


The article delineates the essential components that define the role of a Principal Investigator (PI) within clinical research. It details key responsibilities, qualifications, and the challenges faced by PIs. A successful PI must possess:
Additionally, they must navigate obstacles such as:
These elements are all critical for advancing medical research and improving patient outcomes.
The role of Principal Investigators (PIs) is pivotal in advancing clinical research; however, the complexities of this position often go unnoticed. Understanding the key aspects of the principal investigator job description reveals not only the responsibilities and qualifications required but also the profound impact these leaders have on research outcomes.
As PIs navigate the challenges of funding acquisition, regulatory compliance, and team management, a critical question arises: how can they leverage their unique skills and resources to enhance the efficiency and effectiveness of clinical trials?
This exploration delves into the essential elements that define a successful PI, providing valuable insights into their critical role in shaping the future of medical research.
bioaccess® empowers Principal Investigators by delivering a distinctive blend of regulatory speed, diverse patient populations, and an efficient ethical approval process that aligns with the principal investigator job description. With ethical approvals obtained in merely 4-6 weeks and enrollment rates that are 50% quicker than conventional markets, PIs can focus on their investigations without the usual holdups linked to medical studies.
This agility is crucial for early-phase studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), where timely access to data can significantly influence the trajectory of medical innovations. Successful early-phase studies in Medtech have shown that utilizing these advantages not only speeds up the research timeline but also improves the overall quality of health outcomes.
Additionally, bioaccess® provides $25K savings per patient with FDA-ready data, emphasizing that regulatory speed is vital in the principal investigator job description, as it directly impacts the ability to bring groundbreaking therapies to market swiftly, ultimately benefiting patient care and advancing medical knowledge.
Moreover, bioaccess® provides extensive research management services, which is essential to the principal investigator job description, ensuring that every detail from feasibility studies to project oversight is managed with proficiency, enabling PIs to concentrate on their strengths.

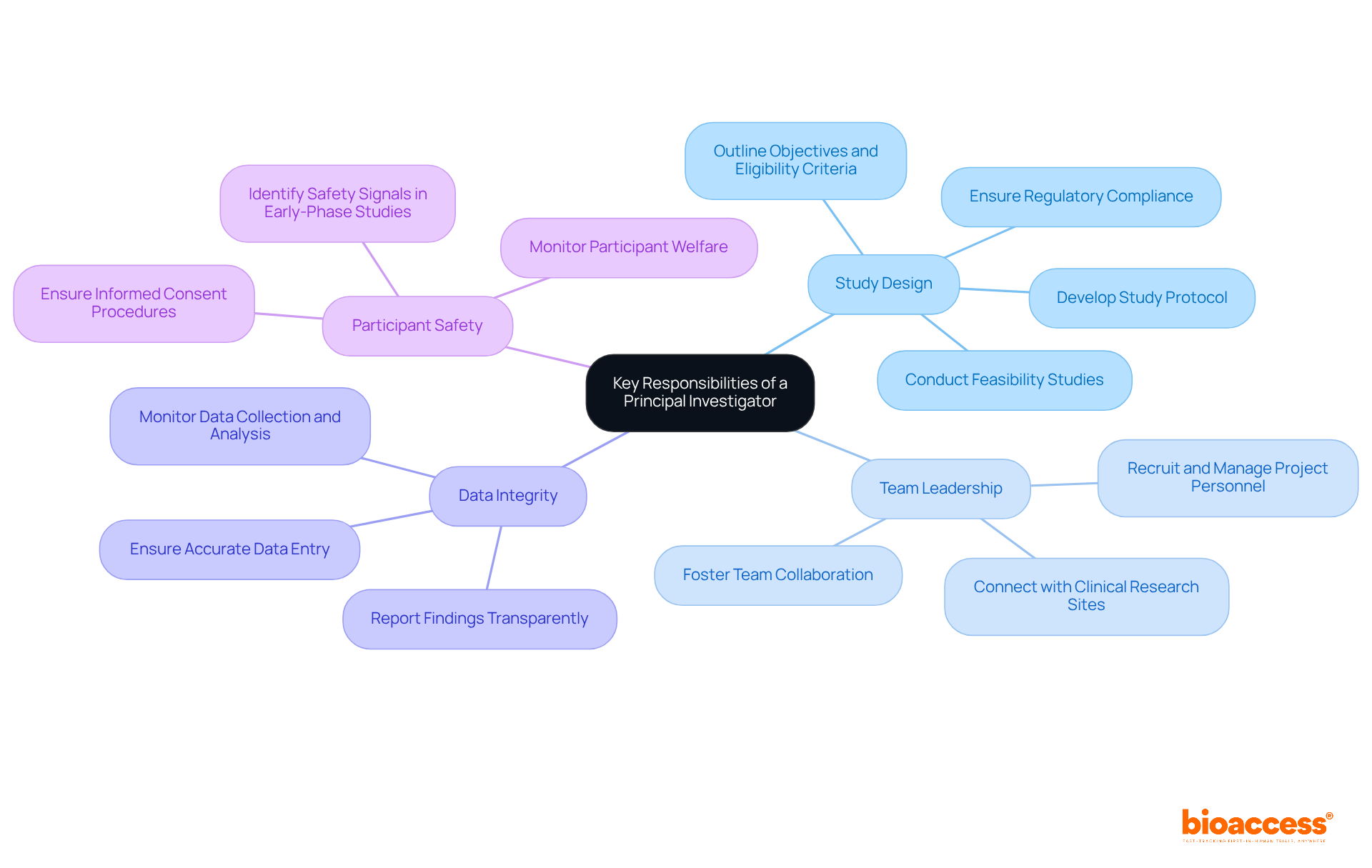
The principal investigator job description highlights the vital role that Principal Investigators (PIs) play in the design, execution, and oversight of clinical studies, ensuring that research is both scientifically rigorous and ethically sound. Their key responsibilities encompass several critical areas:
Current trends show that PIs are increasingly engaged in multi-site studies, which can improve recruitment success but also necessitate skilled management of varied teams and protocols. On average, numerous PIs oversee five or fewer research studies simultaneously, emphasizing the necessity for effective operational strategies to enhance study execution and participant recruitment. As the environment of medical investigation changes, the principal investigator job description necessitates that PIs adjust to new challenges, including the incorporation of technology in recruitment and data management, to improve the efficiency and effectiveness of trials. bioaccess® is dedicated to speeding up study outcomes, offering expert services that enable PIs to successfully navigate these complexities.
