


Innovations in healthcare are rapidly transforming the landscape of patient care, especially through the integration of drug-device combination products. These groundbreaking solutions not only enhance treatment efficacy but also streamline the management of chronic conditions, offering patients a more cohesive approach to their health. However, as these technologies evolve, it’s crucial to consider the challenges and complexities that arise in their implementation and effectiveness.
What are the most innovative drug-device combinations currently shaping the future of healthcare? How can they address the pressing needs of patients and healthcare providers alike? These questions are essential as we navigate the evolving Medtech landscape, where collaboration and innovation are key to overcoming obstacles and improving patient outcomes.
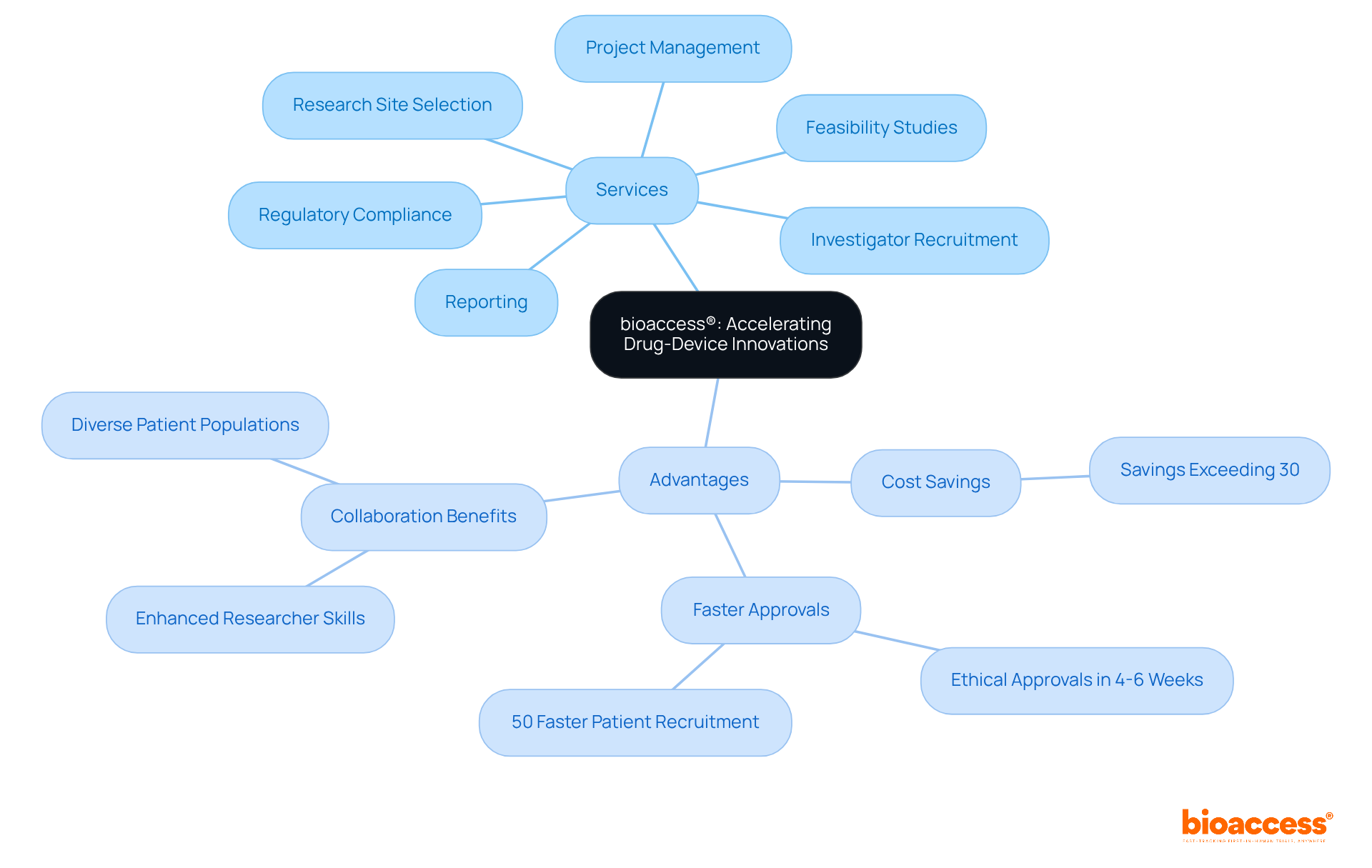
bioaccess® leverages over 15 years of clinical research expertise to accelerate the development of drug-device combination products. With a keen focus on Latin America's swift regulatory processes—especially in Colombia, where the total IRB/EC and MoH (INVIMA) review takes just 90-120 days—bioaccess® is committed to ensuring that innovative healthcare solutions reach the market more quickly. Our extensive services encompass:
In a landscape where ethical approvals can be secured in only 4-6 weeks and enrollment is expedited by 50%, bioaccess® is at the forefront of driving significant advancements in medical technology. This strategic approach not only enhances the speed of introducing new therapies to patients but also highlights the vital role of regulatory agility and cost efficiency—offering savings of over 30% compared to trials in North America or Western Europe. Such advantages are crucial for Directors of Clinical Research as they design studies and aim for improved patient outcomes.
