


The primary objective of the article titled "10 Insights on Phase 3 Clinical Trial Success Strategies" is to delineate effective strategies for ensuring the success of Phase 3 clinical trials. It underscores the significance of:
as essential components that elevate the probability of regulatory approval and favorable outcomes in clinical research.
Phase 3 clinical trials represent a critical juncture in the journey of medical innovations, serving as the final test before therapies can reach the market. These trials not only validate the efficacy and safety of new treatments but also play a pivotal role in shaping the future of healthcare. As the stakes rise, it becomes essential for Medtech companies to understand the strategies that lead to successful Phase 3 trials, enabling them to navigate the complexities of drug development. What are the key insights and best practices that can enhance the likelihood of success in these high-stakes studies?
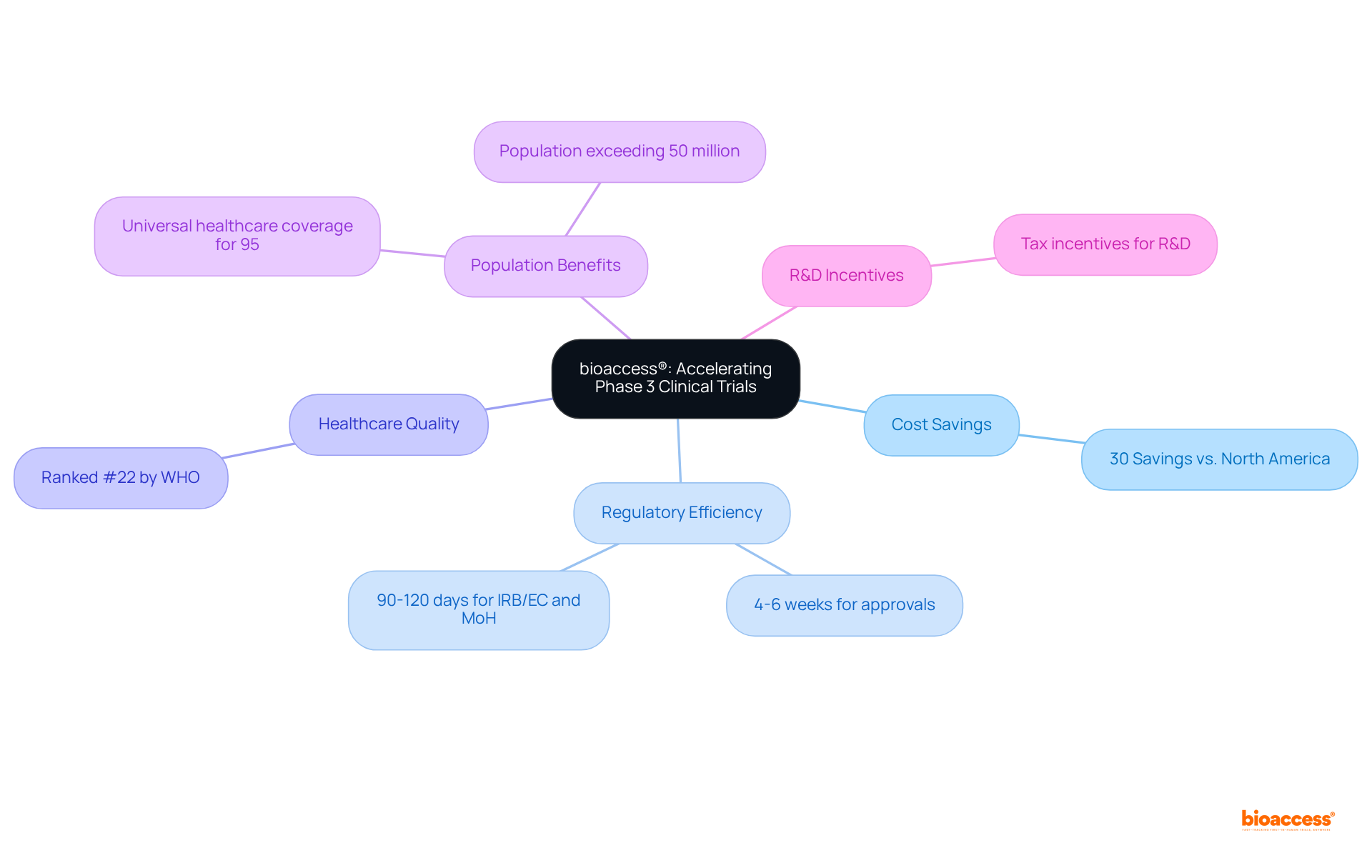
bioaccess® excels at accelerating phase 3 clinical trials by leveraging its strategic sites across Latin America, particularly in Colombia, the Balkans, and Australia. Notably, Colombia provides significant cost savings exceeding 30% compared to North America and Western Europe, coupled with a rapid regulatory approval process that typically spans just 4-6 weeks. This efficiency empowers Medtech innovators to navigate the complexities of clinical trials adeptly, enhancing their prospects for swift commercialization. The overall IRB/EC and MoH (INVIMA) assessment in Colombia requires only 90-120 days, ensuring that research studies can progress without unnecessary delays.
Moreover, Colombia's healthcare system is highly esteemed, ranked #22 by the World Health Organization, with hospitals recognized as some of the finest in Latin America. This high-quality healthcare environment, combined with a population exceeding 50 million and universal healthcare coverage for 95% of the populace, facilitates effective recruitment of participants. Additionally, investments in science, technology, and innovation initiatives in Colombia benefit from R&D tax incentives, making it an even more attractive location for research studies.
By partnering with bioaccess®, Medtech companies can significantly enhance their overall success rate in research studies, ensuring their innovations reach individuals more quickly and effectively.