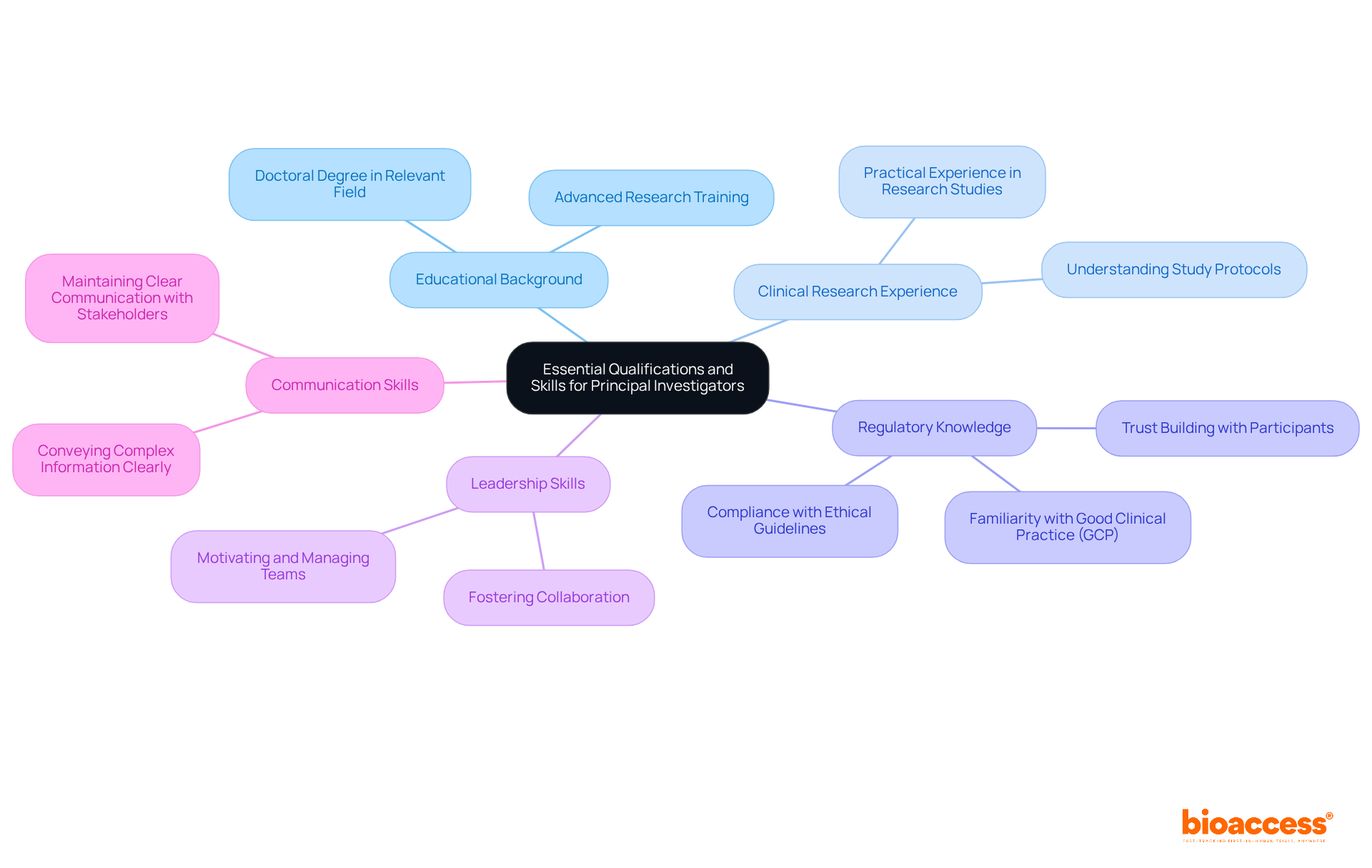
To excel as a Principal Investigator, individuals must possess a robust set of qualifications and skills, including:
Statistics show that a considerable number of Principal Investigators, according to the principal investigator job description, possess substantial medical experience, which boosts their credibility and effectiveness in directing studies. For example, a PI who obtains funding for over 20% of their grants is frequently viewed as exceptional in their area, highlighting the significance of both experience and skill in attaining success in studies.
