


The article highlights ten groundbreaking innovations in implantable medical devices that are revolutionizing patient care. Key advancements include:
These innovations not only enhance treatment efficacy but also improve patient comfort and facilitate real-time health monitoring. As a result, they significantly advance healthcare outcomes and foster greater patient engagement. The relevance of these developments in the Medtech landscape cannot be overstated, as they address critical challenges in clinical research and patient management.
The landscape of healthcare is undergoing a seismic shift, driven by groundbreaking innovations in implantable medical devices that promise to enhance patient care and outcomes. These advancements not only streamline clinical trials and regulatory processes but also introduce cutting-edge technologies that improve monitoring, pain management, and medication adherence.
However, as the industry embraces these transformative solutions, critical questions emerge:
Exploring these dynamics reveals a future where patient-centered care is not merely a goal but a reality shaped by technology.
bioaccess® leverages its extensive expertise and local advantages to expedite clinical studies for implantable medical devices. By capitalizing on Latin America's regulatory speed, the Balkans' diverse healthcare populations, and Australia's efficient ethical approval processes, bioaccess® secures approvals in an impressive 4-6 weeks. This accelerated timeline not only streamlines clinical trials but also significantly shortens the path to market for innovative healthcare solutions, ultimately enhancing patient care and outcomes. The organization’s steadfast commitment to ethical practices guarantees that all studies comply with the highest standards of integrity, fostering trust among stakeholders and participants alike.
bioaccess® excels in the feasibility and selection of research sites, principal investigator selection, and comprehensive project management—key components for successful clinical trials. Notably, bioaccess® has completed over 159 regulatory submissions for more than 75 medical trials, showcasing its adeptness at navigating complex regulatory environments efficiently. This strategic approach positions bioaccess® as a frontrunner in the field, enabling innovators to harness regulatory speed for improved clinical research outcomes. As the Medtech landscape continues to evolve, collaboration and innovative strategies will be essential for overcoming challenges and advancing healthcare solutions.
Neurostimulator systems have emerged as a transformative solution for managing chronic pain and neurological disorders, impacting approximately 20.9% of US adults. These implantable medical devices operate by delivering electrical impulses to targeted regions of the nervous system, effectively modulating pain signals and enhancing quality of life. Recent advancements in neurostimulator technology showcase adaptive stimulation algorithms that customize treatment based on real-time feedback from individuals, thereby improving efficacy and comfort.
Clinical studies, such as the ReActiv8-B trial, have demonstrated significant advancements in pain relief and functional outcomes, with 83% of participants experiencing clinically meaningful benefits over three years. As Dr. Christopher Gilligan from the Division of Pain Medicine observes, "Participants demonstrated improvements in pain, disability, and healthcare-related quality of life that increased with treatment duration."
This compelling evidence paves the way for broader adoption of implantable medical devices in clinical practice, providing renewed hope for individuals suffering from chronic pain and related conditions.
Implantable medical devices, like cardiac monitors, are revolutionizing heart health monitoring and management by continuously tracking heart rhythms and detecting arrhythmias or other abnormalities in real-time. This capability enables prompt interventions with implantable medical devices that can significantly enhance health outcomes.
Recent advancements in miniaturization have led to the development of implantable medical devices that are less invasive and more comfortable for individuals, while improved battery life ensures longer operational periods without the need for frequent replacements.
Moreover, the integration of wireless technology in implantable medical devices facilitates seamless data transmission to healthcare providers, enabling proactive management of cardiac conditions. This real-time monitoring not only elevates the quality of care but also empowers individuals to take an active role in their heart health.
As Dr. Lawrence McAuliffe, MD, FACC, emphasizes, "There are a lot of cardiac conditions that we want to know exist because they require treatment and intervention, and you want to do that as timely as possible."
This is particularly crucial given that the number of Americans who died from obesity-related heart disease has tripled over the past 20 years, underscoring the urgent need for effective heart health monitoring.
The miniaturization of implantable instruments represents a pivotal trend that enhances patient comfort and outcomes. Smaller instruments are inherently less invasive, effectively minimizing surgical risks and reducing recovery durations. Recent innovations in materials and engineering have enabled the development of products that are not only compact but also highly functional.
