


This article presents a crucial overview of ten ISO 13485 audit firms in Mexico, which play an essential role in ensuring compliance within the Medtech sector. Each firm listed provides specialized services, reflecting a strong commitment to assisting medical device manufacturers in efficiently and effectively navigating regulatory requirements. Such support is vital for enhancing product quality and ensuring market readiness, ultimately contributing to the advancement of healthcare solutions.
In the rapidly evolving landscape of medical technology, achieving ISO 13485 certification stands as a pivotal milestone for manufacturers seeking to ensure quality and compliance. This certification not only enhances operational efficiency but also strategically positions companies in a market where regulatory standards are continuously tightening.
However, with a multitude of audit firms available, how can Medtech startups and established companies effectively navigate the maze of options to identify the right partner for their certification journey?
This article presents a curated list of the top ISO 13485 audit firms in Mexico, each offering unique strengths and services that can significantly influence a company’s path to compliance and market readiness.
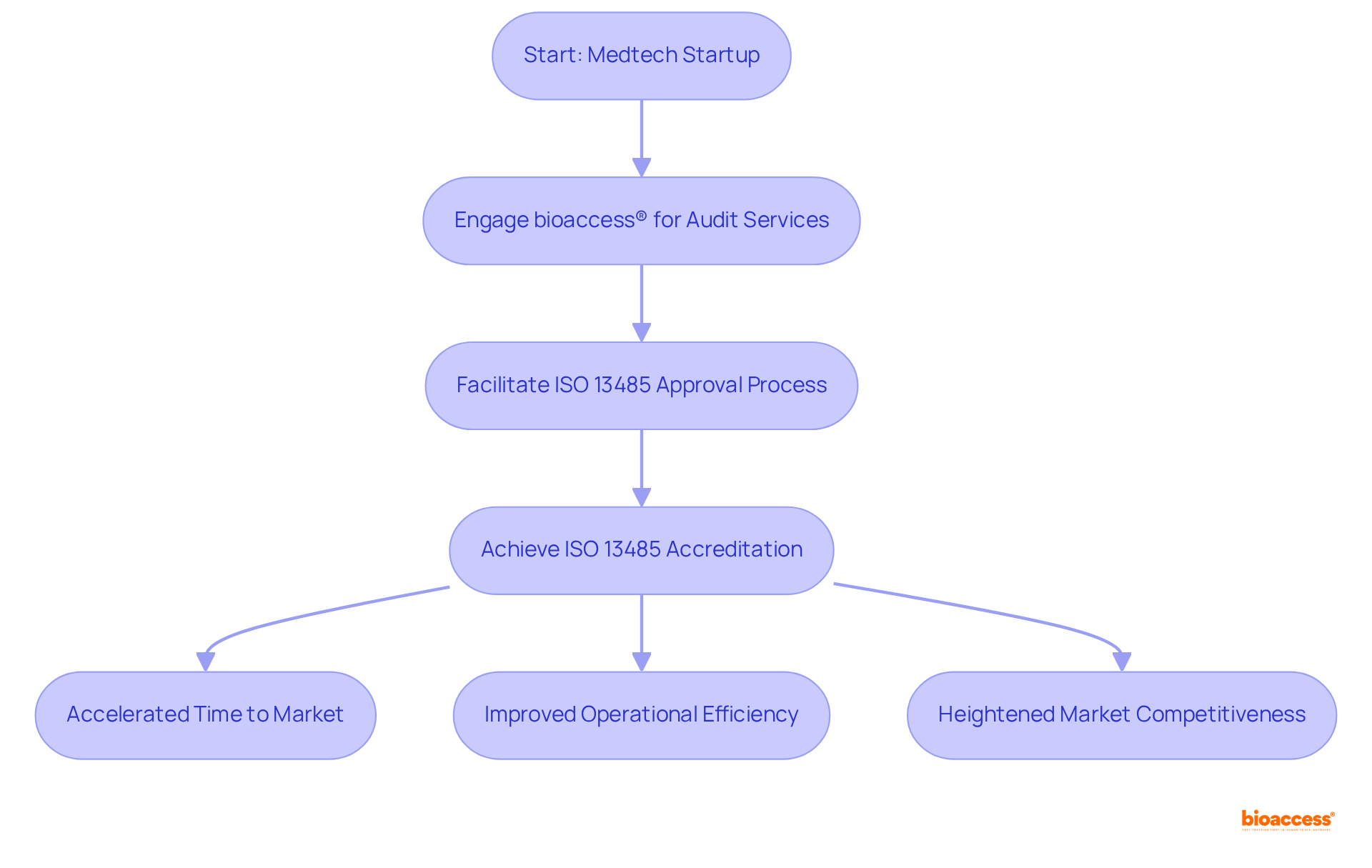
bioaccess® specializes in expedited ISO 13485 audit services tailored for Medtech startups and is recognized in the ISO 13485 audit firms Mexico list, leveraging its profound understanding of regulatory frameworks across Latin America, the Balkans, and Australia.