As we navigate the complexities of clinical research, collaboration becomes essential. By partnering with bioaccess®, stakeholders can harness our expertise to overcome key challenges in the Medtech landscape, ultimately leading to more effective and timely healthcare solutions.
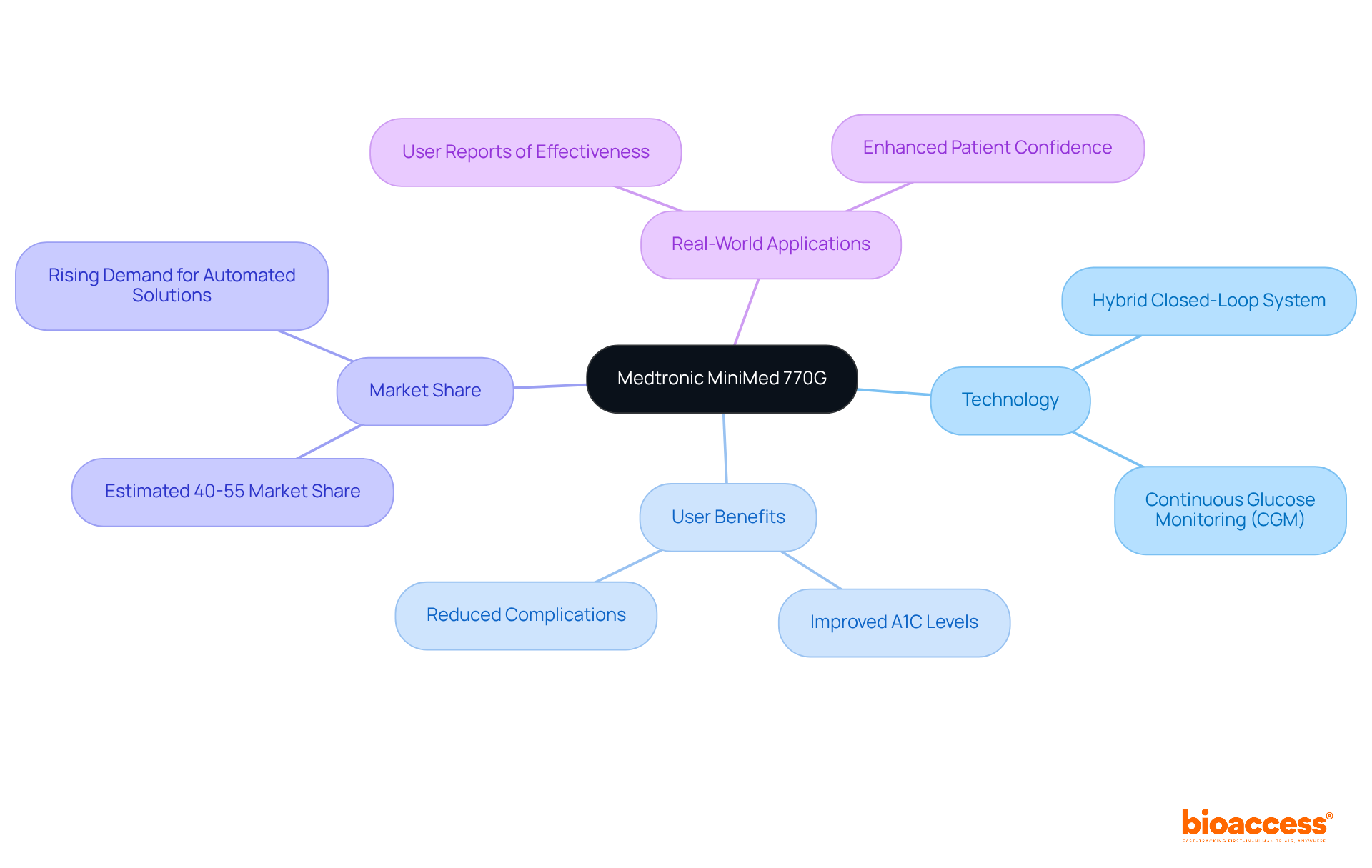
The Medtronic MiniMed 770G device represents a significant advancement in insulin delivery technology. As a hybrid closed-loop system, it autonomously adjusts insulin delivery based on real-time glucose readings, providing users with a tailored diabetes management experience. This integration of continuous glucose monitoring (CGM) technology greatly enhances glycemic control, effectively reducing the risks associated with both hyperglycemia and hypoglycemia. With an estimated market share of around 40-55% among leading insulin pump manufacturers, the MiniMed 770G illustrates the increasing trend towards automated insulin delivery solutions.
Real-world applications underscore its effectiveness; users have reported improved A1C levels and a decrease in diabetes-related complications. The innovative design of this setup not only simplifies insulin administration but also empowers