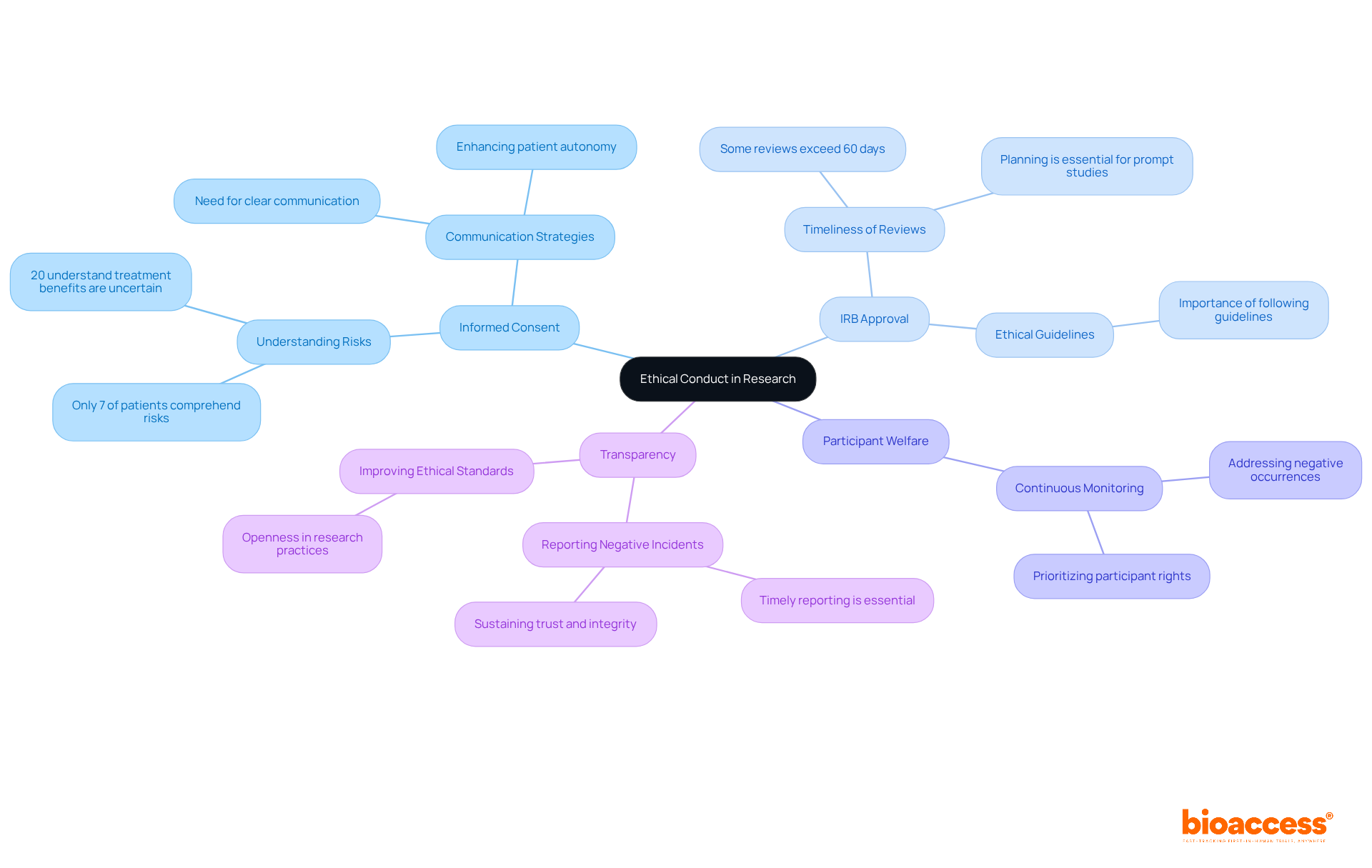
According to the principal investigator job description, Principal Investigators must prioritize ethical conduct by focusing on several key responsibilities.
Informed Consent: Ensuring that participants fully understand the study and voluntarily agree to participate is essential. Research indicates that comprehension of informed consent components is often inadequate, with only about 7% of patients grasping the risks associated with clinical trials. Furthermore, only 20% of participants understood that the benefits of treatment were uncertain. This highlights the critical need for clear communication strategies that enhance understanding and respect patient autonomy.
IRB Approval: Securing Institutional Review Board (IRB) approval is a prerequisite before starting any study. The review process can be lengthy, with some studies reporting review times exceeding the recommended 60 days. As highlighted by Daniel E. Hall, MD, the review durations noted at certain IRBs are significantly longer than this goal, emphasizing the significance of planning and following ethical guidelines to enable prompt studies.
Participant Welfare: Continuous monitoring of participant safety and rights is paramount. Investigators must be attentive to addressing any negative occurrences or issues that arise during the study, prioritizing participant welfare throughout the investigation process.
Transparency: Timely reporting of any negative incidents or protocol deviations is essential for sustaining trust and integrity in the investigative process. Openness not only promotes responsibility but also improves the ethical standards of research trials, ensuring that participants are aware of any alterations that might impact their participation.
By adhering to these principles, the principal investigator job description emphasizes the importance of maintaining the ethical standards required for conducting responsible and effective medical studies.
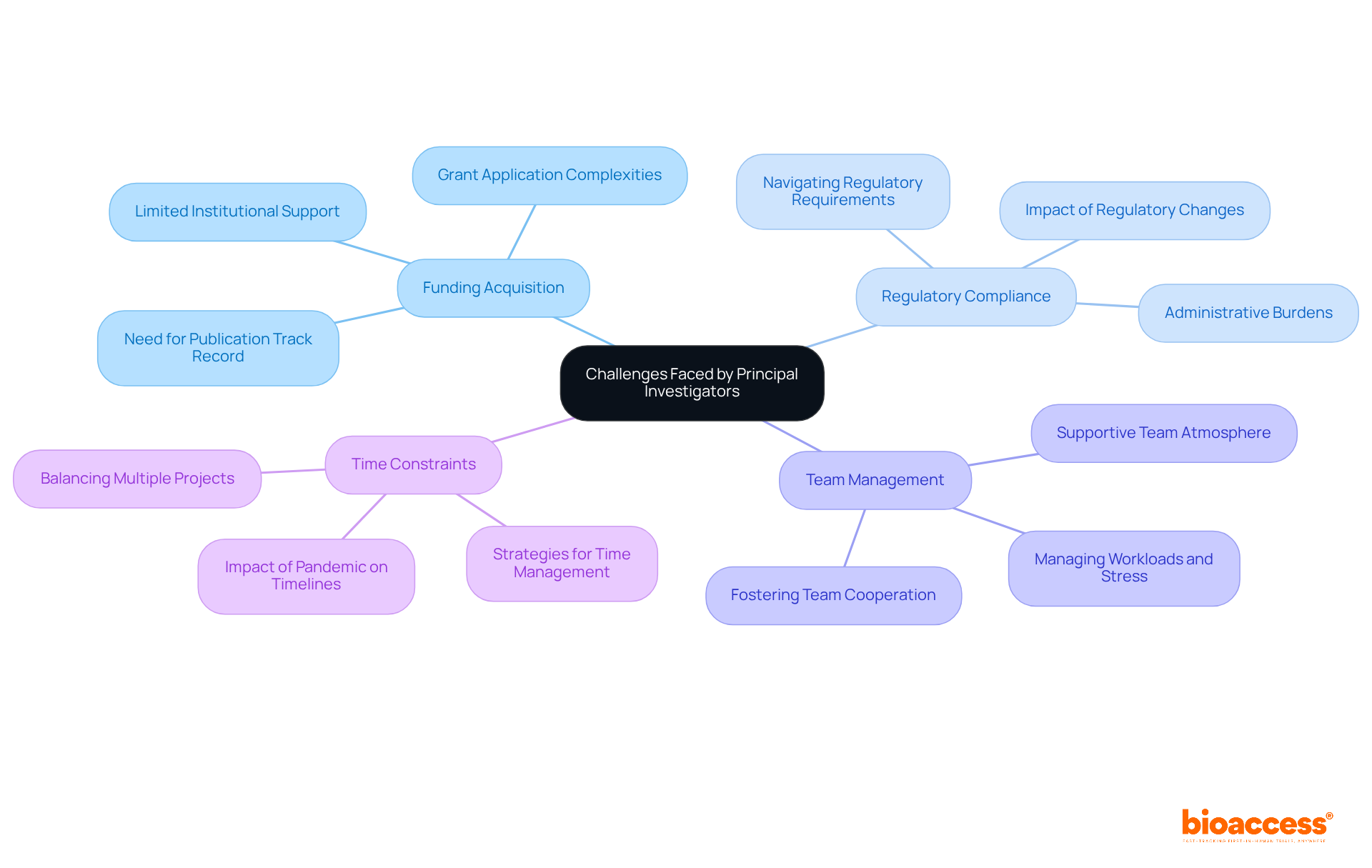
Principal Investigators (PIs) encounter a multitude of obstacles that can significantly impact their efficiency in clinical studies. Among these challenges, funding acquisition stands out prominently. Securing adequate funding for research projects is often a competitive and time-consuming endeavor. Many PIs grapple with the complexities of grant applications, a situation exacerbated by limited institutional support. Insights from funding agencies suggest that early-career researchers should prioritize building a robust track record of publications, particularly as first or corresponding authors, to improve their prospects for securing funding. A study underscores that new principal investigators must publish first-author papers to make a lasting impression on the scientific community.