In 2025, the average duration to achieve ISO 13485 approval in Latin America is significantly reduced, with bioaccess® facilitating this process to ensure startups can secure approval within weeks rather than months. This swift approach not only accelerates time to market but also substantially enhances standards, establishing bioaccess® as a vital ally for innovative medical device firms.
Successful cases of ISO 13485 accreditation among Medtech companies demonstrate the tangible benefits of this qualification, as outlined in the iso 13485 audit firms mexico list, including improved operational efficiency and heightened market competitiveness.
Industry leaders consistently highlight the essential role of ISO 13485 compliance in nurturing a culture of quality and safety, underscoring its significance within the Medtech sector.
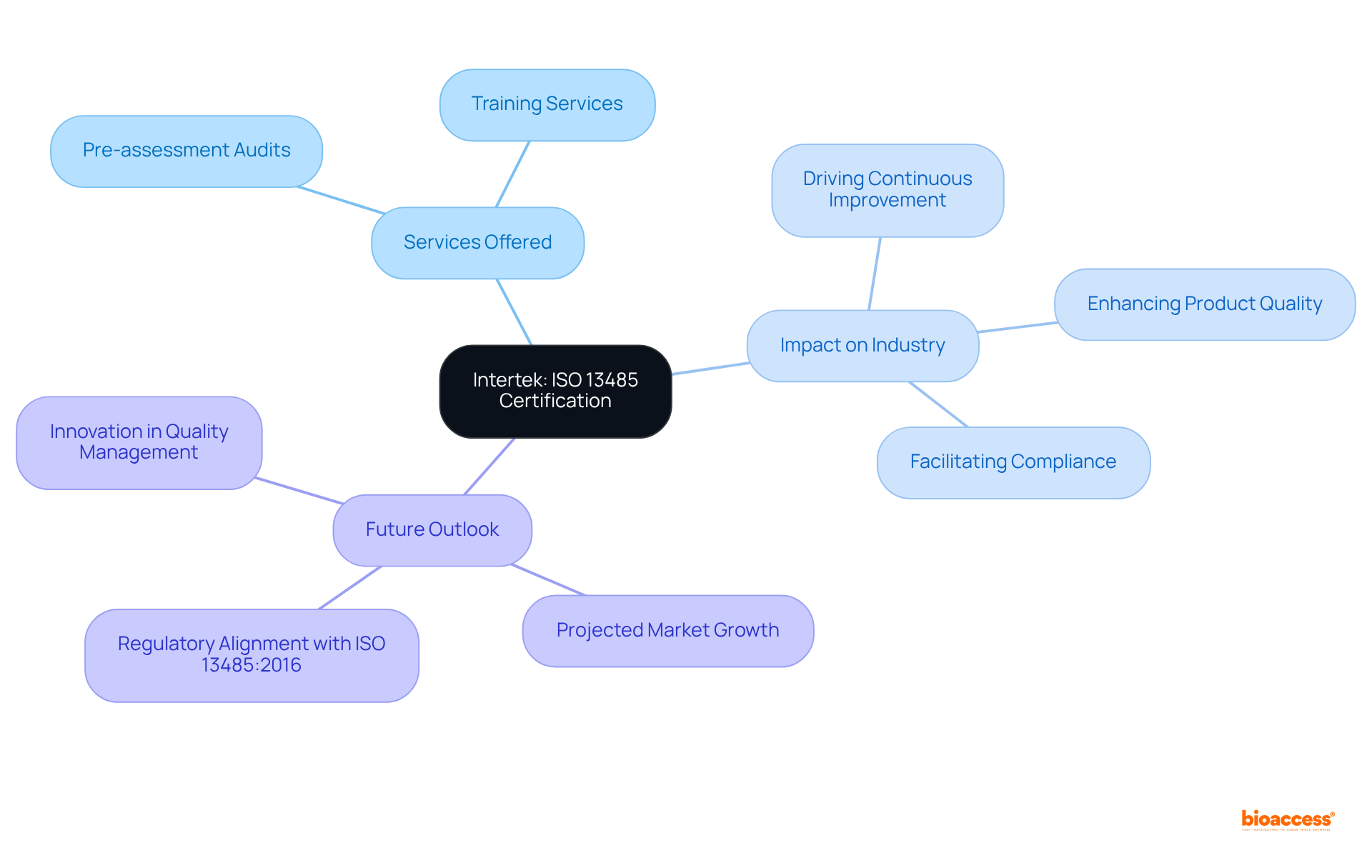
Intertek is recognized on the ISO 13485 audit firms Mexico list as a global leader in ISO 13485 accreditation and auditing services, offering essential support to medical device manufacturers as they navigate the complexities of compliance.
With a vast network of specialists, Intertek ensures that clients receive tailored assistance, including:
This comprehensive approach not only aids producers in meeting local and global regulatory standards but also fosters a culture of continuous improvement in management practices.
In 2025, Intertek issued a substantial number of ISO 13485 approvals, exemplifying its commitment to enhancing product quality and safety within the Medtech sector.
As the industry evolves, Intertek's role in facilitating compliance and driving innovation becomes increasingly vital, positioning manufacturers for success in a competitive landscape.
DNV provides specialized services for ISO 13485 audit firms Mexico list, tailored specifically for the medical device industry. With experienced auditors who possess a profound understanding of the unique challenges manufacturers encounter, DNV offers valuable insights that enhance the approval process. Recognized as an auditing entity by the Medical Device Single Audit Program (MDSAP), DNV bolsters its credibility in the approval process.
In 2025, DNV's auditing services were acknowledged for their effectiveness in navigating the complexities of regulations, with the average duration for ISO 13485 certification typically spanning from three to six months. This efficiency is critical, especially as the global Management Systems Certification market is projected to grow from approximately $25 billion in 2023 to $40 billion by 2032, underscoring the increasing significance of regulatory standards in the industry.
Furthermore, DNV's engagement in the TCP III initiative facilitates the sharing of audit reports, streamlining regulatory procedures and enhancing assurance and safety for medical devices. DNV's unwavering commitment to excellence and safety empowers clients to confidently demonstrate adherence to international standards, thereby enhancing their market readiness and fostering trust among stakeholders.