patients to manage their condition with greater confidence and ease. As we navigate the evolving Medtech landscape, the MiniMed 770G stands out as a beacon of progress, addressing key challenges in diabetes care and enhancing patient outcomes.
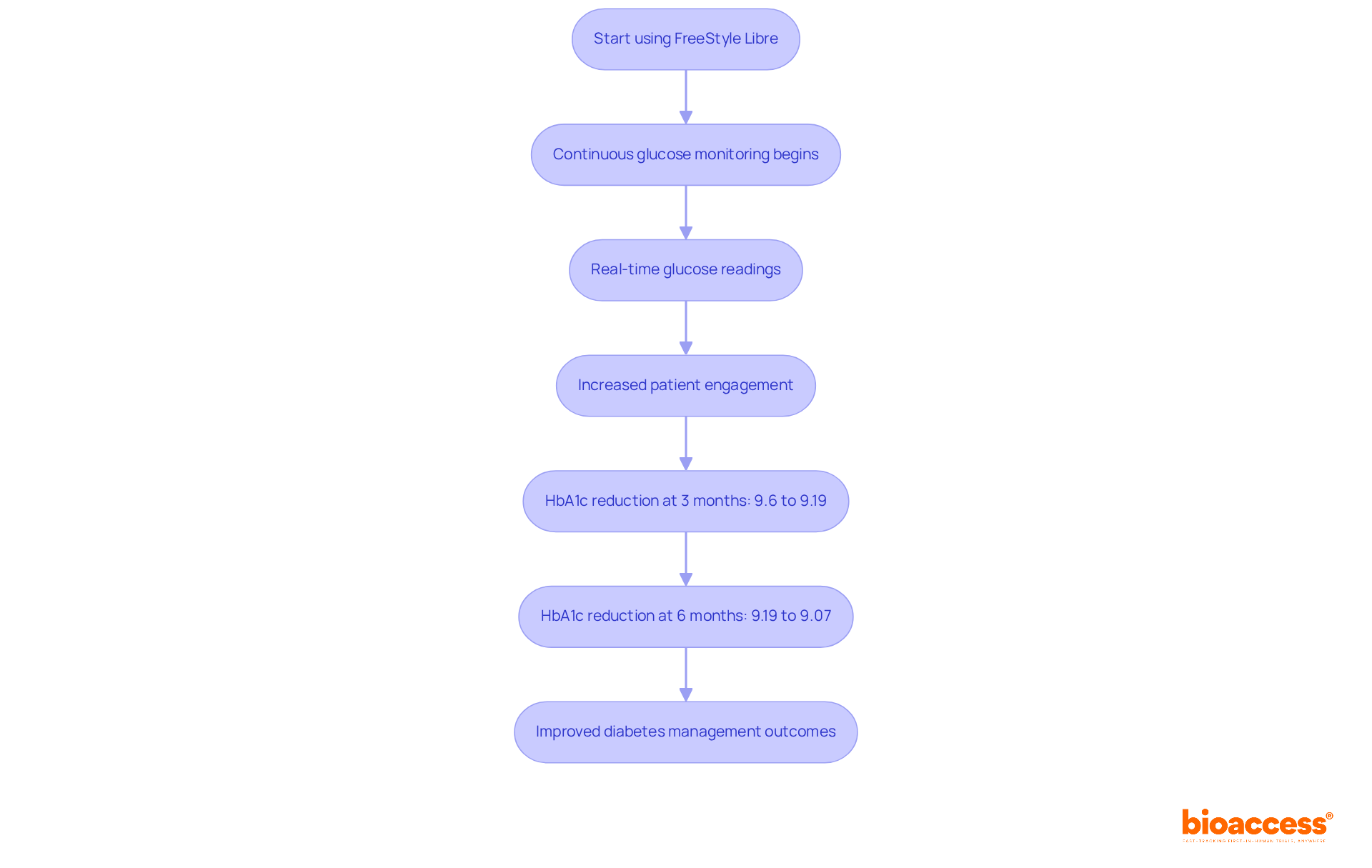
Abbott's FreeStyle Libre device has revolutionized glucose monitoring, offering a continuous glucose monitoring (CGM) solution that eliminates the need for routine fingersticks. Patients now enjoy real-time glucose readings through a small sensor worn on the skin, significantly enhancing their diabetes management capabilities. The platform's intuitive interface and seamless mobile app integration foster greater patient engagement and adherence to treatment plans.
Studies reveal that users of the FreeStyle Libre system experienced a median HbA1c reduction from 9.6% to 9.19% at three months, further decreasing to 9.07% at six months. This showcases the device's effectiveness in improving glycemic control. Moreover, the increased awareness of glucose levels has led to more frequent healthcare provider visits, with a substantial rise observed in the FreeStyle Libre group (p<0.001). This indicates a proactive strategy for managing diabetes.