Regulatory compliance presents another formidable challenge for PIs. Navigating the intricate landscape of regulatory requirements can be daunting, with compliance issues potentially leading to delays in the initiation of studies and increased administrative burdens. Data reveals that the lack of unified national strategies for clinical studies often results in disjointed efforts and erratic decision-making, complicating the compliance environment. For instance, research indicates that changes in the regulatory landscape during health emergencies can cause significant delays in recruitment initiation, adversely affecting study timelines. At bioaccess, we provide essential support in regulatory compliance, ensuring that study documents meet national requirements and streamlining the setup and approval processes through our expertise in regulatory affairs, particularly concerning medical devices and in vitro diagnostics.
Effective team management is crucial for the success of medical studies. PIs must foster efficient cooperation among diverse team members, ensuring alignment with project goals while managing interpersonal relationships. The combined demands of medical practice and research often lead to increased workloads and mental stress, making it imperative for PIs to cultivate a supportive team atmosphere. Interviewees have noted that the immaturity of trial capacity at hospitals exacerbates these challenges, highlighting the need for enhanced support and resources.
Time constraints further complicate the landscape for PIs. The management of multiple projects and deadlines can lead to significant stress and burnout. PIs frequently find themselves balancing various responsibilities, which detracts from their ability to concentrate on the scientific aspects of their work. Strategies for effective time management and prioritization are essential for sustaining productivity and well-being in this demanding role. The study emphasizes that the chaotic environment during the pandemic intensified these time constraints, fostering feelings of powerlessness among investigators.
These challenges underscore the critical need for robust support systems and resources, such as those offered by bioaccess, to empower Principal Investigators in their vital roles, as detailed in the principal investigator job description, within the clinical investigation ecosystem.
