


The biotechnology trends to monitor in 2024 encompass significant advancements in:
These trends signify profound innovations and regulatory changes that are set to transform the biotech landscape. Companies are strategically leveraging these technologies to enhance research and therapeutic applications, underscoring their relevance in the clinical research arena.
As the biotechnology landscape evolves, 2024 promises to be a pivotal year, marked by groundbreaking trends and innovations. The rapid advancements in artificial intelligence are reshaping research methodologies, while the transformative potential of CRISPR technology positions the industry on the brink of a revolution. However, amidst this excitement lies a crucial question: how will emerging technologies and regulatory frameworks adapt to ensure ethical practices and effective market access? This article delves into ten key biotechnology trends to watch, providing insights into the future of healthcare and the opportunities that lie ahead.
bioaccess® is transforming the clinical research landscape by offering rapid ethical approvals and expedited patient enrollment, particularly in Colombia, where the total IRB/EC and MoH (INVIMA) review process takes just 90 to 120 days.
By capitalizing on Colombia's cost efficiency—providing savings exceeding 30% compared to North America and Western Europe—and its high-quality healthcare system, recognized among the best globally, bioaccess® empowers Medtech innovators to bring their products to market more effectively.
Furthermore, with a population exceeding 50 million and 95% coverage under universal healthcare, patient recruitment is accelerated through pre-qualified networks, facilitating site activation in under eight weeks.
This agility not only accelerates the advancement of medical devices but also enhances the overall innovation environment in biotechnology, marking it as a significant phenomenon to watch in 2024.

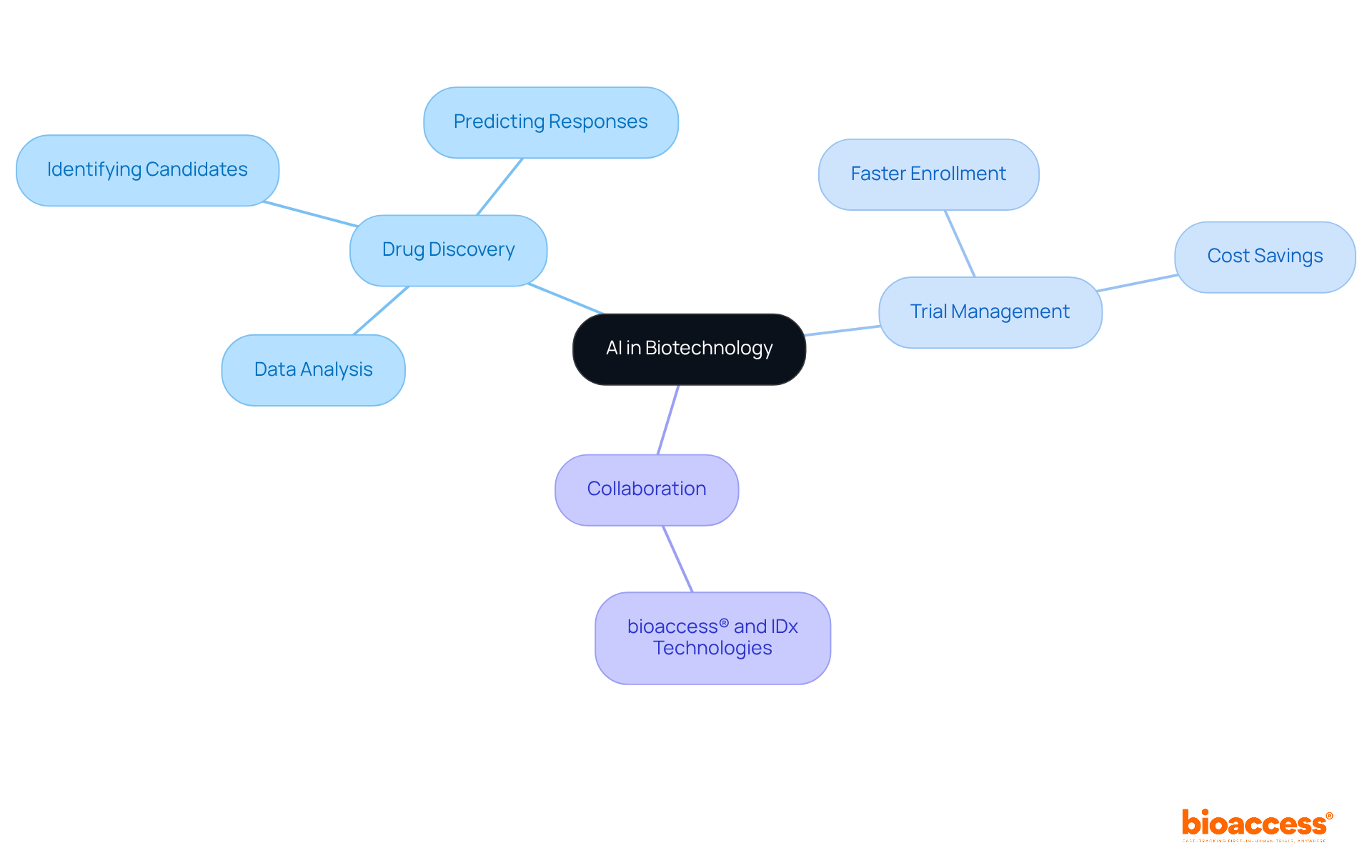
Artificial Intelligence is progressively being incorporated into biotech activities, from drug discovery to trial management. This integration is not merely a trend; it represents a significant advancement in the field. AI algorithms can analyze vast datasets to identify potential drug candidates, predict patient responses, and optimize trial designs. Notably, bioaccess® stands at the forefront of this transformation, achieving 50% faster patient enrollment and $25K savings per patient through its FDA-ready data solutions. Such capabilities do not just accelerate research timelines; they also enhance the precision of outcomes, establishing AI as a pivotal force in biotechnology 2024. Furthermore, bioaccess®'s collaboration with IDx Technologies exemplifies the role of AI in improving accessibility for disease detection in Latin America, underscoring how inventive solutions can revolutionize clinical practices.
CRISPR technology is rapidly advancing, with new applications emerging in gene editing, agriculture, and therapeutic development. Researchers are actively exploring its potential to treat genetic disorders and enhance crop resilience, leading to significant growth in the market for CRISPR-related products. This trend underscores the critical importance of oversight considerations, particularly in Colombia, where INVIMA (Colombia National Food and Drug Surveillance Institute) plays a vital role in supervising the marketing and production of health products, including medical devices.
As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that products adhere to stringent safety, efficacy, and quality standards. This governance framework becomes increasingly essential as the biotechnology landscape expands, emphasizing the ethical implications and the necessity for compliance in the development of innovative solutions.