This groundbreaking technology not only streamlines monitoring but also empowers individuals to take charge of their health, ultimately leading to improved outcomes in diabetes management. Furthermore, with the anticipated launch of the FreeStyle Libre 3 system in February 2025, individuals can look forward to even greater advancements in glucose monitoring technology.
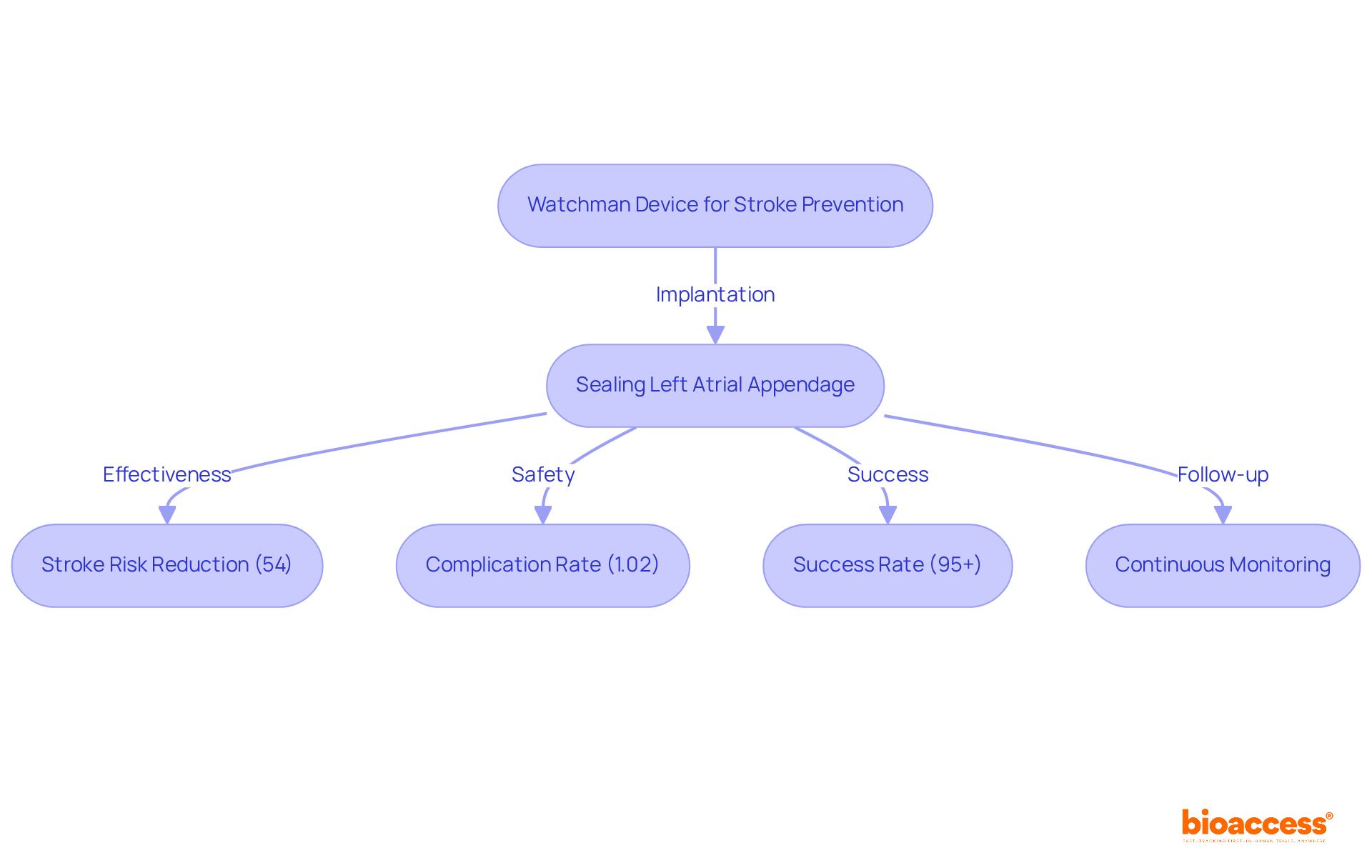
Boston Scientific's Watchman product represents a significant advancement for individuals with non-valvular atrial fibrillation at risk of stroke. By effectively sealing the left atrial appendage, where blood clots are likely to form, this implant reduces the risk of stroke substantially, offering a compelling alternative to long-term anticoagulation therapy. Clinical studies consistently demonstrate its efficacy, revealing a stroke risk reduction of up to 54%, making it a vital option for many patients.
Cardiologists have noted the device's low complication rates, with a procedural complication rate of just 1.02% for pericardial tamponade, underscoring its safety profile. However, potential complications such as bleeding and infection must also be taken into account.
The implantation procedure boasts a success rate of 95% or higher, highlighting both its effectiveness and safety. As innovations in atrial fibrillation treatment continue to evolve in 2025, the Watchman system stands out as a transformative solution, bolstered by robust clinical trial results that confirm its effectiveness in stroke prevention. Continuous monitoring following implantation is crucial to ensure the device functions correctly and to address any potential concerns.
As Dr. Chatani, a clinical proctor for Boston Scientific, states, 'The Watchman system not only decreases the risk of stroke but also provides a minimally invasive alternative for patients who cannot endure long-term anticoagulation therapy.
Stryker's Neuroform Atlas stent system is meticulously engineered for the treatment of wide-neck intracranial aneurysms, representing a significant advancement in neurovascular care. This sophisticated tool enhances support and wall apposition during stent-assisted coiling procedures, which is essential for achieving successful aneurysm occlusion. Its unique design allows for superior navigation through complex vascular anatomy, making it a preferred choice among neurovascular specialists.