The effectiveness of a Principal Investigator (PI) is pivotal in determining research outcomes through several key dimensions:
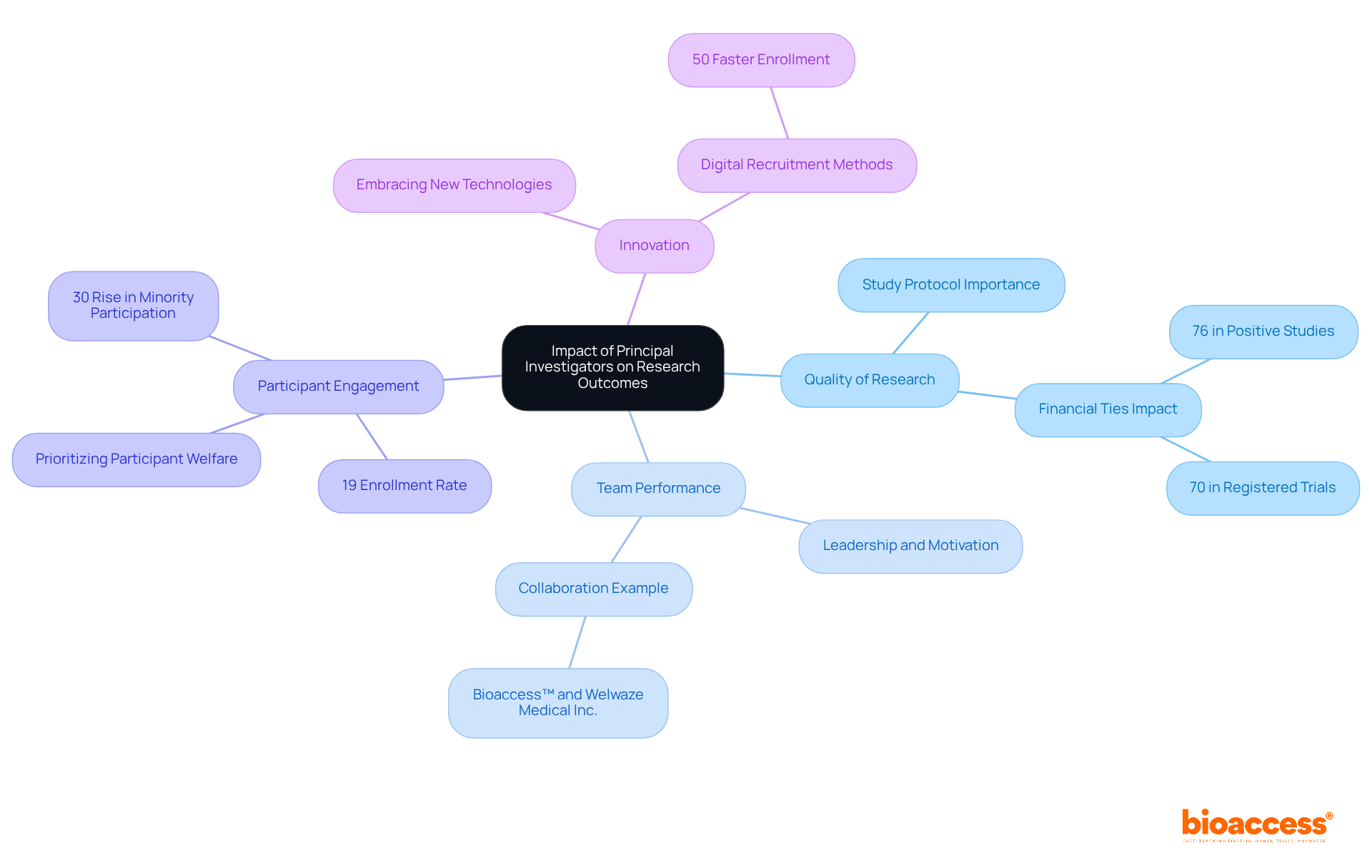
Quality of Research: A meticulously crafted study protocol is essential for generating reliable results. Research indicates that well-structured trials are more likely to yield valid conclusions, underscoring the importance of a PI's expertise in protocol design. Moreover, the prevalence of financial ties among PIs, observed to be 76% in positive studies, can significantly affect the integrity of study outcomes.
Team Performance: Strong leadership from PIs cultivates a motivated and productive research team. Effective PIs promote a setting of teamwork and responsibility, which is essential for managing the intricacies of medical studies. This is particularly relevant in collaborative efforts, such as the partnership between bioaccess™ and Welwaze Medical Inc. for the Celbrea® medical device launch in Colombia, where strategic leadership is essential for success. As Jonathan Kimmelman observed, doubt concerning PIs' assertions is crucial for guaranteeing the dependability of study results.
Participant Engagement: PIs who prioritize participant welfare significantly enhance recruitment and retention rates. Data indicate that studies conducted by dedicated PIs can experience up to a 30% rise in minority participation, emphasizing the significance of inclusive approaches in scientific inquiry. However, obstacles persist, as only around 19% of patients invited to take part in research studies actually sign up, indicating a need for enhanced outreach.
Innovation: PIs who embrace new technologies and methodologies can markedly improve study efficiency and outcomes. For example, studies employing digital recruitment methods have been proven to reach enrollment rates that are 50% quicker on average, highlighting the importance of creative strategies in medical studies. A case study on the over-optimistic predictions of PIs illustrates the gap between expected and actual outcomes, emphasizing the need for realistic forecasting.
In summary, the leadership and strategic vision described in the principal investigator job description are essential to the success of trials, influencing not only the quality of studies but also the overall participant experience and involvement. Directors of clinical studies should consider evaluating the financial ties of PIs when assessing their potential impact on study outcomes.
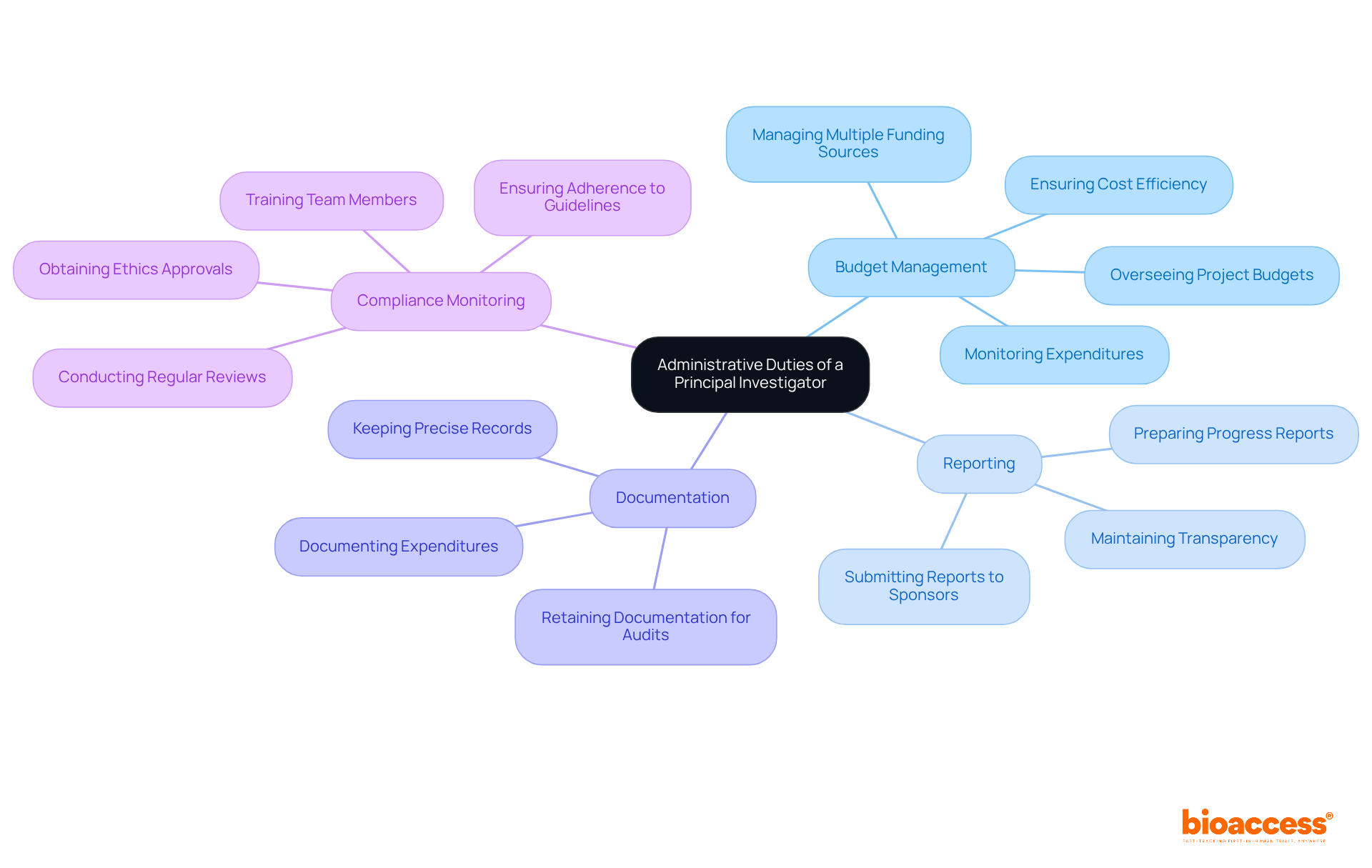
The principal investigator job description encompasses several key administrative duties that are vital for the successful execution of research projects.