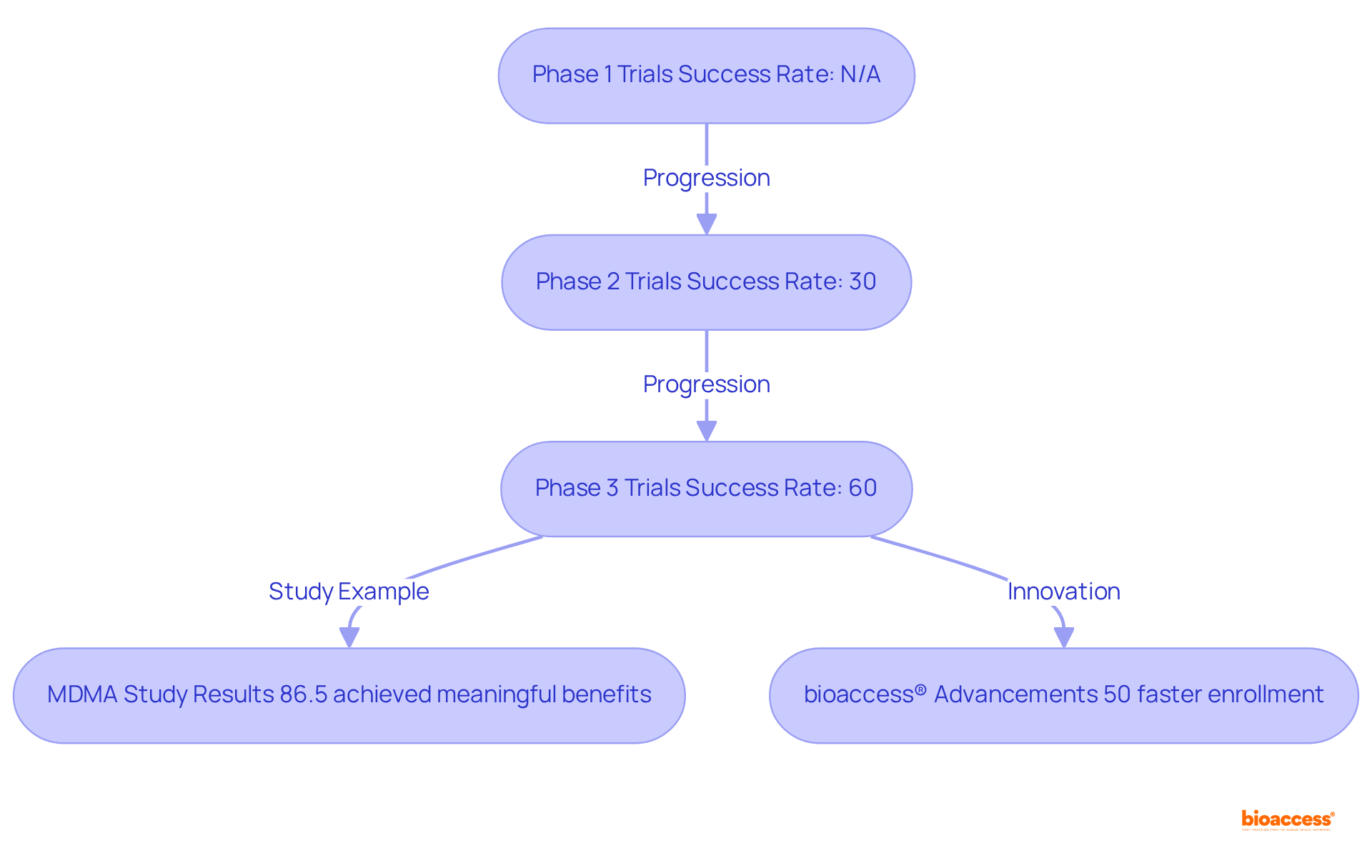
Phase 3 clinical trials are pivotal in determining the efficacy and safety of new therapies prior to regulatory approval. Typically involving extensive participant groups, phase 3 clinical trials are designed to validate findings from earlier Stage 1 and Stage 2 research. Notably, the success rates of phase 3 clinical trials are considerably higher than those of their predecessors, with approximately 60% resulting in regulatory approval, compared to just 30% for phase 2 clinical trials. Recent discoveries underscore the effectiveness and safety of therapies evaluated in Stage 3, with numerous studies revealing significant improvements in patient outcomes and quality of life—elements crucial for patient endorsement.
Successful phase 3 clinical trials yield robust data essential for regulatory submissions, marking them as a critical juncture in the drug development pipeline. For example, the MDMA-assisted therapy (MDMA-AT) study for PTSD showcased a remarkable reduction in symptoms, with 86.5% of participants achieving clinically meaningful benefits. Such results not only validate the therapeutic advantages but also highlight the treatment's impact on individuals' daily lives, reinforcing the importance of these studies within the broader clinical research landscape.
Moreover, bioaccess® enhances the efficiency of these studies by enabling treatment-naive cardiology or neurology groups to be enrolled 50% faster than traditional Western sites, resulting in substantial savings of $25K per individual with FDA-ready data—effectively eliminating rework and delays. This capability is exemplified by ReGelTec's Early Feasibility Study on HYDRAFIL™, where eleven individuals suffering from chronic low back pain were successfully treated in Colombia. This research illustrates the potential for expedited patient enrollment and favorable treatment outcomes, emphasizing how bioaccess contributes to the success of the phase 3 clinical trial. As experts assert, Stage 3 studies are essential in validating treatment efficacy, ultimately shaping the future of therapeutic methodologies.