For instance, miniaturized pacemakers can now be implanted without the need for leads, which significantly reduces complications and boosts satisfaction among patients. As these tools evolve to become more user-friendly, it is anticipated that adherence to treatment regimens will increase, ultimately leading to improved health outcomes.
PEEK-based implantable medical devices signify a remarkable advancement in biocompatibility, offering substantial advantages over traditional materials. Known for its outstanding mechanical properties and wear resistance, PEEK has been utilized in patients for over 20 years, making it exceptionally suitable for use in implantable medical devices. Its biocompatibility greatly minimizes the risk of adverse reactions, thereby enhancing the safety profile of implantable medical devices like spinal implants and orthopedic screws.
For example, PEEK spinal implantable medical devices have shown lower rates of subsidence and migration compared to metal alternatives, which contributes to improved patient outcomes. Recent studies indicate that PEEK implants, which are a type of implantable medical device, exhibit infection rates comparable to titanium, reinforcing their appropriateness for various healthcare applications.
Furthermore, ongoing research is investigating PEEK's potential to enhance drug delivery systems, expanding its applications in healthcare. Experts in materials science emphasize PEEK's unique characteristics, including its lower elastic modulus that closely mirrors that of cortical bone, promoting better load distribution and reducing stress shielding. PEEK can maintain continuous use up to 480°F (250°C), demonstrating its thermal stability and suitability for diverse healthcare applications.