Budget Management: PIs are tasked with overseeing project budgets, ensuring that funds are allocated appropriately and in accordance with the project's objectives. This includes monitoring expenditures to prevent cost overruns and ensuring that all charges are reasonable and necessary. A well-structured budget is essential for operational efficiency, allowing for the acquisition of necessary resources and equipment.
Reporting: PIs must prepare and submit progress reports to sponsors and regulatory bodies, detailing the status of the study and any significant developments. Timely submission of these reports is crucial, as it ensures compliance with funding requirements and maintains transparency with stakeholders.
Documentation: Keeping precise records of all investigative activities and communications is a fundamental duty of PIs. This documentation serves as a critical reference for audits and compliance checks, and it must be retained for a specified period after project completion.
Compliance Monitoring: PIs are responsible for ensuring that all study activities adhere to institutional and regulatory guidelines. This includes obtaining necessary ethics approvals and ensuring that all team members are trained in compliance protocols. Regular reviews of project activities help to identify any deviations from established guidelines, allowing for prompt corrective actions.
In addition to these core responsibilities, PIs often face challenges related to budget management, particularly when dealing with multiple funding sources. Effective communication and clear instructions for allocating charges among different funding streams are essential to maintain financial integrity throughout the project lifecycle. By prioritizing these administrative duties as detailed in the principal investigator job description, Principal Investigators can significantly enhance the quality and efficiency of clinical studies.
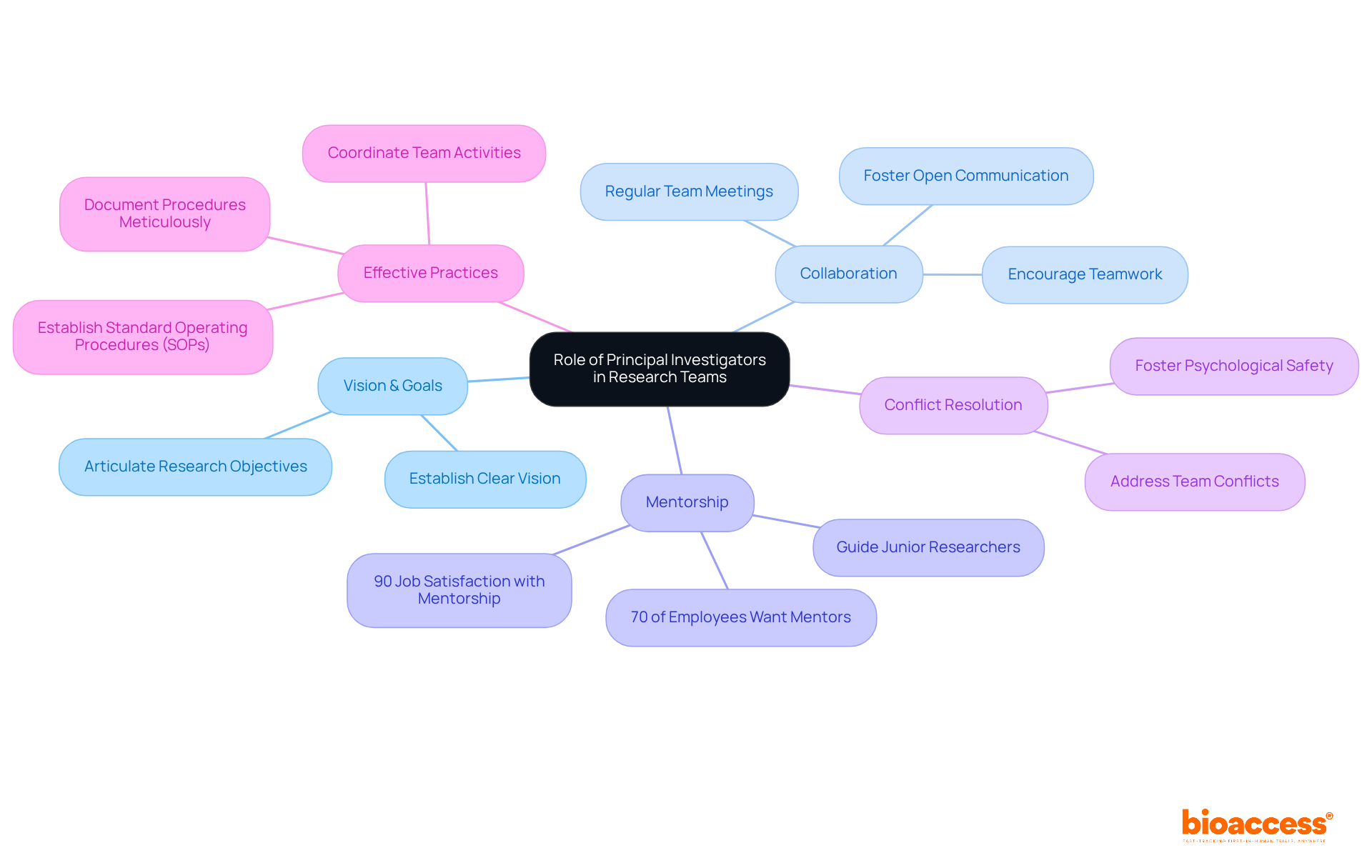
The principal investigator job description outlines the pivotal leadership role that Principal Investigators (PIs) serve in research teams by establishing a clear vision. They articulate specific research goals and objectives, ensuring that every team member comprehends the project’s direction and purpose. This foundational step is essential for aligning the team’s efforts and maximizing productivity.