Psychedelics and neuroplastogens are emerging as promising treatments for mental health disorders, including depression and PTSD. The expanding research into their therapeutic effects is bolstered by evolving regulatory attitudes and a surge in public interest. This trend signifies a shift towards more comprehensive strategies within biotechnology 2024, underscoring the critical need for rigorous trials to validate these treatments.
Companies like bioaccess® are leading this charge, providing accelerated clinical trial services that bridge innovative Medtech, Biopharma, and Radiopharma startups with premier clinical research sites across Latin America, Eastern Europe, and Australia. By facilitating faster approvals and enhancing patient recruitment, bioaccess® plays a pivotal role in advancing these groundbreaking therapies to the next phase of development efficiently.
RNA technology, particularly mRNA, has fundamentally transformed vaccine development and is now being investigated for a range of therapeutic applications, including cancer treatment and genetic disorders. The capacity to design RNA molecules that instruct cells to produce specific proteins paves the way for advancements in personalized medicine. This trend is poised to gain momentum in biotechnology 2024 as ongoing research continues to validate the efficacy of RNA-based therapies.
Bioprinting and tissue engineering stand at the forefront of regenerative medicine, facilitating the creation of intricate tissue structures for both research and therapeutic applications. These groundbreaking technologies promise to tackle organ shortages and enhance drug testing models. As advancements continue, particularly in Latin America, navigating the legal landscape becomes increasingly crucial.
Regulatory authorities, such as INVIMA in Colombia, play an essential role in overseeing medical devices and ensuring adherence to safety and efficacy standards. As these regulatory frameworks adapt to incorporate innovative technologies, they will significantly influence access strategies for companies seeking to introduce bioprinting and tissue engineering solutions in the region.
This evolving regulatory environment in biotechnology 2024 warrants close attention.
The biotechnology sector in 2024 is currently experiencing a notable surge in mergers and acquisitions as companies strive to enhance their capabilities and broaden their market reach. This trend is significantly shaped by financial leaders such as John Myklusch, a seasoned Chief Financial Officer with over 20 years of experience in steering businesses through intricate exit strategies, including partnership sales and private equity investments. His profound expertise in financial strategy and risk management proves crucial as companies navigate these strategic maneuvers.
These efforts often culminate in increased investments in research and development, thereby fostering innovation. As companies consolidate resources and expertise, the biotechnology landscape will inevitably continue to evolve, making biotechnology 2024 a pivotal trend to monitor in mergers and acquisitions.