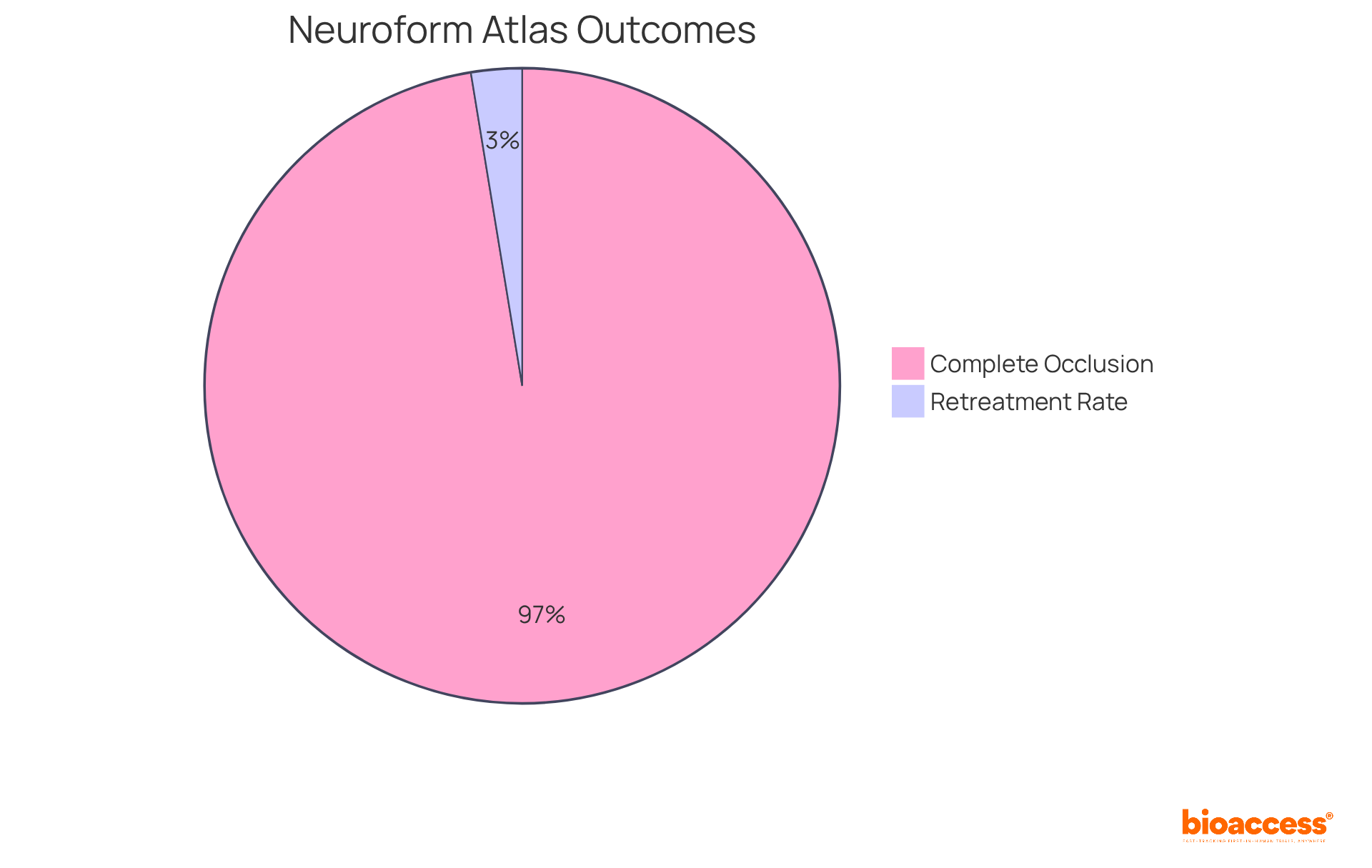
Recent studies reveal that the Neuroform Atlas stent boasts an impressive complete occlusion rate of 90.3% at the 12-month follow-up, with 92.6% of individuals who completed the follow-up achieving the primary efficacy endpoint. Furthermore, the low retreatment rate of just 2.4% significantly outperforms traditional devices like the LVIS and WEB, underscoring its effectiveness in managing challenging aneurysm cases. As the demand for innovative neurovascular solutions continues to rise, the Neuroform Atlas emerges as a pivotal instrument in enhancing outcomes for patients and improving procedural success in the treatment of intracranial aneurysms.
Boehringer Ingelheim's Respimat inhaler represents a significant advancement in inhalation therapy, particularly for those dealing with respiratory conditions like asthma and COPD. This gentle mist inhaler delivers medication in a fine mist, allowing for deeper lung penetration and enhancing user compliance. Its innovative design reduces the effort needed for inhalation, making it especially beneficial for individuals who may find traditional inhalers challenging to use.