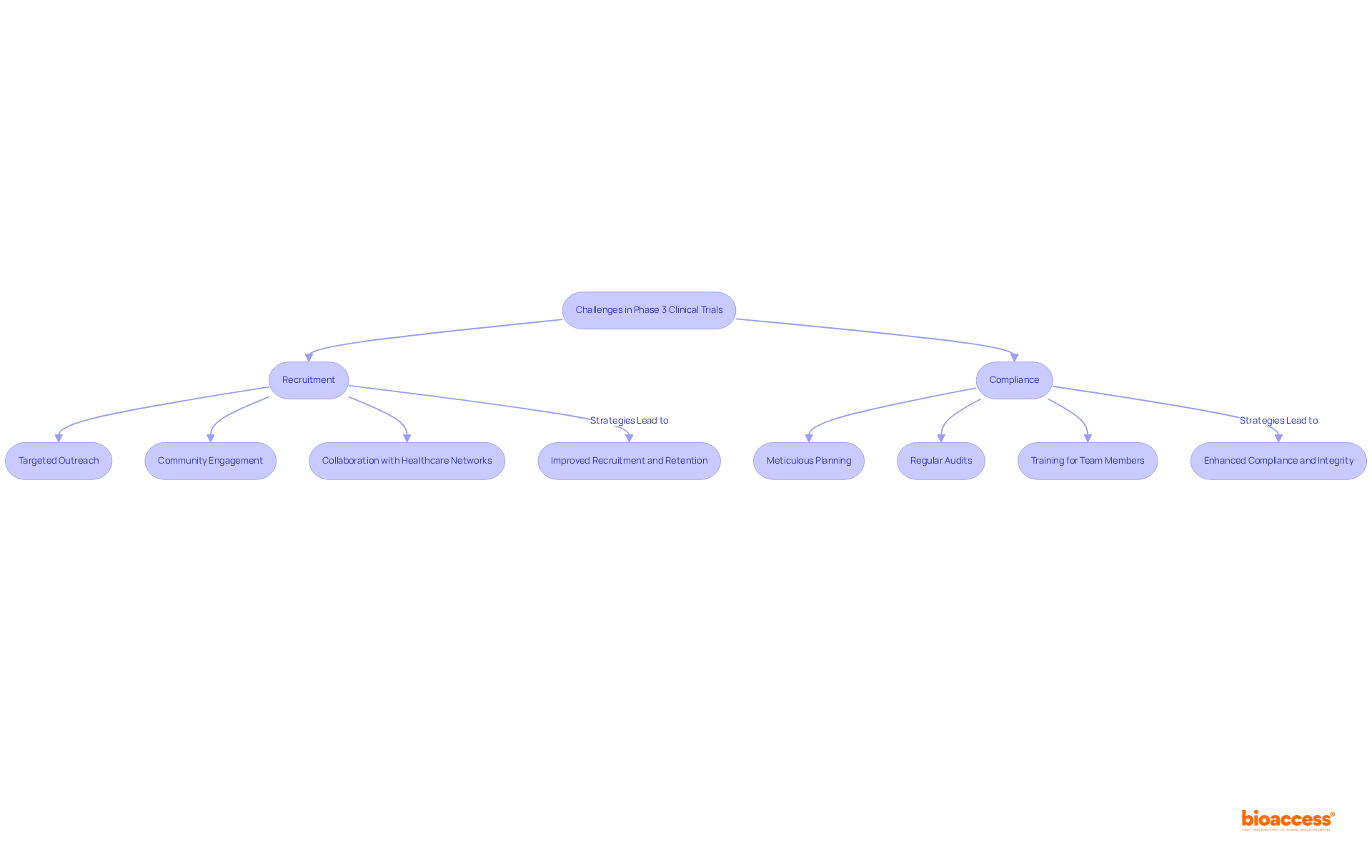
Recruitment and compliance stand as two of the most pressing challenges in the phase 3 clinical trial. Enrolling a sufficient number of eligible participants, especially from diverse populations, poses significant difficulties. As Marta Garcia Manrique, R&D Chief Officer for Individuals at Servier, notes, "Today, participant recruitment for clinical studies occurs mainly through the medical staff of the research center." While this method is crucial, it does have its limitations. To bolster recruitment efforts, strategies such as:
are vital. These approaches not only enhance awareness but also build trust within communities, thereby encouraging participation.
Equally critical is the necessity of maintaining adherence to regulatory requirements, which safeguards the integrity of the study. This requires meticulous planning, regular audits, and comprehensive training for all team members to ensure compliance with protocols and ethical standards. Utilizing efficient management software can facilitate communication and adherence to protocols, while compassionate personnel significantly enhance the experiences of participants, ultimately leading to improved retention rates. Notably, statistics reveal that individuals who found site visits stressful had a dropout rate of 38%, compared to just 16% for those who completed the process. By proactively addressing these challenges, research studies can achieve greater success and significantly contribute to medical advancements, particularly in phase 3 clinical trials. Furthermore, bioaccess®'s expertise in navigating regulatory landscapes and expediting clinical studies positions it uniquely to support these initiatives, ensuring that groundbreaking treatments reach patients more effectively.