As the medical community continues to explore PEEK's capabilities, its role in innovative products as an implantable medical device is poised for significant growth.
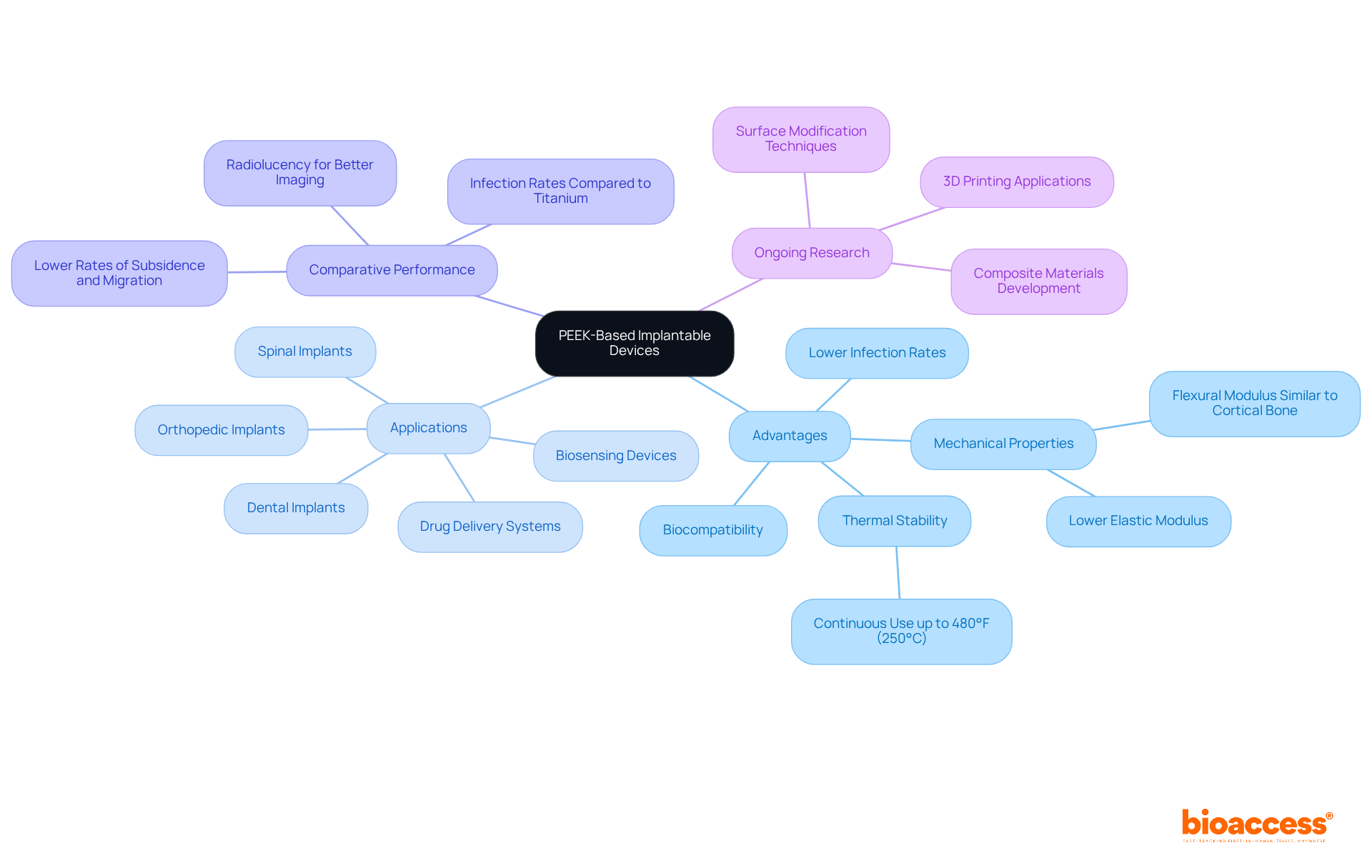
An implantable medical device, specifically drug delivery systems, serves a critical role in ensuring medication adherence by facilitating controlled and sustained release of therapeutics directly at the site of action. These advanced systems can be programmed to deliver precise dosages over extended periods, thereby reducing the necessity for frequent dosing and minimizing associated side effects. Noteworthy innovations in this field include biodegradable implantable medical devices that dissolve post-medication delivery, effectively eliminating the need for surgical removal. Clinical studies substantiate that individuals utilizing these systems experience enhanced adherence and improved health outcomes, particularly in the management of chronic diseases.
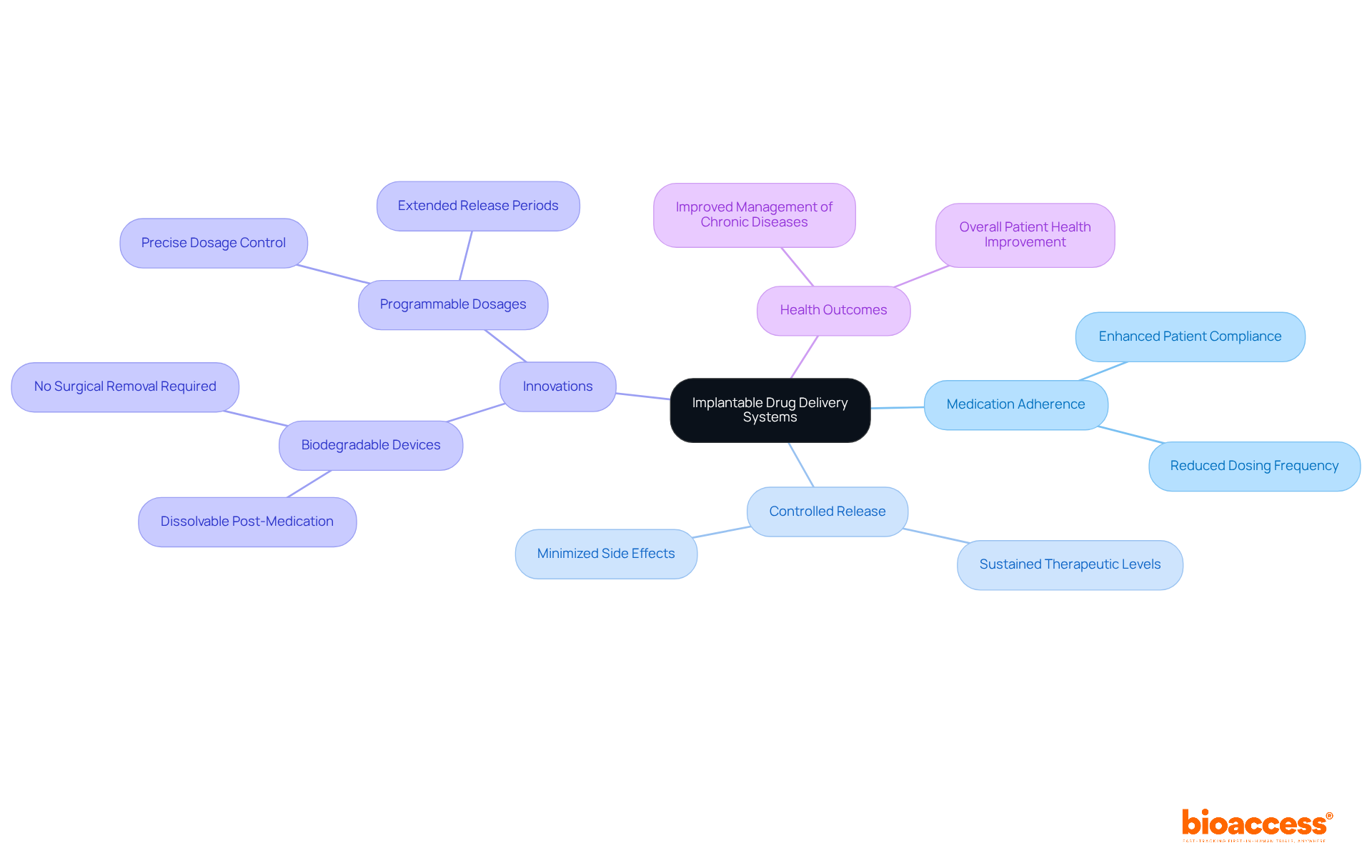
Wireless implantable medical devices are revolutionizing the monitoring landscape by facilitating real-time data transmission to healthcare providers. This innovative technology, such as an implantable medical device, allows for continuous tracking of vital signs and other health metrics, which is crucial for timely interventions when necessary.