Clinical trials have shown that patients utilizing the Respimat inhaler demonstrate improved adherence to their medication regimens, which is vital for effectively managing chronic respiratory diseases. Recent statistics reveal that the digital dose inhaler market is projected to grow at a CAGR of 19.4% from 2023 to 2030, reflecting the increasing demand for cutting-edge inhalation devices. Respiratory specialists commend the device for its user-friendly operation and its capacity to deliver consistent dosages, further establishing its significance in the realm of modern inhalation therapy innovations.
For instance, a recent study indicated that individuals using the Respimat inhaler experienced a 30% increase in adherence compared to those using traditional inhalers. This underscores the inhaler's effectiveness in improving outcomes for users, making it a compelling choice for those seeking reliable respiratory management solutions.
Teva's ProAir RespiClick is a groundbreaking breath-activated inhaler that significantly simplifies asthma management for individuals. By enabling users to deliver medication with a simple inhalation, it eliminates the complexities associated with traditional inhalers, such as priming and coordination. This user-friendly design not only enhances usability but also promotes adherence to prescribed treatment regimens, leading to improved asthma control.
According to David I. Bernstein, MD, "the approval of ProAir RespiClick is significant as it eliminates the need for hand-breath coordination during inhalation," which underscores a key benefit of this innovative device. Real-world studies have demonstrated that breath-activated inhalers like ProAir RespiClick can lead to better outcomes for individuals, with adherence rates improving significantly. A systematic review indicated that the proportion of individuals achieving well-controlled asthma increased from 22.7% to 43.7% after using digital inhalers, highlighting the effectiveness of such innovations in asthma management.
Currently, between 30% and 62% of individuals in Europe and North America have unmanaged asthma, emphasizing the urgent need for effective management solutions like ProAir RespiClick. Teva's ProAir RespiClick commands a substantial market share, reflecting its acceptance and effectiveness among healthcare professionals and patients alike. As the demand for user-friendly asthma management solutions continues to rise, ProAir RespiClick emerges as a vital tool in transforming asthma care.
Novartis' Aimovig (erenumab) stands as a groundbreaking treatment for migraine prevention, employing a novel mechanism of action that specifically targets the calcitonin gene-related peptide (CGRP). This self-injection device has shown remarkable efficacy in significantly reducing the number of migraine days for individuals, offering renewed hope for those grappling with chronic migraines. Its user-friendly design and proven effectiveness position Aimovig as an essential component in contemporary migraine management strategies.
As we delve deeper into the Medtech landscape, it's crucial to recognize the transformative role of innovative treatments like Aimovig in addressing the pressing challenges faced by patients. The integration of such advancements not only enhances patient outcomes but also underscores the importance of collaboration among healthcare professionals to optimize treatment approaches.
In conclusion, Aimovig exemplifies the potential of modern medicine to revolutionize migraine care. By embracing these innovations, we can pave the way for more effective management strategies, ultimately improving the quality of life for countless individuals suffering from migraines.

Eli Lilly's Trulicity (dulaglutide) stands out as a once-weekly injectable medication specifically designed to manage blood sugar levels in adults with type 2 diabetes, which constitutes 90 to 95 percent of all diabetes cases in the U.S. As a GLP-1 receptor agonist, it not only aids in glycemic control but also promotes weight loss, making it a comprehensive treatment option.
Clinical studies reveal that higher doses of dulaglutide can lead to significant reductions in HbA1c levels, with decreases ranging from 0.5% to 2.2% over a period of 3 to 24 months. Notably, 23.4-55.7% of individuals achieved HbA1c levels below 7.0%, showcasing the medication's effectiveness in meeting clinical targets.
Moreover, weight reductions of 2.1 to 10.4 pounds have been documented, enhancing the overall health profile of users. The convenience of once-weekly dosing significantly boosts adherence rates, which range from 27.2% to 61.0%. This improved compliance is crucial, as it correlates with better health outcomes and sustained management of the condition.
However, it is essential to acknowledge that discontinuation rates for dulaglutide fall between 26.2% and 37.0%, indicating some challenges in user experiences. Overall, Trulicity exemplifies how innovative drug-device combination products can transform care for individuals facing blood sugar challenges by integrating effective pharmacotherapy with improved user compliance.

