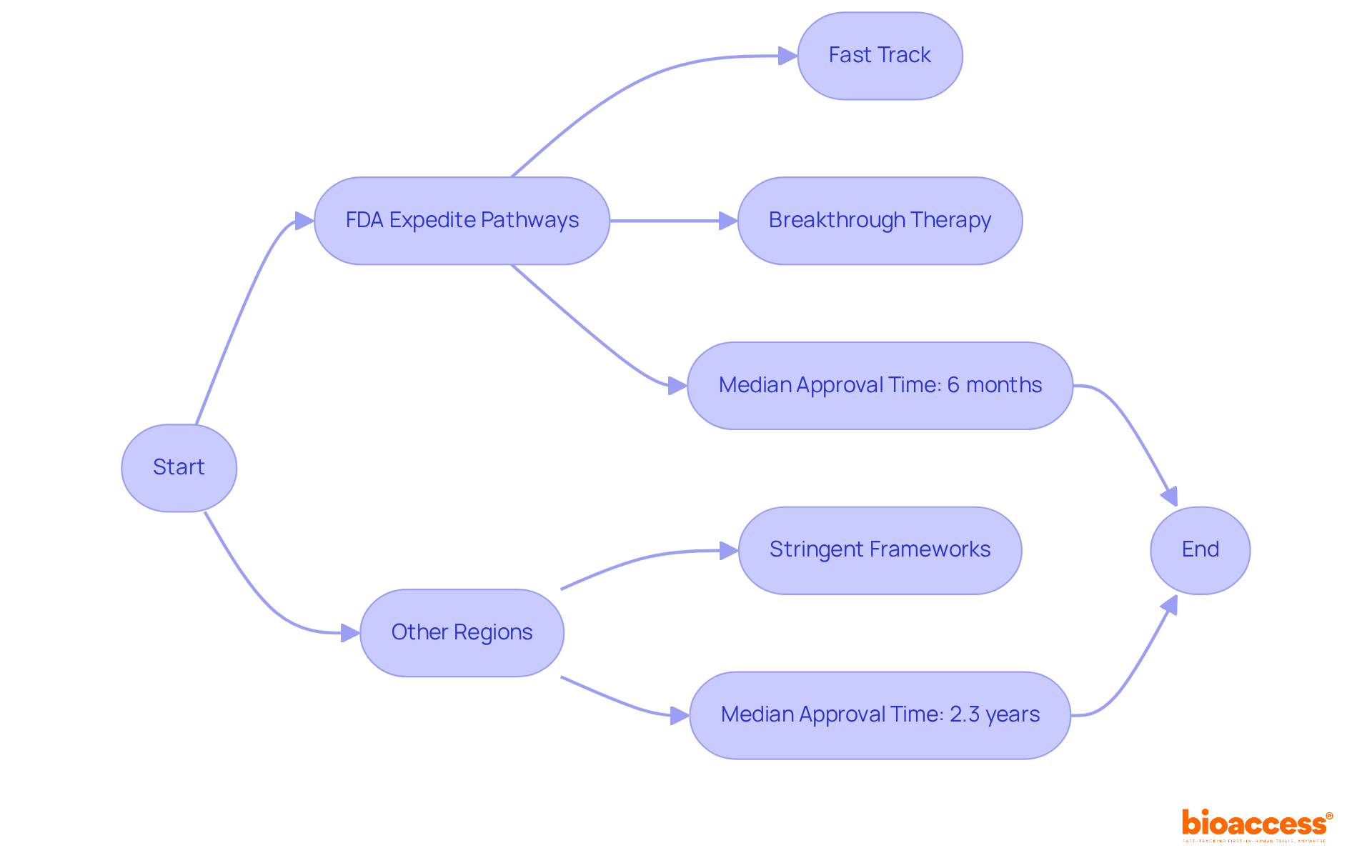
A comparative examination of regulatory routes in medical studies shows significant differences in approval procedures and timelines through head to head comparisons. The FDA's expedited pathways, including Fast Track and Breakthrough Therapy designations, enhance access to investigational therapies in the U.S., allowing for approvals in as little as six months. In contrast, other regions often adhere to more stringent frameworks, which can considerably extend the approval timeline. For instance, over the past decade, 51 accelerated approvals were converted to traditional approvals in a median time of 2.3 years, underscoring the efficiency of the FDA's expedited programs. Moreover, in 2018 and 2019, 73% and 60% of novel drugs, respectively, received expedited approval, reflecting the FDA's commitment to addressing unmet medical needs.