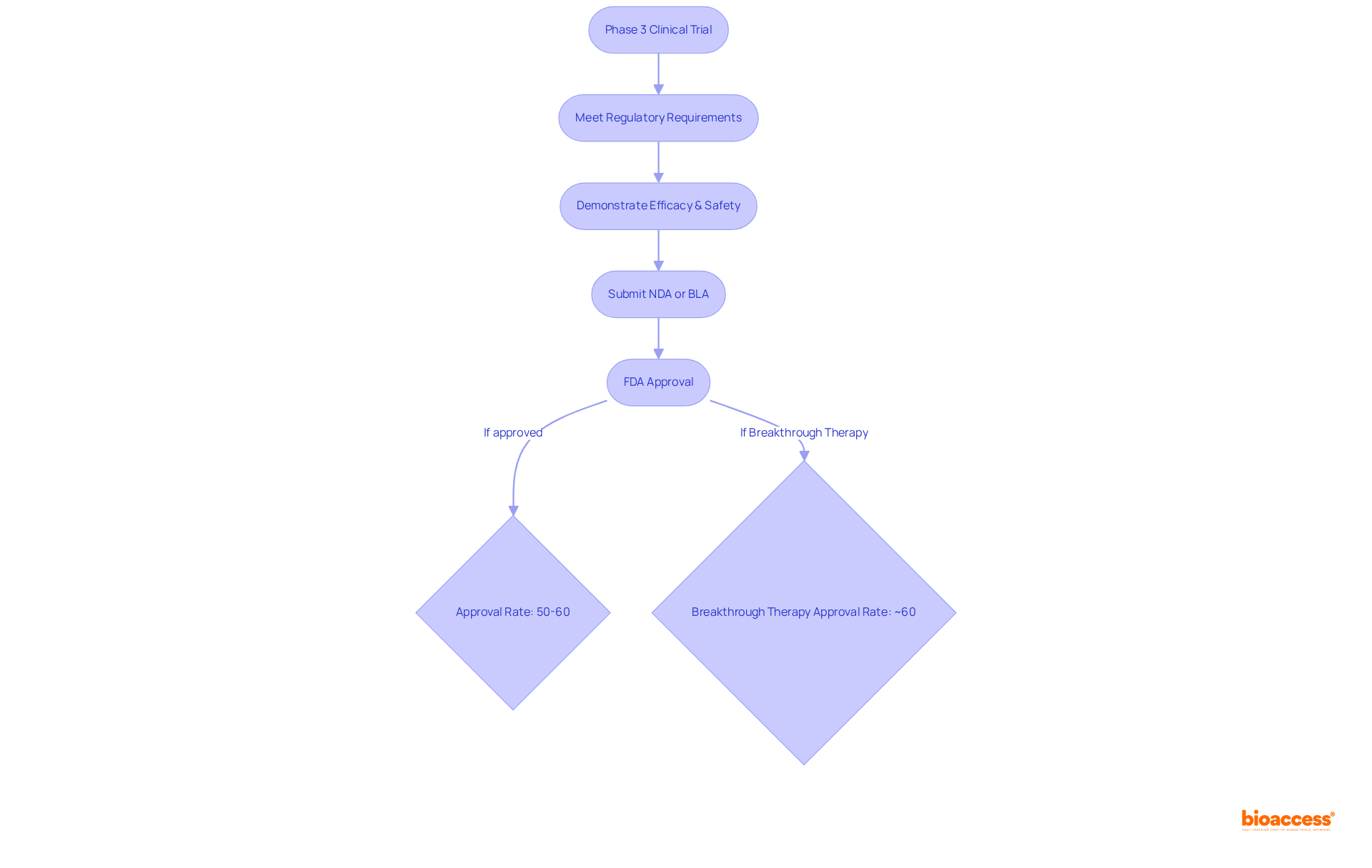
The phase 3 clinical trial is pivotal in the regulatory pathway to FDA approval, serving as the final step before a drug can be marketed. These studies are meticulously crafted to meet specific regulatory requirements, particularly the demonstration of statistical significance in both efficacy and safety. The information generated during the phase 3 clinical trial is indispensable, forming the backbone of the New Drug Application (NDA) or Biologics License Application (BLA) submitted to the FDA.
Approximately 50%-60% of medications that enter a phase 3 clinical trial ultimately secure FDA approval, underscoring the critical nature of this stage in the drug development process. Regulatory specialists emphasize that a well-organized phase 3 clinical trial can significantly enhance the likelihood of approval, as it provides comprehensive evidence of a drug's benefits and risks.
Recent updates reveal that the FDA is increasingly focused on accelerating the review process for drugs demonstrating substantial improvements over existing therapies, particularly those designated as Breakthrough Therapy, which enjoy an approval rate of around 60%. Understanding these regulatory pathways is essential for Medtech firms to ensure their studies are compliant and strategically aligned for successful approval.