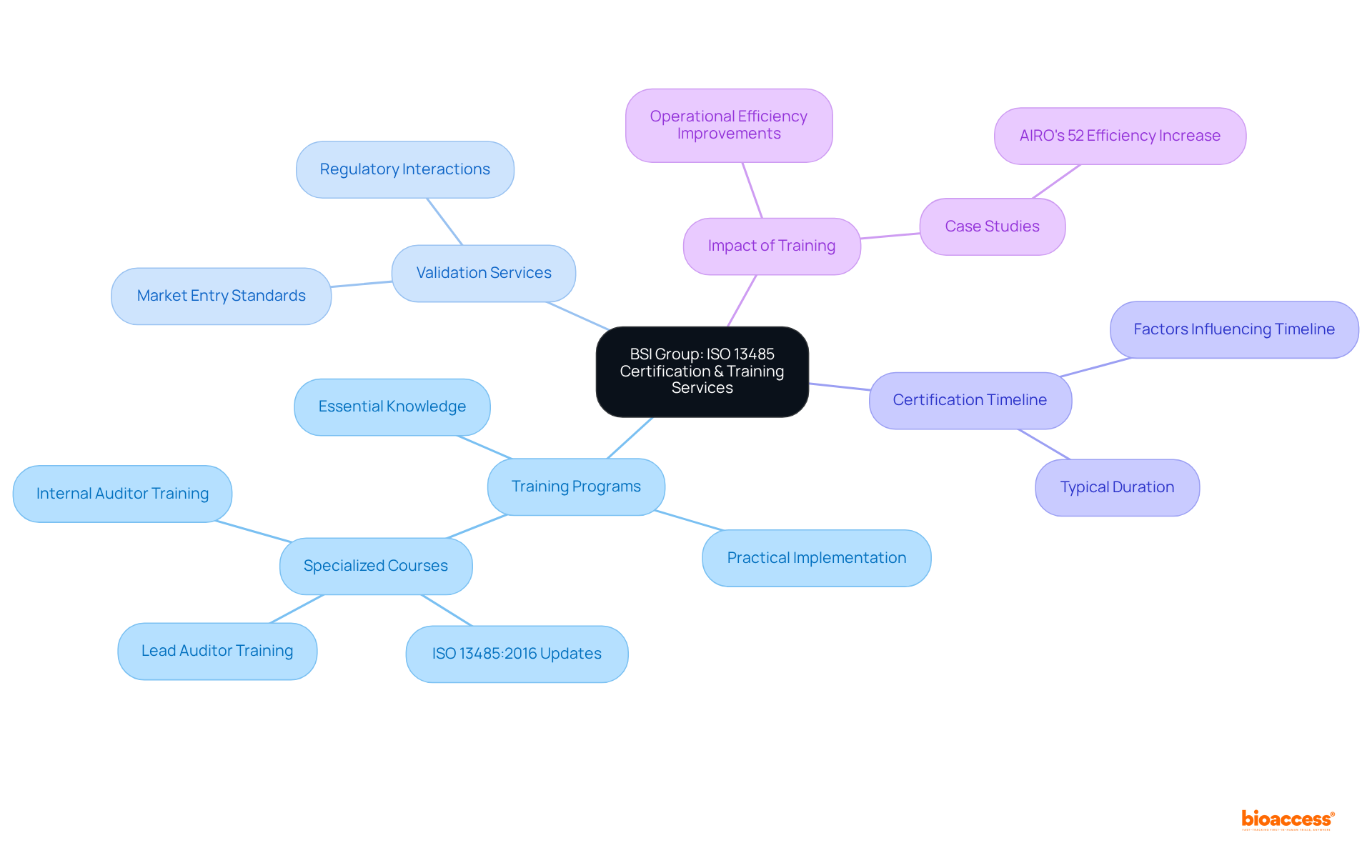
BSI Group provides a comprehensive range of ISO 13485 certification and training services for medical device manufacturers, as listed among the ISO 13485 audit firms Mexico list. Their training programs equip organizations with the essential knowledge required to establish and maintain effective quality management systems (QMS). By emphasizing practical implementation, these programs support both startups and established companies in navigating the complexities of compliance, ensuring that their performance systems are robust and appropriately scaled to meet operational demands.
The validation services offered by BSI are crucial for companies aiming to fulfill the stringent standards necessary for market entry. This not only bolsters their credibility within the industry but also streamlines interactions with regulatory bodies. Typically, organizations can expect to achieve ISO 13485 certification within a few months, according to the ISO 13485 audit firms Mexico list, depending on their level of preparedness and the complexity of their management systems.