Targeted protein degradation is emerging as a promising strategy for drug development, facilitating the selective removal of disease-causing proteins. This innovative approach holds the potential to tackle previously 'undruggable' targets, thereby expanding the therapeutic landscape. As research progresses, the implications for trials and approval processes will be significant, particularly in light of bioaccess®'s cutting-edge 6-8 week sprint method for authorization. This expedited procedure not only accelerates patient enrollment for cardiology and neurology groups but also aids in overcoming compliance challenges that often hinder early-phase trials.
With comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, and project management—bioaccess® is strategically positioned to support the evolving landscape of biotechnology 2024.
Stem cell technology is revolutionizing regenerative medicine and therapeutic applications, heralding groundbreaking advancements. Research into the differentiation and application of stem cells is rapidly expanding, with promising potential in treating degenerative diseases and injuries. As the field progresses, oversight considerations will be paramount in the development and commercialization of stem cell therapies.
In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) oversees the regulatory framework for health products, including medical devices and therapies that utilize stem cells. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA ensures that these innovations adhere to stringent safety, efficacy, and quality standards.
This supervisory framework represents a significant trend for biotechnology 2024, influencing how stem cell technologies are developed and introduced into the market.
As biotechnology 2024 continues to evolve, effective access strategies are crucial for companies seeking to penetrate emerging economies. Understanding local regulations, such as those enforced by INVIMA in Colombia, is essential for ensuring compliance and facilitating smoother product launches. Recognized as a Level 4 health authority by PAHO/WHO, INVIMA plays a vital role in overseeing medical devices, ensuring their safety and efficacy.
Furthermore, utilizing services provided by bioaccess®, which encompass:
can significantly enhance the pace at which companies navigate regulatory processes. This trend underscores the importance of strategic planning and collaboration in navigating the complexities of market access in biotechnology 2024, particularly as Medtech clinical studies contribute to local economies through job creation, economic growth, and improved healthcare outcomes.

The landscape of biotechnology in 2024 is characterized by transformative trends poised to reshape the industry. Rapid advancements in clinical research, facilitated by bioaccess®, alongside groundbreaking applications of artificial intelligence, position the sector for significant growth and innovation. These developments enhance the efficiency and effectiveness of research while paving the way for new therapeutic possibilities that can globally improve patient outcomes.
Key insights from the article underscore the pivotal role of:
Each trend highlights the critical need for regulatory oversight, particularly in regions like Colombia, where INVIMA ensures compliance with safety and efficacy standards. Furthermore, the surge in mergers and acquisitions within the biotech sector indicates a strategic shift towards consolidating resources and expertise to drive further advancements.
As biotechnology evolves, stakeholders must remain vigilant and proactive in navigating the regulatory landscapes and market access strategies that will define the industry's future. Embracing collaboration and leveraging innovative solutions will be essential for companies aiming to capitalize on these trends and contribute to a healthier, more sustainable world. The advancements in biotechnology not only hold promise for medical breakthroughs but also represent a significant opportunity for economic growth and improved healthcare outcomes across emerging markets.
What is bioaccess® and how does it impact clinical research?
bioaccess® is transforming the clinical research landscape by providing rapid ethical approvals and expedited patient enrollment, particularly in Colombia, where the total review process takes just 90 to 120 days.
What advantages does Colombia offer for Medtech innovations?
Colombia offers cost efficiency with savings exceeding 30% compared to North America and Western Europe, alongside a high-quality healthcare system. These factors enable Medtech innovators to bring their products to market more effectively.
How does bioaccess® facilitate patient recruitment in Colombia?
With a population exceeding 50 million and 95% coverage under universal healthcare, patient recruitment is accelerated through pre-qualified networks, allowing for site activation in under eight weeks.
What role does Artificial Intelligence play in biotech operations?
Artificial Intelligence is being integrated into biotech activities, such as drug discovery and trial management, to analyze vast datasets, identify drug candidates, predict patient responses, and optimize trial designs.
How does bioaccess® utilize AI in clinical research?
bioaccess® achieves 50% faster patient enrollment and saves $25K per patient through its FDA-ready data solutions, enhancing research timelines and precision of outcomes.
What is the significance of bioaccess®'s collaboration with IDx Technologies?
This collaboration exemplifies how AI can improve accessibility for disease detection in Latin America, showcasing inventive solutions that can revolutionize clinical practices.
What are the current applications of CRISPR technology?
CRISPR technology is advancing in gene editing, agriculture, and therapeutic development, with researchers exploring its potential to treat genetic disorders and enhance crop resilience.
What role does INVIMA play in the biotechnology landscape in Colombia?
INVIMA ensures that health products, including medical devices, adhere to stringent safety, efficacy, and quality standards as a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization.
Why is oversight critical in the development of biotechnological innovations?
As the biotechnology landscape expands, oversight becomes essential to address ethical implications and ensure compliance in the development of innovative solutions.