Recent advancements in wireless communication protocols have markedly improved the reliability and security of data transmission, thereby safeguarding individual privacy. For instance, research indicates that the integration of remote monitoring solutions has resulted in a 76% reduction in hospital readmission rates, as highlighted by the University of Pittsburgh Medical Center. This statistic underscores the effectiveness of timely data in health management.
Moreover, healthcare professionals have noted that real-time data transmission not only boosts patient engagement but also empowers individuals to make informed decisions based on immediate insights, which is especially relevant for the effective use of implantable medical devices, ultimately leading to enhanced care and outcomes.
With projections estimating the RPM market to reach $42 billion by 2028, the significance of wireless implantable medical devices in providing high-quality, patient-centered care is set to grow exponentially.
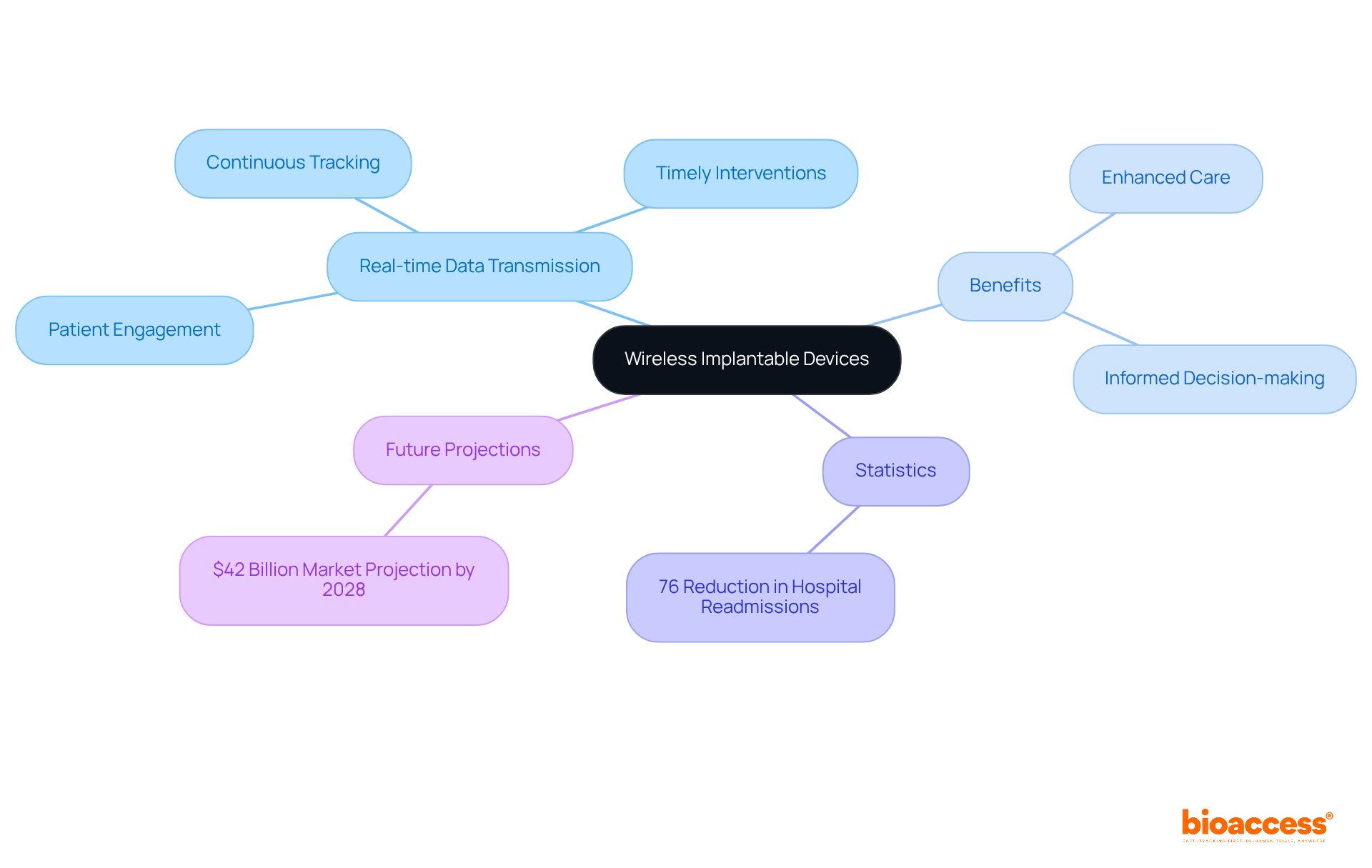
Real-time health monitoring sensors are revolutionizing care by delivering continuous feedback on vital health parameters such as heart rate, blood pressure, and glucose levels. This groundbreaking technology facilitates the prompt detection of abnormalities, significantly enhancing safety for individuals. Furthermore, the integration of artificial intelligence and machine learning algorithms amplifies the predictive capabilities of these sensors, enabling proactive management of chronic conditions.
Clinical trials have demonstrated that individuals utilizing real-time monitoring experience a reduction in complications and improved health outcomes, with studies indicating an accuracy of up to 95% in predicting health deterioration. Healthcare professionals acknowledge the critical role of these advancements, emphasizing that AI-driven insights can lead to more personalized treatment plans and better resource allocation in clinical settings.
As the healthcare landscape continues to evolve, the significance of these innovative monitoring technologies cannot be overstated.
Regulatory adherence is paramount in the advancement of implantable medical devices, ensuring their safety and efficacy for user application. Manufacturers encounter the intricate challenge of navigating complex regulatory pathways, which include:
Compliance with guidelines established by regulatory bodies such as the FDA and EMA is crucial for securing market approval. Best practices for compliance encompass:
Experts underscore that early interaction with regulatory bodies clarifies expectations and refines study designs, ultimately mitigating the risk of delays. By prioritizing compliance, manufacturers not only reduce risks but also enhance the credibility and marketability of their products.
The impact of regulatory routes on approval durations is significant; streamlined procedures can expedite market entry, allowing innovative products to reach users more swiftly. For example, the FDA's expedited pathways are designed to facilitate quicker access to promising therapies, though they require careful consideration of informed consent and the communication of uncertainties to patients.
Bioaccess® offers a comprehensive process for enhancing clinical trials, which includes:
In summary, a strategic approach to regulatory navigation, supported by bioaccess's expert services, is essential for success in the competitive field of implantable medical devices.