Recent training initiatives by BSI have included specialized courses that focus on the latest updates in ISO 13485:2016, particularly its implications for medical device manufacturers. These programs underscore the importance of ensuring patient safety and compliance with regulations, both of which are vital for the successful commercialization of innovative products. Case studies illustrate how organizations participating in BSI's training have significantly improved their adherence rates and operational efficiencies, highlighting the tangible benefits of investing in management training. For instance, AIRO achieved a 52% increase in bone ablation efficiency, demonstrating the impact of effective management systems. Moreover, with over 15 years of experience in clinical research services, bioaccess® recognizes the necessity of a suitably scaled quality management system, as emphasized by Medtech consultant David Amor, who also stresses the importance of documentation and practical execution in meeting regulatory standards.
TÜV SÜD is recognized for its comprehensive ISO 13485 auditing and approval services, which are crucial for medical device manufacturers and feature in the ISO 13485 audit firms Mexico list to help them meet stringent regulatory standards. Their experienced auditors conduct thorough assessments, ensuring adherence to all pertinent regulations and industry best practices. This meticulous approach not only enhances product safety and quality but also fosters client trust throughout the approval process.
With an average audit duration of approximately [insert specific duration] that meets industry benchmarks, TÜV SÜD facilitates a streamlined path to approval, promoting smoother market access for innovative medical technologies. Their commitment to quality and safety is exemplified by successful instances, such as [insert specific examples], showcasing their vital role in aiding Medtech companies in achieving regulatory compliance and enhancing their market visibility.
Furthermore, TÜV SÜD's recent recognition as a Notified Body under MDR 2017/745 highlights their expertise and authority in the sector.
Maven Professional Services stands at the forefront of customized ISO 13485 compliance solutions for medical device manufacturers. Their team of experts collaborates closely with clients to thoroughly understand their unique requirements, crafting tailored strategies that ensure compliance. This bespoke approach not only streamlines the accreditation process but also enhances the overall quality management system, positioning clients for success in a competitive landscape.
PJR (Perry Johnson Registrars) distinguishes itself through its customer-focused ISO 13485 accreditation services, meticulously tailored to meet the specific needs of medical device manufacturers. Their dedicated team prioritizes client support, providing expert guidance throughout the qualification process. This unwavering commitment to excellence and customer satisfaction not only simplifies the regulatory journey but also empowers manufacturers to secure ISO 13485 approval efficiently.
With a typical qualification timeframe that aligns with industry standards, PJR assures clients of their ability to navigate the complexities of compliance with confidence. The advantages of PJR's support extend beyond mere certification; they cultivate a collaborative environment that enhances understanding and implementation of management systems, ultimately leading to improved product quality and regulatory readiness.
Case studies exemplify how PJR's customized approach has effectively facilitated ISO 13485 compliance for various Medtech firms, emphasizing the importance of client-centered services, which are crucial according to the ISO 13485 audit firms Mexico list for achieving and maintaining high industry standards.
Importantly, PJR's efficiency is on par with the swift ethical approvals provided by bioaccess®, which occur within 4-6 weeks, highlighting the critical importance of speed in the Medtech landscape.