Johnson & Johnson's OneTouch Verio solution represents a significant advancement in integrated blood sugar management, seamlessly combining glucose monitoring with a user-friendly mobile application. This innovative framework allows patients to effectively track their glucose levels while providing personalized insights that enhance self-management. By facilitating data exchange with healthcare professionals, the OneTouch Verio solution fosters a collaborative approach to managing blood sugar conditions. The focus on connectivity and user engagement not only boosts adherence to treatment plans but also leads to improved health outcomes.
Current market trends reveal a growing demand for integrated care solutions, particularly drug-device combination products, for individuals facing blood sugar challenges, with the related devices market projected to reach USD 61.2 billion by 2030, expanding at a CAGR of 12.3%. Experts in blood sugar management recognize the OneTouch Verio device as a prime example of drug-device combination products that can elevate patient experiences and outcomes in glucose care. A recent study highlighted clinically significant HbA reductions of between 1.0% and 1.4% among users, underscoring the effectiveness of this approach.
As the competitive landscape evolves, the OneTouch Verio solution distinguishes itself among key players in the diabetes care devices market, emphasizing the significance of drug-device combination products in diabetes management. For clinical research directors, leveraging such integrated systems can significantly enhance patient engagement and improve overall treatment efficacy. What challenges do you face in your clinical research efforts? Consider how solutions like OneTouch Verio could address those needs and drive better outcomes.
Innovative drug-device combination products are reshaping the future of healthcare, offering integrated solutions that enhance treatment effectiveness and patient management. These advancements streamline chronic condition care and underscore the importance of collaboration among stakeholders to tackle the complexities of implementation. This article showcases various groundbreaking products, from bioaccess®'s accelerated development processes to Medtronic's MiniMed 770G insulin delivery system, each playing a pivotal role in improving patient outcomes.
Key insights from the discussion reveal the transformative potential of these innovations. For instance, the Abbott FreeStyle Libre has revolutionized glucose monitoring, while Boston Scientific's Watchman device significantly reduces stroke risk for patients with atrial fibrillation. Each product emphasizes the necessity for regulatory agility and user-friendly designs, contributing to enhanced adherence and overall health management. Moreover, the rise of integrated solutions, such as Johnson & Johnson's OneTouch Verio, underscores the growing demand for cohesive care strategies in diabetes management.
As the healthcare landscape continues to evolve, embracing these innovative drug-device combinations is crucial for improving patient care and outcomes. Stakeholders are encouraged to explore how such technologies can address existing challenges in clinical research and patient management. The future of healthcare lies in the synergy between technology and patient-centric solutions, paving the way for more effective and accessible treatment options for all.
What is bioaccess® and what does it focus on?
bioaccess® is a company that leverages over 15 years of clinical research expertise to accelerate the development of drug-device combination products, with a particular focus on Latin America's regulatory processes, especially in Colombia.
How long does the regulatory review process take with bioaccess® in Colombia?
The total IRB/EC and MoH (INVIMA) review process in Colombia takes just 90-120 days.
What services does bioaccess® provide?
bioaccess® offers services including feasibility studies, research site selection, investigator recruitment, regulatory compliance, project management, and thorough reporting on study status and adverse events.
What advantages does bioaccess® provide in clinical research?
bioaccess® enhances the speed of introducing new therapies to patients, highlights the importance of regulatory agility, and offers cost efficiency, with savings of over 30% compared to trials in North America or Western Europe.
What is the Medtronic MiniMed 770G?
The Medtronic MiniMed 770G is a hybrid closed-loop insulin delivery system that autonomously adjusts insulin delivery based on real-time glucose readings, improving diabetes management.
How does the MiniMed 770G impact diabetes management?
The device enhances glycemic control, effectively reducing risks associated with hyperglycemia and hypoglycemia, and has been associated with improved A1C levels and a decrease in diabetes-related complications.
What is the Abbott FreeStyle Libre and how does it function?
The Abbott FreeStyle Libre is a continuous glucose monitoring (CGM) device that provides real-time glucose readings through a small sensor worn on the skin, eliminating the need for routine fingersticks.
What are the benefits of using the FreeStyle Libre system?
Users have experienced significant reductions in HbA1c levels, increased awareness of glucose levels, and more frequent healthcare provider visits, leading to improved diabetes management outcomes.
What upcoming advancements can users expect from Abbott's FreeStyle Libre?
The anticipated launch of the FreeStyle Libre 3 system in February 2025 promises even greater advancements in glucose monitoring technology.