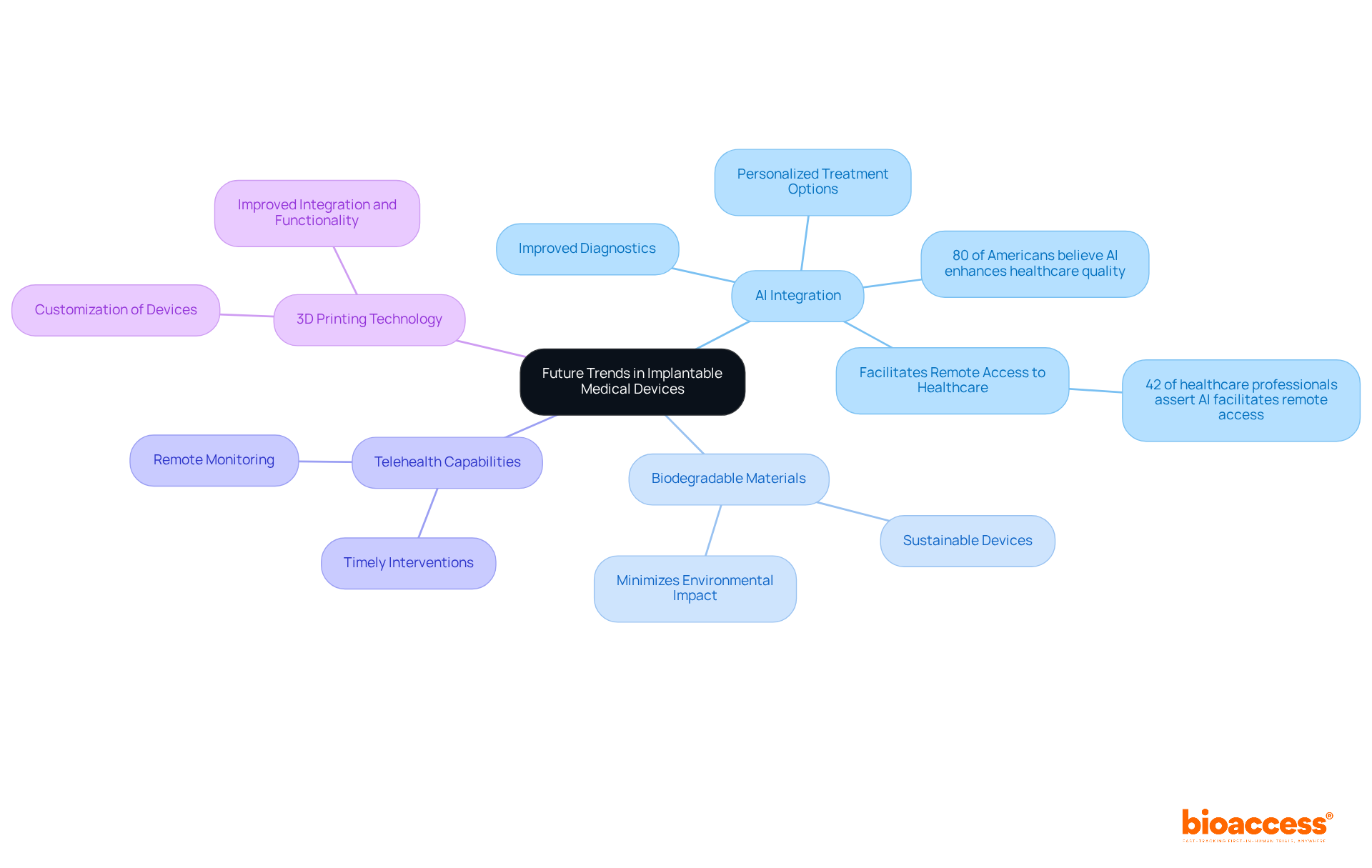
The future of implantable medical devices is on the brink of remarkable advancements, driven by technological breakthroughs and evolving individual demands. A key trend is the integration of artificial intelligence, which is significantly enhancing personalized treatment options. For instance, AI algorithms can analyze individual data to tailor therapies, thereby improving outcomes and patient satisfaction. Notably, 80% of Americans believe that AI has the potential to enhance healthcare quality, reduce costs, and increase accessibility, highlighting its critical role in this sector.