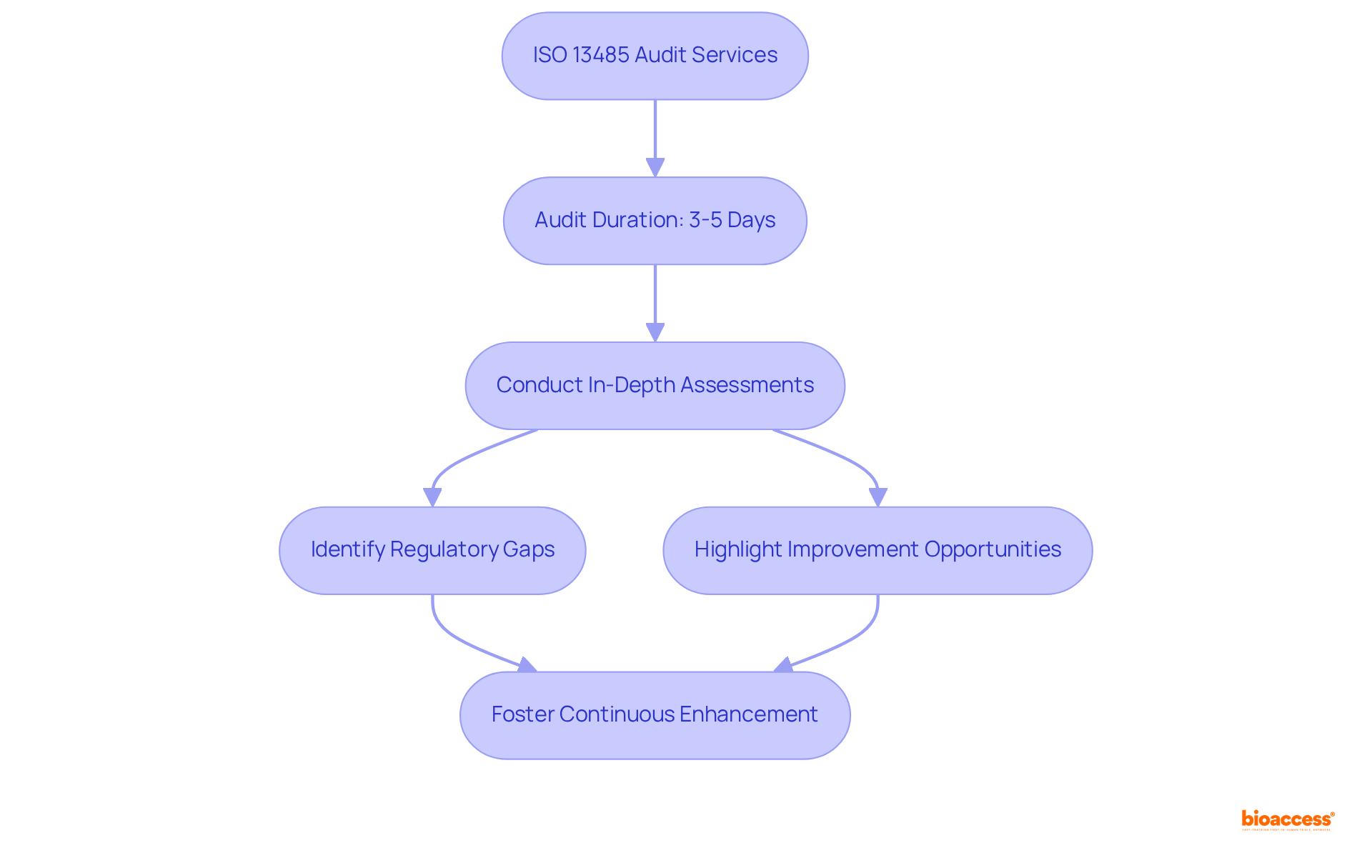
Pro QC International offers a comprehensive list of ISO 13485 audit firms in Mexico, providing audit services specifically designed for medical device manufacturers to ensure compliance with rigorous quality management standards. Their seasoned auditors perform in-depth assessments that not only identify regulatory gaps but also highlight opportunities for improvement, fostering a culture of continuous enhancement.
With an average audit duration of approximately 3 to 5 days, consistent with industry best practices, Pro QC underscores the necessity of meticulous audits to uphold regulatory standards within the Medtech sector. This commitment to excellence is reflected in the successful compliance stories of numerous manufacturers, illustrating how thorough evaluations can lead to significant operational improvements.
As noted in the 2025 Medical Device Industry Report, companies are increasingly struggling to meet management system requirements, making comprehensive audits vital for navigating the complexities of regulatory demands. Moreover, the report on the impact of internal silos indicates that dismantling these barriers through thorough evaluations can markedly improve quality outcomes.