This understanding is vital for sponsors aiming to navigate the regulatory landscape effectively and capitalize on the advantages offered by these expedited pathways. Bioaccess® links pioneering Medtech, Biopharma, and Radiopharma startups with leading research facilities in Latin America, Eastern Europe, and Australia. By utilizing bioaccess's extensive management services for research studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—sponsors can accelerate their research processes and ensure adherence to regulatory standards. Ultimately, this collaboration results in quicker access to innovative therapies.
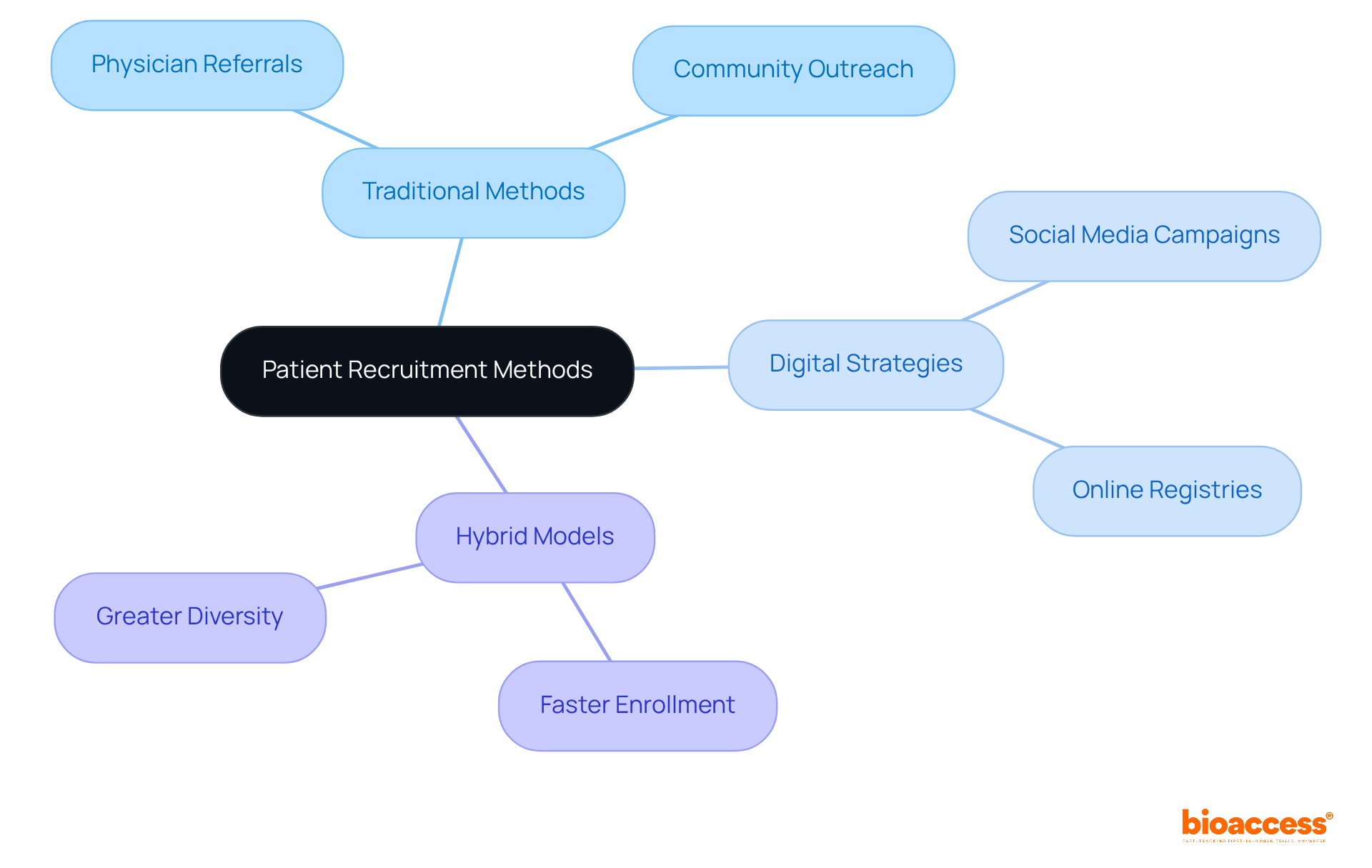
A comparative analysis of participant recruitment methods reveals a significant shift from traditional approaches, such as physician referrals and community outreach, to innovative digital strategies, including social media campaigns and online registries. Research indicates that hybrid recruitment models, which blend traditional and digital methods, are particularly effective. These models yield faster enrollment and greater participant diversity.