Furthermore, the development of biodegradable materials is paving the way for sustainable devices, minimizing environmental impact while ensuring efficacy. The expansion of telehealth capabilities also plays a crucial role, enabling remote monitoring of individuals, which is vital for timely interventions and ongoing care management. Indeed, 42% of healthcare professionals assert that AI facilitates remote access to healthcare, underscoring its importance in enhancing engagement with patients receiving care.
In addition, advancements in 3D printing technology are revolutionizing the customization of implantable medical devices, allowing for a more precise fit to individual anatomies. This level of personalization is anticipated to lead to improved integration and functionality of devices within the body. As these trends continue to evolve, they are poised to significantly shape the landscape of patient care, resulting in more effective and tailored treatment options that address the unique needs of each patient.
The landscape of implantable medical devices is undergoing a rapid transformation, propelled by innovative technologies that significantly enhance patient care and outcomes. From neurostimulators alleviating chronic pain to wireless monitoring systems enabling real-time health tracking, these advancements are revolutionizing healthcare delivery. The integration of artificial intelligence, miniaturization, and improved biocompatibility exemplifies how these devices are evolving to become more effective, user-friendly, and safer for patients.
Key insights from the article underscore the critical role of regulatory compliance in ensuring the safety and efficacy of these devices, as well as the impact of organizations like bioaccess® in expediting clinical research processes. With a steadfast commitment to ethical practices and a focus on innovative strategies, bioaccess® serves as a model for how collaboration can propel progress within the Medtech sector. Additionally, the exploration of future trends—such as biodegradable materials and telehealth capabilities—highlights the ongoing dedication to enhancing healthcare delivery and patient engagement.
As the medical community increasingly embraces these advancements, it is imperative for stakeholders—including manufacturers, healthcare providers, and patients—to remain informed about the latest innovations in implantable medical devices. This awareness empowers them to advocate effectively for their needs, contributing to a healthcare environment that prioritizes safety, efficacy, and personalized care. The potential for these technologies to reshape patient experiences and improve health outcomes is substantial, marking an exhilarating period for the future of medical technology.
What is bioaccess® and what does it specialize in?
bioaccess® is an organization that specializes in accelerating clinical research for implantable medical devices by leveraging local advantages and regulatory speed in various regions, including Latin America, the Balkans, and Australia.
How quickly can bioaccess® secure approvals for clinical studies?
bioaccess® can secure approvals for clinical studies in an impressive 4-6 weeks, significantly streamlining the clinical trial process.
What are the key components of bioaccess®'s clinical trial management?
Key components include feasibility and selection of research sites, principal investigator selection, and comprehensive project management.
How many regulatory submissions has bioaccess® completed?
bioaccess® has completed over 159 regulatory submissions for more than 75 medical trials.
What types of conditions do neurostimulator devices address?
Neurostimulator devices are designed to manage chronic pain and neurological disorders, impacting approximately 20.9% of US adults.
How do neurostimulator systems work?
They operate by delivering electrical impulses to targeted regions of the nervous system, modulating pain signals and enhancing quality of life.
What recent advancements have been made in neurostimulator technology?
Recent advancements include adaptive stimulation algorithms that customize treatment based on real-time feedback from individuals.
What were the outcomes of the ReActiv8-B clinical trial?
The ReActiv8-B trial demonstrated that 83% of participants experienced clinically meaningful benefits in pain relief and functional outcomes over three years.
How do implantable cardiac monitors enhance heart health monitoring?
They continuously track heart rhythms and detect arrhythmias in real-time, enabling prompt interventions and significantly improving health outcomes.
What advancements have been made in implantable cardiac monitors?
Advancements include miniaturization for less invasive devices, improved battery life, and the integration of wireless technology for seamless data transmission to healthcare providers.
Why is real-time monitoring important for heart health?
Real-time monitoring allows for proactive management of cardiac conditions and empowers individuals to take an active role in their heart health, which is crucial given the rising incidence of obesity-related heart disease.