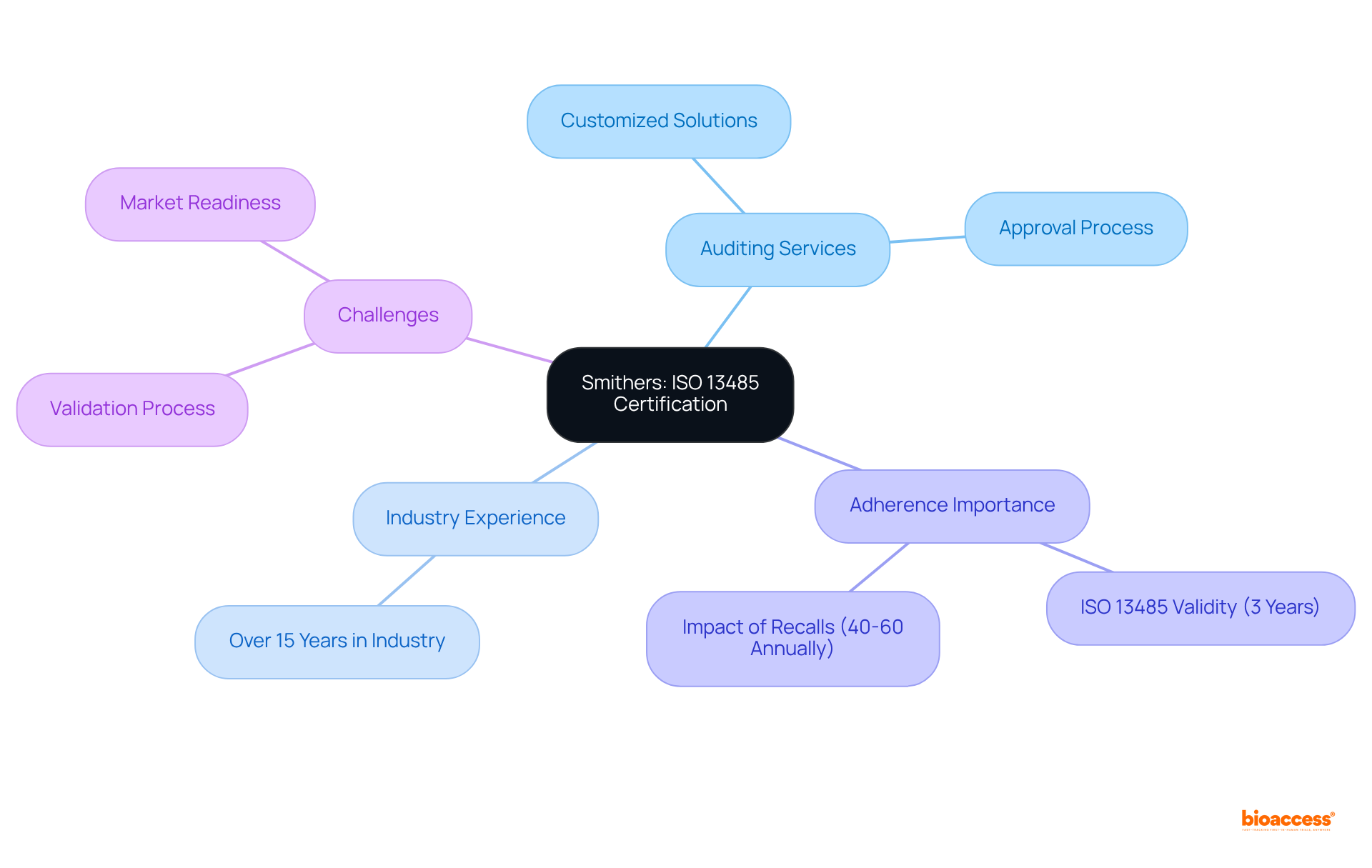
Smithers specializes in providing ISO 13485 audit firms Mexico list and auditing services tailored specifically for medical device producers. With over 15 years of industry experience, their expert team is adept at navigating the unique challenges faced by this sector. They provide customized solutions that facilitate adherence, allowing clients to manage the approval process with confidence. Notably, ISO 13485 accreditation is valid for three years, requiring manufacturers to uphold rigorous management systems to ensure compliance.