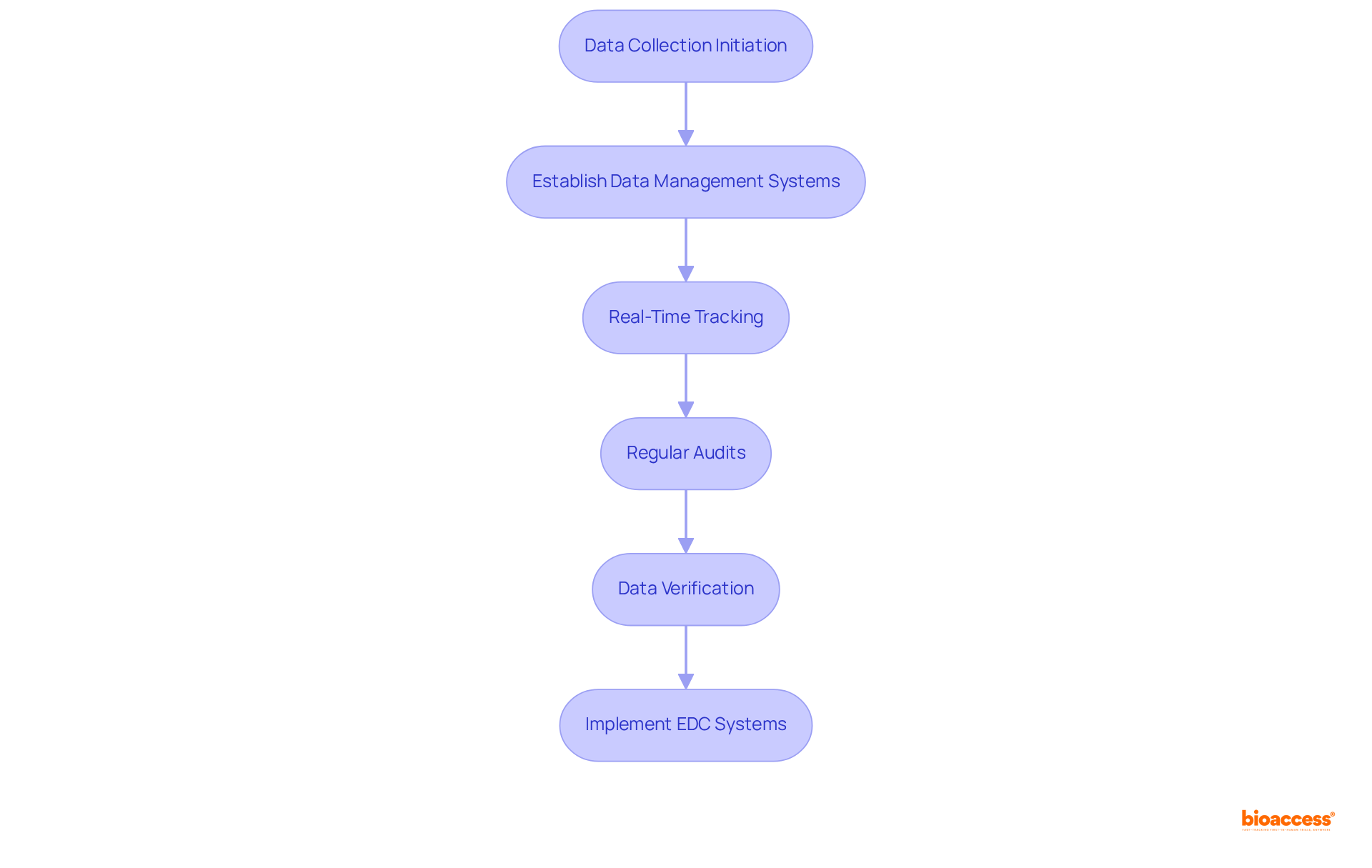
Efficient data gathering and oversight are paramount for ensuring the reliability of Stage 3 study outcomes. Establishing robust data management systems facilitates real-time tracking of study progress and participant results, which is essential given that the average phase 3 clinical trial protocol now collects approximately 3.5 million data points—seven times more than 15 years ago.
Regular audits and data verification processes are essential for early identification of discrepancies, enabling timely corrective actions. Furthermore, the implementation of electronic data capture (EDC) systems significantly streamlines data collection, enhances accuracy, and improves overall efficiency.
This shift towards advanced data management solutions is critical to managing the increasing volume and complexity of information generated in modern medical studies, ultimately leading to more reliable outcomes.
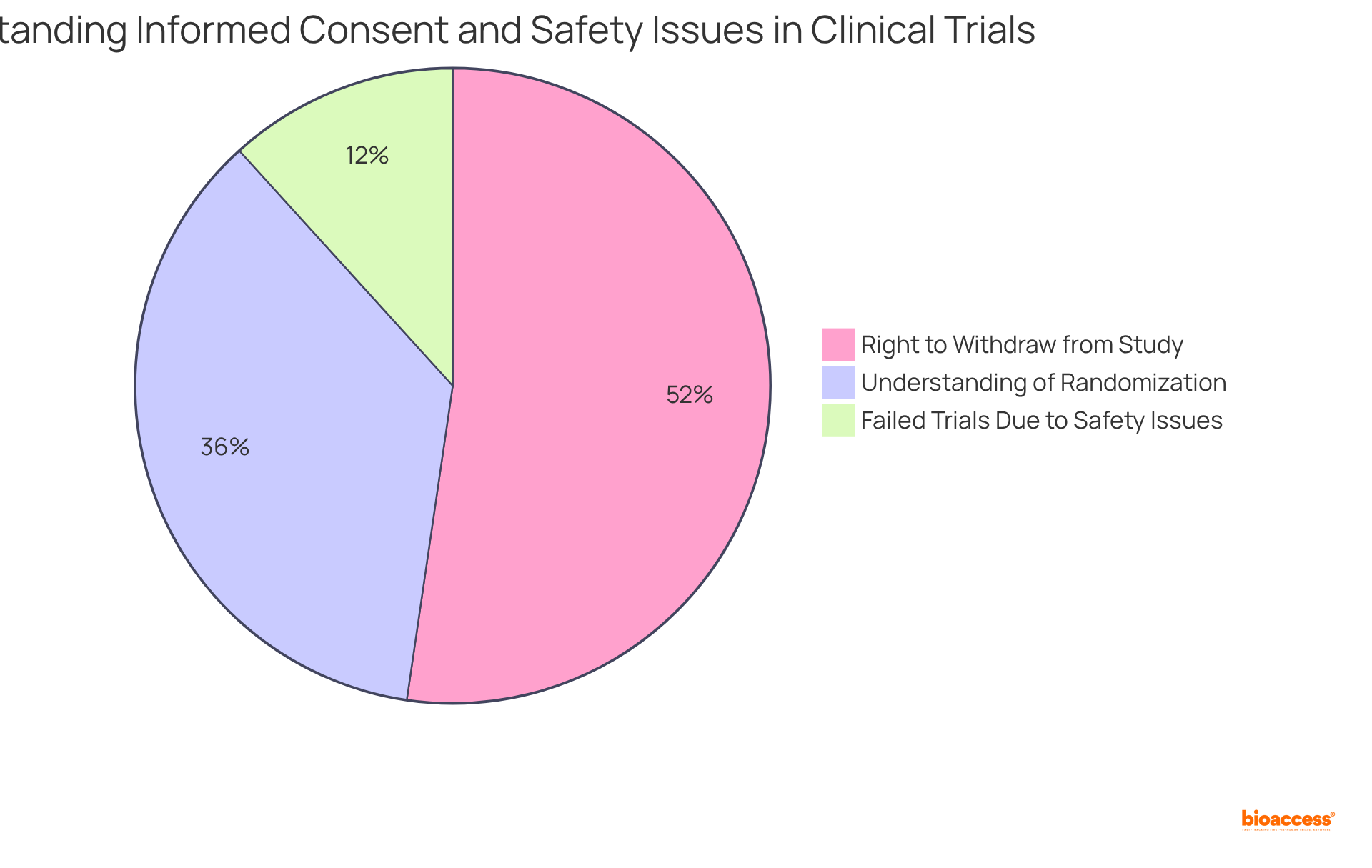
Informed consent stands as a cornerstone of ethical practice in phase 3 clinical trial studies, ensuring that participants are fully aware of the study's purpose, procedures, risks, and benefits. Recent studies reveal a significant variance in understanding the components of informed consent, with only 52.1% of participants grasping the concept of randomization and 75.8% recognizing their right to withdraw at any time. This highlights the urgent need for improved communication strategies aimed at enhancing participant comprehension.