Fostering collaboration is a critical responsibility specified in the principal investigator job description. They encourage open communication and teamwork, creating an environment where researchers feel comfortable sharing ideas and insights. Such a cooperative atmosphere is vital for enhancing the quality of studies and driving innovation. In fact, 70% of employees express a desire for multiple mentors, highlighting the importance of diverse perspectives in research endeavors.
Additionally, the principal investigator job description highlights the significant role PIs play in mentoring junior researchers. By offering guidance and support, they help cultivate the next generation of scientists. Effective mentorship has been shown to significantly enhance the success rates of junior researchers, with 90% of employees with mentors reporting job satisfaction. This statistic underscores the crucial role outlined in the principal investigator job description that PIs play in facilitating career advancement within the research community.
Conflict resolution is yet another essential function described in the principal investigator job description. They are tasked with addressing and resolving conflicts within the team, ensuring that disagreements do not impede progress. Their mediation skills foster a psychologically safe environment characterized by mutual trust and respect, which is vital for maintaining team morale and productivity.
Successful PIs understand that the principal investigator job description involves collaboration that transcends mere teamwork; it includes actively engaging with team members to leverage diverse perspectives and expertise. For instance, implementing regular team meetings and defining clear roles can significantly enhance coordination and accountability. Furthermore, the principal investigator job description emphasizes the importance of mentorship and collaboration, which often leads to improved outcomes in both the quality of studies and team dynamics, ultimately resulting in more substantial scientific contributions. As noted, thorough documentation and the establishment of Standard Operating Procedures (SOPs) are effective practices that PIs can adopt to bolster collaboration and ensure integrity in their work.
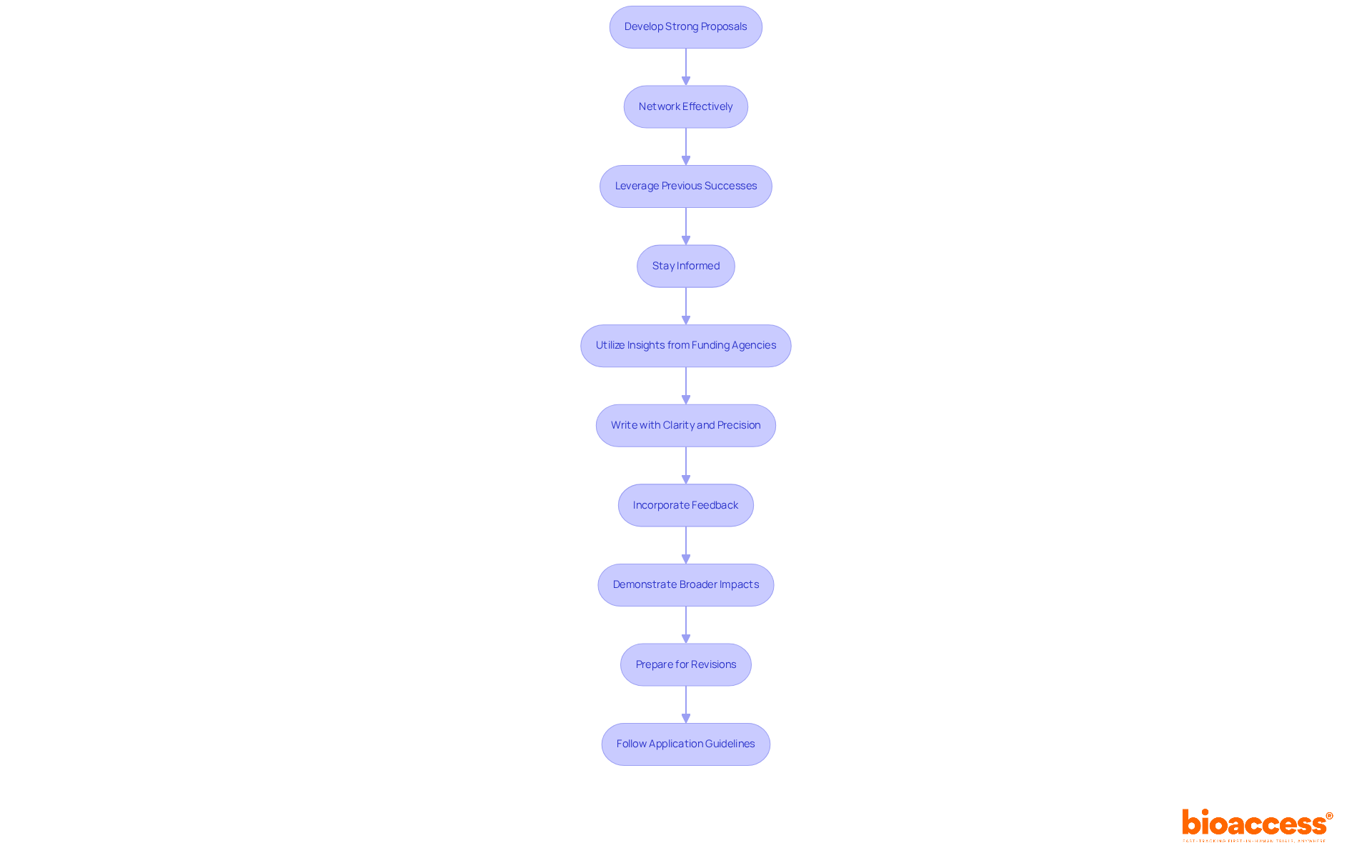
To secure research funding, Principal Investigators should:
Develop Strong Proposals: Craft compelling grant proposals that clearly articulate the research objectives, significance, and potential impact. Successful proposals often include a well-defined methodology and a clear budget, significantly enhancing the chances of funding approval.