Furthermore, with approximately 40-60 medical device recalls occurring annually in the United States over the past five years, the critical importance of adherence and quality management in the medical device sector cannot be overstated. Smithers addresses key challenges in the validation process, guiding clients in overcoming obstacles and optimizing their path to market readiness.
DAC Audit Services excels in providing expert ISO 13485 conformity audits specifically tailored for medical device producers, which can be found in the ISO 13485 audit firms Mexico list. With a team of seasoned auditors, they conduct comprehensive assessments to guarantee that organizations adhere to all regulatory requirements. This unwavering commitment to quality and compliance not only facilitates efficient certification but also empowers clients in their mission to introduce safe and effective medical devices to the market.
The landscape of ISO 13485 audit firms in Mexico is crucial for ensuring that medical device manufacturers adhere to the stringent regulatory standards essential for market success. By highlighting ten key firms, this article emphasizes the necessity of specialized auditing services tailored to the unique challenges of the Medtech sector. These firms not only facilitate compliance but also enhance operational efficiencies, ultimately contributing to improved product quality and safety.
Key insights reveal that companies such as:
provide comprehensive services that streamline the certification process, reduce time to market, and foster a culture of continuous improvement. The focus on tailored solutions, rigorous auditing processes, and expert guidance underscores the critical nature of ISO 13485 compliance in upholding high industry standards and enhancing competitiveness.
As the Medtech industry continues to evolve, the importance of engaging with proficient ISO 13485 audit firms cannot be overstated. Manufacturers are urged to view compliance not merely as a regulatory obligation but as a strategic advantage that can accelerate their innovations to market swiftly and safely. Investing in quality management systems and expert auditing services is essential for sustaining trust and ensuring the safety of medical devices that impact lives.
What is bioaccess and what services does it provide?
bioaccess® specializes in expedited ISO 13485 audit services tailored for Medtech startups, leveraging its understanding of regulatory frameworks across Latin America, the Balkans, and Australia.
How does bioaccess facilitate the ISO 13485 approval process for startups?
In 2025, bioaccess® significantly reduces the duration to achieve ISO 13485 approval in Latin America, enabling startups to secure approval within weeks rather than months.
What are the benefits of ISO 13485 accreditation for Medtech companies?
Successful ISO 13485 accreditation leads to improved operational efficiency and heightened market competitiveness for Medtech companies.
Why is ISO 13485 compliance important in the Medtech sector?
Industry leaders emphasize that ISO 13485 compliance nurtures a culture of quality and safety, making it essential within the Medtech sector.
Who is Intertek and what role do they play in ISO 13485 certification?
Intertek is a global leader in ISO 13485 accreditation and auditing services, providing essential support to medical device manufacturers in navigating compliance complexities.
What services does Intertek offer to clients?
Intertek offers pre-assessment audits and training services to help producers meet local and global regulatory standards and foster continuous improvement.
What was Intertek's achievement in 2025 regarding ISO 13485 approvals?
In 2025, Intertek issued a substantial number of ISO 13485 approvals, showcasing its commitment to enhancing product quality and safety in the Medtech sector.
What specialized services does DNV provide for ISO 13485 certification?
DNV offers specialized ISO 13485 audit services tailored for the medical device industry, providing valuable insights to enhance the approval process.
How long does the ISO 13485 certification process typically take with DNV?
The average duration for ISO 13485 certification with DNV typically spans from three to six months.
What initiatives does DNV engage in to enhance regulatory procedures?
DNV's involvement in the TCP III initiative facilitates the sharing of audit reports, streamlining regulatory procedures and enhancing assurance and safety for medical devices.
How does DNV support clients in demonstrating adherence to international standards?
DNV's commitment to excellence and safety empowers clients to confidently demonstrate adherence to international standards, enhancing their market readiness and fostering trust among stakeholders.