Moreover, the safety of participants is paramount. Continuous monitoring for adverse occurrences is crucial, as data indicates that 17% of unsuccessful phase 3 clinical trials are linked to safety concerns. Implementing robust protocols to swiftly address any safety issues not only protects participants but also bolsters the credibility of the study results. By adhering to ethical standards, researchers can foster trust and transparency, ultimately contributing to the success of medical studies.
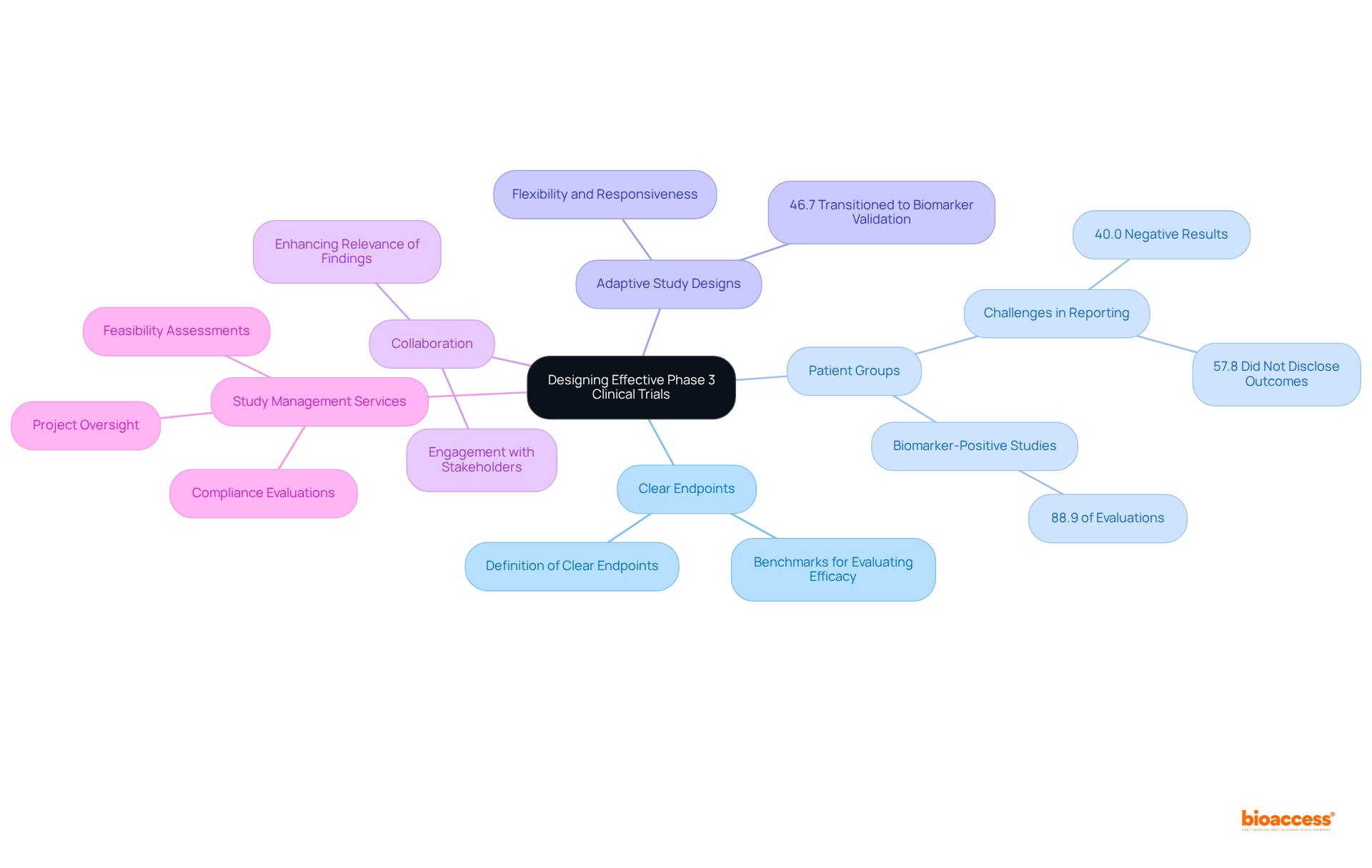
Creating effective phase 3 clinical trial studies necessitates a meticulous approach to several essential components. Central to this is the definition of clear endpoints, which act as benchmarks for evaluating treatment efficacy. Choosing appropriate groups of individuals is crucial; recent discoveries indicate that studies utilizing biomarker-specific designs can significantly enhance the likelihood of favorable results. For instance, research reveals that 88.9% of evaluations employed a biomarker-positive and overall design, underscoring the importance of focusing on specific patient subgroups. Nonetheless, it is vital to acknowledge that 40.0% of studies reported negative results in both biomarker-positive and overall populations, illustrating the challenges encountered in achieving successful outcomes. Furthermore, 57.8% of studies did not disclose primary outcome measure values by treatment arm in biomarker-negative populations, emphasizing the necessity for comprehensive reporting in study designs.