Network Effectively: Building relationships with funding agencies and fellow researchers is crucial. Statistics show that networking can enhance the chances of obtaining funding, as 22.3% of grants in recent years were awarded to clinically active investigators or teams, emphasizing the significance of collaboration and visibility within the scientific community.
Leverage Previous Successes: Highlighting past accomplishments instills confidence in potential funders. Demonstrating a track record of successful projects showcases capability and reliability, essential for gaining trust from funding bodies.
Stay Informed: Keeping abreast of funding trends and opportunities is vital. For example, the NIH has streamlined peer review procedures for numerous project grants, influencing application strategies. Understanding these changes is crucial for the principal investigator job description, as it helps Principal Investigators tailor their proposals to align with current funding priorities. Additionally, referencing NIGMS's analysis of trends in its RPG portfolio provides insights into funding decisions.
Utilize Insights from Funding Agencies: Engaging with insights from funding agencies offers valuable guidance on what constitutes a strong proposal. For instance, the NIGMS emphasizes the importance of transparency in funding trends, which is crucial for understanding the principal investigator job description and how proposals are structured and presented.
Write with Clarity and Precision: Successful grant proposals are characterized by clear and concise writing. Avoiding jargon and ensuring accessibility to reviewers from diverse backgrounds enhances understanding and support for the project.
Incorporate Feedback: Seeking feedback from colleagues or mentors on proposal drafts provides new perspectives and improves the overall quality of the submission. This collaborative approach leads to stronger proposals that resonate with funding bodies.
Demonstrate Broader Impacts: Clearly articulating the broader impacts of the study can be a decisive factor for funding agencies. This includes potential societal benefits, contributions to the field, and implications for policy or practice. Including relevant statistics related to success rates for different demographics strengthens this argument.
Prepare for Revisions: Many successful proposals undergo multiple revisions before submission. Being open to constructive criticism and willing to refine the proposal significantly enhances its quality and competitiveness.
Follow Application Guidelines: Adhering strictly to the application guidelines provided by funding agencies is essential. Non-compliance can lead to automatic disqualification, regardless of the proposal's quality, making it crucial for applicants to understand and follow these guidelines.
The pathway to becoming a Principal Investigator (PI) is defined by the principal investigator job description, which involves several key steps that are essential for success in the field of clinical research.
Incorporating these elements not only enhances the relevance of the content but also provides a comprehensive overview of the pathway to becoming a Principal Investigator.

The role of a Principal Investigator (PI) is integral to the success of clinical research. This is underscored by the focus on leadership, ethical conduct, and effective management. PIs navigate complex responsibilities that directly influence research outcomes and participant safety. Insights illustrate how PIs can leverage resources, such as those offered by bioaccess®, to streamline processes and enhance the quality of their studies.
Key aspects of the principal investigator job description encompass:
The challenges faced by PIs—such as securing funding and managing multiple projects—underscore the importance of building strong networks and developing clear communication strategies. The emphasis on ethical considerations, from informed consent to participant welfare, further reinforces the critical nature of the PI’s role in upholding the integrity of clinical research.
Ultimately, the contributions of Principal Investigators extend beyond individual studies; they shape the future of medical advancements and patient care. As the landscape of clinical research evolves, PIs are encouraged to embrace innovative methodologies and foster collaborative environments within their teams. By doing so, they enhance their effectiveness and contribute to the broader goals of scientific inquiry and public health. Engaging with resources like bioaccess® can provide valuable support in overcoming obstacles and achieving successful research outcomes, ensuring that the vital work of Principal Investigators remains impactful in advancing medical knowledge.
What is bioaccess® and how does it benefit Principal Investigators (PIs)?
bioaccess® is a platform that accelerates clinical research for PIs by providing regulatory speed, diverse patient populations, and an efficient ethical approval process. It enables PIs to obtain ethical approvals in 4-6 weeks and achieve enrollment rates that are 50% quicker than conventional markets, allowing them to focus on their investigations without delays.
Why is regulatory speed important for Principal Investigators?
Regulatory speed is crucial because it directly impacts the ability to bring innovative therapies to market quickly, benefiting patient care and advancing medical knowledge. With bioaccess®, PIs can save $25K per patient with FDA-ready data, which enhances the overall quality of health outcomes.
What are the key responsibilities of a Principal Investigator?
Key responsibilities include study design, team leadership, ensuring regulatory compliance and ethical standards, monitoring data integrity, and ensuring participant safety. PIs must develop study protocols, manage project personnel, maintain regulatory documents, supervise data collection, and protect study participants' rights.
What qualifications and skills are essential for Principal Investigators?
Essential qualifications include a doctoral degree in a relevant field, clinical research experience, regulatory knowledge, leadership skills, and strong communication skills. These qualifications help PIs navigate complex study protocols and effectively manage diverse teams.
How does bioaccess® support Principal Investigators in their role?
bioaccess® supports PIs by offering comprehensive feasibility studies, compliance reviews, project management services, and connecting them with innovative Medtech, Biopharma, and Radiopharma startups. This support helps PIs streamline study execution and participant recruitment.
What trends are currently affecting the role of Principal Investigators?
Current trends show that PIs are increasingly engaged in multi-site studies to improve recruitment success. They must also adapt to new challenges, including incorporating technology in recruitment and data management to enhance trial efficiency and effectiveness.