For example, studies demonstrate that hybrid strategies can successfully recruit a younger demographic, with median ages significantly lower than those achieved through traditional methods. Moreover, the integration of digital tools has been linked to improved engagement and retention rates, effectively addressing logistical challenges faced by potential participants.
This examination underscores the importance of flexible hiring approaches tailored to the unique requirements of specific groups, ensuring broader representation and enhancing the overall success of medical studies.
Varied groups in research are essential for guaranteeing that study findings are relevant to a wide population. Trials conducted in regions with diverse demographics, such as Latin America, provide more representative data, enhancing the validity of findings. The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances access to various patient groups but also addresses the historical underrepresentation of racial and ethnic minorities in research studies, which has led to significant disparities in health outcomes.
For instance, African Americans and Hispanics represent a disproportionately low percentage of research study participants, despite their higher prevalence of specific diseases. Studies in these regions have demonstrated that including underrepresented groups can significantly improve health outcomes and tackle disparities in access to innovative treatments. The FDA has underscored the necessity for increased diversity in medical studies, reinforcing the urgency of this issue. The inclusion of varied groups not only enriches the information gathered but also fosters trust in the research process, as communities see their needs represented in medical studies.
Moreover, bioaccess® offers comprehensive research study management services, including feasibility assessments, site selection, compliance evaluations, and project oversight, which are crucial for effective recruitment strategies. Highlighting diversity in recruitment is vital for the success of trials, yielding more comprehensive insights into treatment effectiveness across various demographics. This approach aligns with the growing recognition that health equity is a fundamental aspect of clinical research, ensuring that all populations benefit from advancements in medical science.
The exploration of head-to-head comparisons in clinical research strategies reveals a critical need for innovation and adaptability in the medical field. By examining various methodologies and approaches, it becomes evident that leveraging regional advantages and technological advancements can significantly enhance the efficiency and effectiveness of clinical trials. This strategic agility is essential for organizations aiming to accelerate research timelines and improve patient outcomes.
Key insights from the comparisons underscore the importance of:
The advantages of utilizing regions with faster regulatory processes, such as Latin America, combined with advanced diagnostic technologies and AI-driven methodologies, demonstrate the potential for optimizing clinical research. Furthermore, a patient-centric approach that prioritizes stakeholder engagement can lead to improved recruitment and retention rates, ultimately enhancing the overall success of studies.
As the landscape of clinical research continues to evolve, it is imperative for organizations to embrace these innovative strategies. By committing to diversity, efficiency, and technological integration, the medical community can ensure that research outcomes are not only relevant but also equitable. This proactive approach will address existing disparities in health outcomes and foster trust within communities, paving the way for groundbreaking advancements in medical science.
What is bioaccess® and how does it enhance clinical research?
bioaccess® is a platform that accelerates clinical research by providing unmatched flexibility through a global-first approach. It leverages the regulatory speed of Latin America, where ethical approvals can be obtained in just 4-6 weeks, and combines this with diverse patient populations in the Balkans and efficient processes in Australia, achieving a 50% faster enrollment rate compared to traditional markets.
Why is the diversity of patient populations important for clinical trials?
The rich diversity of patient populations enhances the understanding of treatment effects across various ethnic groups and fosters a robust recruitment environment, making it a vital hub for clinical trials.
What are second-generation nucleic acid amplification tests (NAATs)?
Second-generation NAATs, including PCR and LAMP, are advanced diagnostic tests that have improved the detection of infectious diseases with enhanced sensitivity and specificity compared to traditional methods.
How do self-collected samples compare to traditional collection methods in NAATs?
Studies indicate that self-collected samples, such as vaginal swabs and first-void urine, yield comparable results to traditional collection methods, positioning them as viable options for patient-centered care.
What advancements have AI-based algorithms brought to the assessment of coronary stenosis?
AI-based algorithms have significantly improved inter-reader agreement among radiologists, enhancing sensitivity and specificity in detecting obstructive coronary artery disease. For example, AI-QCT demonstrated a 94% sensitivity at the individual level.
What are some limitations of using AI-based algorithms in clinical settings?
Potential limitations include selection bias from using invasive coronary angiography as the reference standard and the need for specialized training to enhance diagnostic performance, especially for inexperienced readers.
What was the context of the study involving AI-QCT and coronary angiography?
The study involved 196 individuals who underwent both coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA) within a six-month period, providing critical context for evaluating the effectiveness of AI-assisted evaluations.