Incorporating adaptive study designs further augments the flexibility and responsiveness of research to emerging data. These designs allow for real-time modifications based on interim assessments, potentially leading to more effective study completion and improved patient outcomes. Notably, 46.7% of experiments initially structured as conventional phase 3 clinical trials transitioned to predictive biomarker validation studies during their execution, highlighting the evolving nature of study methodologies. The FDA's endorsement of pembrolizumab in 2018 as the first medication for a tissue-agnostic indication exemplifies the impact of adaptive study designs in oncology.
Collaboration with key stakeholders, including regulatory agencies and patient advocacy organizations, is essential for gathering insights that inform study design and implementation. Engaging these groups can enhance the relevance and applicability of research findings, ultimately facilitating the successful advancement of innovative therapies. Additionally, leveraging extensive study management services—such as feasibility assessments, compliance evaluations, study setup, import permits, project oversight, and reporting procedures provided by bioaccess—can optimize the design process. By concentrating on these strategies, researchers can refine the phase 3 clinical trial designs, ensuring they are well-equipped to confront the challenges of contemporary medical research.
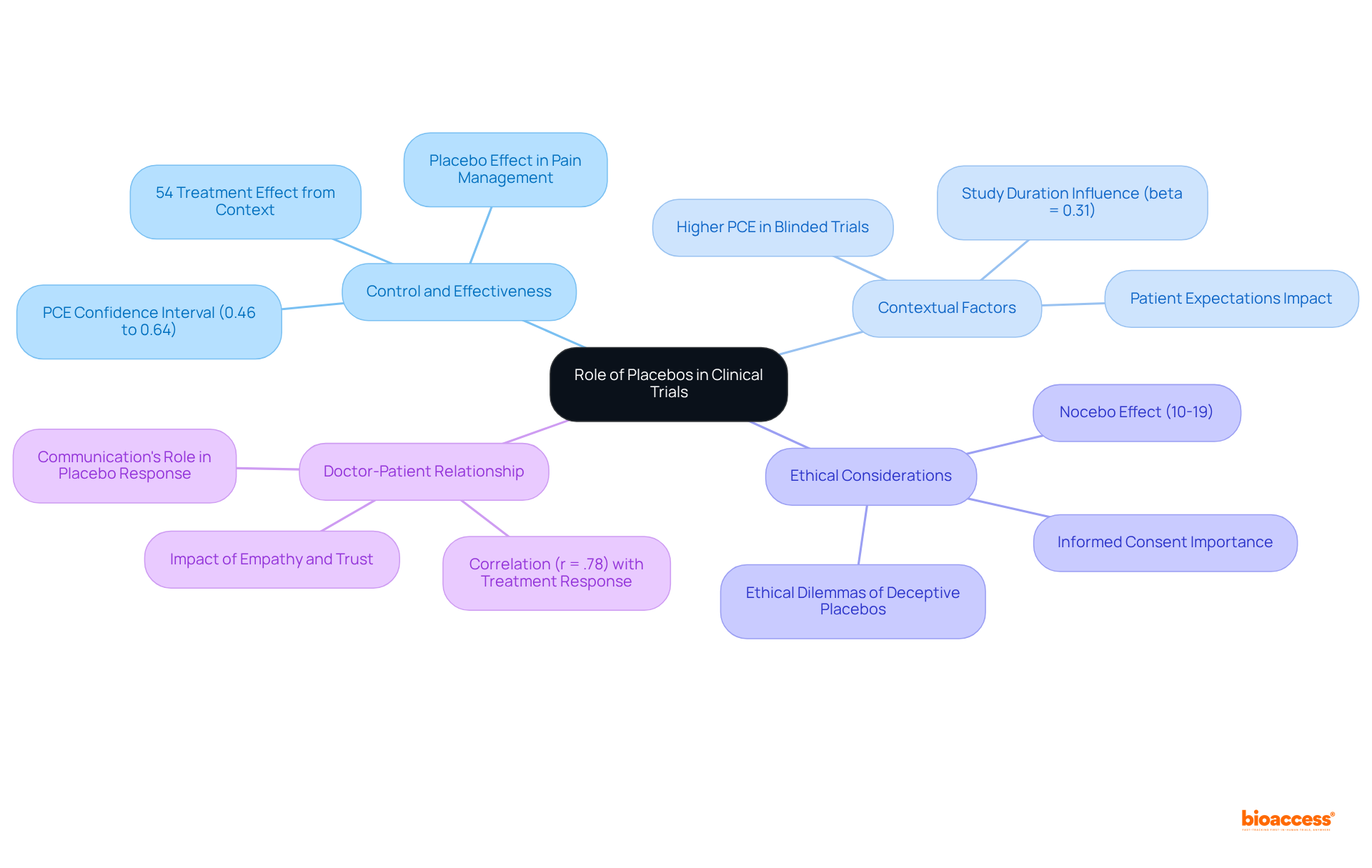
Placebos play a critical role in phase 3 clinical trial studies, acting as a control to evaluate the effectiveness of investigational treatments. Their application reduces bias and establishes a benchmark for measuring treatment effects. A meta-analysis of 186 studies, stemming from an initial review of 202 studies, indicated that roughly 54% of the overall treatment effect can be linked to contextual factors. This corresponds to a pooled Proportional Contextual Effect (PCE) with a 95% confidence interval of 0.46 to 0.64, underscoring the significant influence placebos exert on study outcomes.
However, ethical considerations regarding their use are of utmost importance, particularly in relation to informed consent and the potential ramifications for patient well-being. For instance, the nocebo effect—where negative expectations lead to adverse outcomes—affects 10-19% of studies, highlighting the necessity for meticulous communication with participants.
Striking a balance between the scientific rigor of placebo utilization and ethical responsibilities is vital for preserving the integrity of clinical research. Recent investigations emphasize that the doctor-patient relationship and patient expectations substantially impact placebo responses, evidenced by a strong correlation (r = .78) between improvement rates with placebo and treatment. These elements should be integrated into study designs to bolster overall treatment efficacy.
Stage 3 studies are pivotal in shaping market competition by determining which therapies achieve market accessibility. These trials are fundamental for introducing groundbreaking therapies that expand treatment options for patients. For instance, successful Phase 3 studies can lead to the approval of innovative treatments, significantly enhancing care and outcomes for individuals. Conversely, studies that fail to demonstrate effectiveness can obstruct the introduction of new therapies, thereby impacting market dynamics and limiting choices for healthcare providers and patients alike.
Recent trends indicate a decline in the success rates of treatments progressing to market post-Phase 3 evaluations, with only about 10% of drug candidates securing FDA approval. This underscores the critical need for strategic planning and robust study design to navigate the complexities of clinical development. A comprehensive understanding of the competitive landscape is vital for Medtech companies, empowering them to effectively position their products and capitalize on emerging opportunities in a swiftly evolving market.
Phase 3 clinical trials are pivotal in advancing medical therapies, representing the final hurdle before regulatory approval. These trials generate essential evidence regarding the safety and efficacy of new treatments, which is vital for improving patient outcomes. Successful Stage 3 studies not only facilitate the launch of innovative therapies but also significantly advance the overall understanding of medicine.
By enabling the introduction of new treatments, Stage 3 studies enhance the quality of care and effectively address unmet medical needs. For instance, recent phase 3 clinical trials have demonstrated remarkable effectiveness in tackling challenges such as advanced cancer, underscoring how rigorous research can lead to substantial improvements in survival rates and quality of life for patients.
The insights derived from these trials are invaluable; they inform clinical practices and direct future research endeavors, ultimately benefiting both healthcare professionals and patients.
Phase 3 clinical trials are a pivotal stage in the journey of medical innovations, acting as the final checkpoint before a treatment can gain approval for public use. The insights shared illustrate how leveraging strategic locations, such as Colombia, can substantially enhance the efficiency and cost-effectiveness of these trials. By grasping the intricacies of Phase 3 studies, Medtech companies can adeptly navigate the complexities of regulatory approval and expedite the delivery of life-changing therapies to patients.
Key arguments presented throughout the article underscore the importance of:
Furthermore, the role of bioaccess® in streamlining processes and tackling challenges such as compliance and participant engagement is crucial. These factors collectively contribute to the elevated success rates observed in Phase 3 trials, reinforcing their significance in the broader context of drug development.
Ultimately, the success of Phase 3 clinical trials represents not just a victory for the companies involved but a vital step toward enhancing patient care and broadening treatment options within the healthcare market. As the medical field continues to evolve, embracing best practices and innovative strategies will be essential for overcoming challenges and ensuring that new therapies reach those in need. Engaging with these insights empowers stakeholders to make informed decisions that enrich the landscape of medical research and elevate patient outcomes.
What is bioaccess® and how does it support phase 3 clinical trials?
bioaccess® accelerates phase 3 clinical trials by leveraging strategic sites across Latin America, particularly in Colombia, the Balkans, and Australia. It helps Medtech innovators navigate the complexities of clinical trials, enhancing their prospects for swift commercialization.
What are the cost benefits of conducting clinical trials in Colombia?
Conducting clinical trials in Colombia offers significant cost savings exceeding 30% compared to North America and Western Europe. Additionally, the regulatory approval process typically takes just 4-6 weeks.
How long does the overall assessment process take for research studies in Colombia?
The overall IRB/EC and MoH (INVIMA) assessment in Colombia requires only 90-120 days.
What is the significance of phase 3 clinical trials in drug development?
Phase 3 clinical trials are crucial for determining the efficacy and safety of new therapies before regulatory approval. They validate findings from earlier research stages and have a higher success rate, with approximately 60% resulting in regulatory approval.
How does bioaccess® improve participant enrollment in phase 3 trials?
bioaccess® enables treatment-naive cardiology or neurology groups to be enrolled 50% faster than traditional Western sites, resulting in substantial savings and effective data collection.
What challenges are associated with phase 3 clinical trials?
The main challenges include participant recruitment and compliance with regulatory requirements. Enrolling a diverse participant group can be difficult, and maintaining adherence to protocols is essential for study integrity.
What strategies can enhance participant recruitment for clinical trials?
Effective recruitment strategies include targeted outreach, community engagement, and collaboration with local healthcare networks to build trust and awareness within communities.
Why is compliance important in phase 3 clinical trials?
Compliance is critical to safeguard the integrity of the study. It requires meticulous planning, regular audits, and comprehensive training for team members to adhere to protocols and ethical standards.
How does bioaccess® address the challenges in phase 3 clinical trials?
bioaccess® leverages its expertise in navigating regulatory landscapes and expediting clinical studies to support recruitment and compliance initiatives, ensuring that innovative treatments reach patients more effectively.